| Issue |
A&A
Volume 647, March 2021
|
|
|---|---|---|
| Article Number | A10 | |
| Number of page(s) | 25 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/202039756 | |
| Published online | 02 March 2021 | |
Bottlenecks to interstellar sulfur chemistry
Sulfur-bearing hydrides in UV-illuminated gas and grains
1
Instituto de Física Fundamental (CSIC). Calle Serrano 121-123,
28006
Madrid,
Spain
e-mail: javier.r.goicoechea@csic.es
2
Facultad de Ciencias. Universidad Autónoma de Madrid,
28049
Madrid,
Spain
3
Institut de Radioastronomie Millimétrique (IRAM),
Grenoble, France
4
LERMA, Observatoire de Paris, PSL Research University, CNRS, Sorbonne Universités,
92190
Meudon, France
5
Observatorio Astronómico Nacional (OAN),
Alfonso XII, 3,
28014
Madrid, Spain
6
Max-Planck-Institut für Radioastronomie,
Auf dem Hügel 69,
53121
Bonn,
Germany
7
OASU/LAB-UMR5804, CNRS, Université Bordeaux,
33615
Pessac, France
8
European Southern Observatory,
Alonso de Cordova 3107,
Vitacura,
Santiago, Chile
Received:
25
October
2020
Accepted:
23
December
2020
Hydride molecules lie at the base of interstellar chemistry, but the synthesis of sulfuretted hydrides is poorly understood and their abundances often crudely constrained. Motivated by new observations of the Orion Bar photodissociation region (PDR) – 1″ resolution ALMA images of SH+; IRAM 30 m detections of bright H232S, H234S, and H233S lines; H3S+ (upper limits); and SOFIA/GREAT observations of SH (upper limits) – we perform a systematic study of the chemistry of sulfur-bearing hydrides. We self-consistently determine their column densities using coupled excitation, radiative transfer as well as chemical formation and destruction models. We revise some of the key gas-phase reactions that lead to their chemical synthesis. This includes ab initio quantum calculations of the vibrational-state-dependent reactions SH+ + H2(v) ⇄ H2S+ + H and S + H2 (v) ⇄ SH + H. We find that reactions of UV-pumped H2(v ≥ 2) molecules with S+ ions explain the presence of SH+ in a high thermal-pressure gas component, Pth∕k ≈ 108 cm−3 K, close to the H2 dissociation front (at AV < 2 mag). These PDR layers are characterized by no or very little depletion of elemental sulfur from the gas. However, subsequent hydrogen abstraction reactions of SH+, H2S+, and S atoms with vibrationally excited H2, fail to form enough H2S+, H3S+, and SH to ultimately explain the observed H2S column density (~2.5 × 1014 cm−2, with an ortho-to-para ratio of 2.9 ± 0.3; consistent with the high-temperature statistical value). To overcome these bottlenecks, we build PDR models that include a simple network of grain surface reactions leading to the formation of solid H2S (s-H2S). The higher adsorption binding energies of S and SH suggested by recent studies imply that S atoms adsorb on grains (and form s-H2S) at warmer dust temperatures (Td < 50 K) and closer to the UV-illuminated edges of molecular clouds. We show that everywhere s-H2S mantles form(ed), gas-phase H2S emission lines will be detectable. Photodesorption and, to a lesser extent, chemical desorption, produce roughly the same H2S column density (a few 1014 cm−2) and abundance peak (a few 10−8) nearly independently of nH and G0. This agrees with the observed H2S column density in the Orion Bar as well as at the edges of dark clouds without invoking substantial depletion of elemental sulfur abundances.
Key words: astrochemistry / line: identification / ISM: clouds / photon-dominated region
© ESO 2021
1 Introduction
Hydride molecules play a pivotal role in interstellar chemistry (e.g., Gerin et al. 2016), being among the first molecules to form in diffuse interstellar clouds and at the UV-illuminated edges of dense star-forming clouds, so-called photodissociation regions (PDRs; Hollenbach & Tielens 1997). Sulfur is on the top ten list of most abundant cosmic elements and it is particularly relevant for astrochemistry and star-formation studies. Its low ionization potential (10.4 eV) makes the photoionization of S atoms a dominant source of electrons in molecular gas at intermediate visual extinctions AV ≃ 2–4 mag (Sternberg & Dalgarno 1995; Goicoechea et al. 2009; Fuente et al. 2016).
The sulfur abundance, [S/H], in diffuse clouds (Howk et al. 2006) is very close to the [S/H] measured in the solar photosphere ([S/H]⊙ ≃ 1.4 ×10−5; Asplund et al. 2009). Still, the observed abundances of S-bearing molecules in diffuse and translucent molecular clouds (nH ≃102 − 103 cm−3) make up avery small fraction, <1 %, of the sulfur nuclei (mostly locked as S+; Tieftrunk et al. 1994; Turner 1996; Lucas & Liszt 2002; Neufeld et al. 2015). In colder dark clouds and dense cores shielded from stellar UV radiation, most sulfur is expected in molecular form. However, the result of adding the abundances of all detected gas-phase S-bearing molecules is typically a factor of ~102–103 lower than [S/H]⊙ (e.g., Fuente et al. 2019). Hence, it is historically assumed that sulfur species deplete on grain mantles at cold temperatures and high densities (e.g., Graedel et al. 1982; Millar & Herbst 1990; Agúndez & Wakelam 2013). However, recent chemical models predict that the major sulfur reservoir in dark clouds can be either gas-phase neutral S atoms (Vidal et al. 2017; Navarro-Almaida et al. 2020) or organo-sulfur species trapped on grains (Laas & Caselli 2019). Unfortunately, it is difficult to overcome this dichotomy from an observational perspective. In particular, no ice carrier of an abundant sulfur reservoir other than solid OCS (hereafter s-OCS, with an abundance of ~10−8 with respect to H nuclei; Palumbo et al. 1997) has been convincingly identified. Considering the large abundances of water ice (s-H2 O) grain mantles in dense molecular clouds and cold protostellar envelopes (see reviews by van Dishoeck 2004; Gibb et al. 2004; Dartois 2005), one may also expect hydrogen sulfide (s-H2 S) to be the dominant sulfur reservoir. Indeed, s-H2S is the most abundant S-bearing ice in comets such as 67P/Churyumov-Gerasimenko (Calmonte et al. 2016). However, only upper limits to the s-H2 S abundance of ≲1% relative to water ice have so far been estimated toward a few interstellar sightlines (e.g., Smith 1991; Jiménez-Escobar & Muñoz Caro 2011). These values imply a maximum s-H2 S ice abundance of several 10−6 with respect to H nuclei. Still, this upper limit could be higher if s-H2S ices are well mixed with s-H2O and s-CO ices (Brittain et al. 2020).
The bright rims of molecular clouds illuminated by nearby massive stars are intermediate environments between diffuse and cold dark clouds. Such environments host the transition from ionized S+ to neutral atomic S, as well as the gradual formation of S-bearing molecules (Sternberg & Dalgarno 1995). In one prototypical low-illumination PDR, the edge of the Horsehead nebula, Goicoechea et al. (2006) inferred very modest gas-phase sulfur depletions. In addition, the detection of narrow sulfur radio recombination lines in dark clouds (implying the presence of S+ ; Pankonin & Walmsley 1978) is an argument against large sulfur depletions in the mildly illuminated surfaces of these clouds. The presence of new S-bearing molecules such as S2H, the first (and so far only) doubly sulfuretted species detected in a PDR (Fuente et al. 2017), suggests that the chemical pathways leading to the synthesis of sulfuretted species are not well constrained; and that the list of S-bearing molecules is likely not complete.
Interstellar sulfur chemistry is unusual compared to that of other elements in that none of the simplest species, X = S, S+, SH, SH+, or H2 S+, react exothermically with H2 (v = 0) in the initiation reactions X + H2 → XH + H (so-called hydrogen abstraction reactions). Hence, one would expect a slow sulfur chemistry and very low abundances of SH+ (sulfanylium) and SH (mercapto) radicals in cold interstellar gas. However, H2 S (Lucas & Liszt 2002),SH+ (Menten et al. 2011; Godard et al. 2012), and SH (Neufeld et al. 2012, 2015) have been detected in low-density diffuse clouds (nH ≲ 100 cm−3) through absorption measurements of their ground-state rotational lines1. In UV-illuminated gas, most sulfur atoms are ionized, but the very high endothermicity of reaction
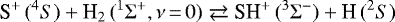 (1)
(1)
(E∕k = 9860 K, e.g., Zanchet et al. 2013a, 2019) prevents this reaction from being efficient unless the gas is heated to very high temperatures. In diffuse molecular clouds (on average at Tk ~ 100 K), the formation of SH+ and SH onlyseems possible in the context of local regions of overheated gas subjected to magnetized shocks (Pineau des Forets et al. 1986) or in dissipative vortices of the interstellar turbulent cascade (Godard et al. 2012, 2014). In these tiny pockets (~100 AU in size), the gas would attain the hot temperatures (Tk ≃ 1000 K) and/or ion-neutral drift needed to overcome the endothermicities of the above hydrogen abstraction reactions (see, e.g., Neufeld et al. 2015).
Dense PDRs (nH ≃ 103−106 cm−3) offer a complementary environment to study the first steps of sulfur chemistry. Because of their higher densities and more quiescent gas, fast shocks or turbulence dissipation do not contribute to the gas heating. Instead, the molecular gas is heated to Tk ≲ 500 K by mechanisms that depend on the flux of far-UV photons (FUV; E < 13.6 eV). A different perspective of the H2 (v) reactivity emerges because certain endoergic reactions become exoergic and fast when a significant fraction of the H2 reagents are radiatively pumped to vibrationally excited states v ≥ 1 (Stecher & Williams 1972; Freeman & Williams 1982; Tielens & Hollenbach 1985; Sternberg & Dalgarno 1995). In this case, state-specific reaction rates for H2 (ν, J) are needed to make realistic predictions of the abundance of the product XH (Agúndez et al. 2010; Zanchet et al. 2013b; Faure et al. 2017). The presence of abundant FUV-pumped H2 (v ≥ 1) triggers a nonthermal “hot” chemistry. Indeed, CH+ and SH+ emission lines have been detected in the Orion Bar PDR (Nagy et al. 2013; Goicoechea et al. 2017) where H2 lines up to v = 10 have been detected as well (Kaplan et al. 2017).
In this study we present a systematic (observational and modeling) study of the chemistry of S-bearing hydrides in FUV-illuminated gas. We try to answer the question of whether gas-phase reactions of S atoms and SH+ molecules with vibrationally excited H2 can ultimately explain the presence of abundant H2S, or if grain surface chemistry has to be invoked.
The paper is organized as follows. In Sects. 2 and 3 we report on new observations of H S, H
S, H S, H
S, H S, SH+, SH, and H3 S+ emission lines toward the Orion Bar. In Sect. 4 we study their excitation and derive their column densities. In Sect. 6 we discuss their abundances in the context of updated PDR models, with emphasis on the role of hydrogen abstraction reactions
S, SH+, SH, and H3 S+ emission lines toward the Orion Bar. In Sect. 4 we study their excitation and derive their column densities. In Sect. 6 we discuss their abundances in the context of updated PDR models, with emphasis on the role of hydrogen abstraction reactions
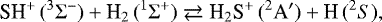 (2)
(2)
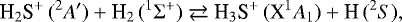 (3)
(3)
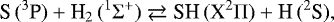 (4)
(4)
photoreactions, and grain surface chemistry. In Sect. 5 we summarize the ab initio quantum calculations we carried out to determine the state-dependent rates of reactions (2) and (4). Details of these calculations are given in Appendices A and B.
2 Observations of S-bearing hydrides
2.1 The Orion Bar
At an adopted distance of ~414 pc, the Orion Bar is an interface of the Orion molecular cloud and the Huygens H II region that surrounds the Trapezium cluster (Genzel & Stutzki 1989; O’Dell 2001; Bally 2008; Goicoechea et al. 2019, 2020; Pabst et al. 2019, 2020). The Orion Bar is a prototypical strongly illuminated dense PDR. The impinging flux of stellar FUV photons (G0) is a few 104 times the mean interstellar radiation field (Habing 1968). The Bar is seen nearly edge-on with respect to the FUV illuminating sources, mainly θ1 Ori C, the most massive star in the Trapezium. This favorable orientation allows observers to spatially resolve the H+ -to-H transition (the ionization front or IF; see, e.g., Walmsley et al. 2000; Pellegrini et al. 2009) from the H-to-H2 transition (the dissociation front or DF; see, e.g., Allers et al. 2005; van der Werf et al. 1996, 2013; Wyrowski et al. 1997; Cuadrado et al. 2019). It also allows one to study the stratification of different molecular species as a function of cloud depth (i.e., as the flux of FUV photons is attenuated; see, e.g., Tielens et al. 1993; van der Wiel et al. 2009; Habart et al. 2010; Goicoechea et al. 2016; Parikka et al. 2017; Andree-Labsch et al. 2017).
Regarding sulfur2, several studies previously reported the detection of S-bearing molecules in the Orion Bar. These include CS, C34 S, SO, SO2, and H2 S (Hogerheijde et al. 1995; Jansen et al. 1995), SO+ (Fuente et al. 2003), C33S, HCS+, H2 CS, and NS (Leurini et al. 2006), and SH+ (Nagy et al. 2013). These detections refer to modest angular resolution pointed observations using single-dish telescopes. Higher-angular-resolution interferometric imaging of SH+, SO, and SO+ (Goicoechea et al. 2017) was possible thanks to the Atacama Compact Array (ACA).
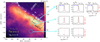 |
Fig. 1 Overview of the Orion Bar. The (0″, 0″) position corresponds to α2000 = 05h 35m 20.1s ; δ2000 = -05°25′07.0″. Left panel: integrated line intensity maps in the 13CO J = 3–2 (color scale) and SO 89–78 emission (gray contours; from 6 to 23.5 K km s−1 in steps of 2.5 K km s−1) obtained with the IRAM 30 m telescope at 8″ resolution. The white dotted contours delineate the position of the H2 dissociation front as traced by the infrared H2 v = 1–0 S(1) line (from 1.5 to 4.0 × 10−4 erg s−1 cm−2 sr−1 in steps of 0.5 × 10−4 erg s−1 cm−2 sr−1; from Walmsley et al. 2000). The black-dashed rectangle shows the smaller FoV imaged with ALMA (Fig. 3). The DF position has been observed with SOFIA, IRAM 30 m, and Herschel. Cyan circles represent the ~15″ beam at 168 GHz. Right panel: H2S lines lines detected toward three positions of the Orion Bar. |
2.2 Observations of H2S isotopologues and H3 S+
We observed the Orion Bar with the IRAM 30 m telescope at Pico Veleta (Spain). We used the EMIR receivers in combination with the Fast Fourier Transform Spectrometer (FTS) backends at 200 kHz resolution (~0.4, ~0.3, and ~0.2 km s−1 at ~168, ~217, and ~293 GHz, respectively). These observations are part of a complete line survey covering the frequency range 80–360 GHz (Cuadrado et al. 2015, 2016, 2017, 2019) and include deep integrations at 168 GHz toward three positions of the PDR located at a distance of 14″, 40″, and 65″ from the IF (see Fig. 1). Their offsets with respect to the IF position at α2000 = 05h 35m 20.1s, δ2000 = -05°25′07.0″ are (+10″, −10″), (+30″, −30″’), and (+35″, −55″). The first position is the DF.
We carried out these observations in the position switching mode taking a distant reference position at (−600″, 0″). The half power beam width (HPBW) at ~168, ~217, and ~293 GHz is ~15″, ~11″, and ~8″, respectively. The latest observations (those at 168 GHz) were performed in March 2020. The data were first calibrated in the antenna temperature scale 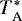 and then converted to the main beam temperature scale, Tmb, using Tmb =
and then converted to the main beam temperature scale, Tmb, using Tmb = 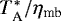 , where ηmb is the antenna efficiency (ηmb = 0.74 at ~168 GHz). We reduced andanalyzed the data using the GILDAS software as described in Cuadrado et al. (2015). The typical rms noise of the spectra is ~3.5, 5.3, and 7.8 mK per velocity channel at ~168, ~217, and ~293 GHz, respectively.Figures 1 and 2 show the detection of o-H2S 11,0 −10,1 (168.7 GHz), p-H2S 22,0 −21,1 (216.7 GHz), and o-H2 34 S 11,0 −10,1 lines (167.9 GHz) (see Table E.1 for the line parameters), as well as several o-H2 33 S 11,0 −10,1 hyperfine lines (168.3 GHz).
, where ηmb is the antenna efficiency (ηmb = 0.74 at ~168 GHz). We reduced andanalyzed the data using the GILDAS software as described in Cuadrado et al. (2015). The typical rms noise of the spectra is ~3.5, 5.3, and 7.8 mK per velocity channel at ~168, ~217, and ~293 GHz, respectively.Figures 1 and 2 show the detection of o-H2S 11,0 −10,1 (168.7 GHz), p-H2S 22,0 −21,1 (216.7 GHz), and o-H2 34 S 11,0 −10,1 lines (167.9 GHz) (see Table E.1 for the line parameters), as well as several o-H2 33 S 11,0 −10,1 hyperfine lines (168.3 GHz).
We complemented our dataset with higher frequency H2 S lines detected by the Herschel Space Observatory (Nagy et al. 2017) toward the “CO+ peak” position (Stoerzer et al. 1995), which is located at only ~4″ from our DF position (i.e., within the HPBW of these observations). These observations were carried out with the HIFI receiver (de Graauw et al. 2010) at a spectral-resolution of 1.1 MHz (0.7 km s−1 at 500 GHz). HIFI’s HPBW range from ~42″ to ~20″ in the 500–1000 GHz window (Roelfsema et al. 2012). The list of additional hydrogen sulfide lines detected by Herschel includes the o-H2S 22,1 −21,2 (505.5 GHz), 21,2 −10,1 (736.0 GHz), and 30,3 −21,2 (993.1 GHz), as well as the p-H2S 20,2 −11,1 (687.3 GHz) line. We used the line intensities, in the Tmb scale, shown in Table A.1 of Nagy et al. (2017).
In order to get a global view of the Orion Bar, we also obtained 2.5′ × 2.5′ maps of the region observed by us with the IRAM 30 m telescope using the 330 GHz EMIR receiver and the FTS backend at 200 kHz spectral-resolution (~0.2 km s−1). On-the-fly (OTF) scans were obtained along and perpendicular to the Bar. The resulting spectra were gridded to a data cube through convolution with a Gaussian kernel providing a final resolution of ~8″. The total integration time was ~6 h. The achieved rms noise is ~1 K per resolution channel. Figure 1 shows the spatial distribution of the 13 CO J = 3–2 (330.5 GHz) and SO 89-78 (346.5 GHz) integrated line intensities.
 |
Fig. 2 Detection of H2 33 S (at ~168.3 GHz) toward the DF position of the Orion Bar. Red lines indicate hyperfine components. Blue lines show interloping lines from 13CCH. The length of each line is proportional to the transition line strength (taken from the Cologne Database for Molecular Spectroscopy, CDMS; Endres et al. 2016). |
 |
Fig. 3 ALMA 1″-resolution images zooming into the edge of the Orion Bar in 12CO 3–2 (left panel, Goicoechea et al. 2016) and SH+ 10 –01 F = 1/2–3/2 line (middle panel, integrated line intensity). The right panel shows the H2 v = 1–0 S(1) line (Walmsley et al. 2000). We rotated these images (all showing the same FoV) with respect to Fig. 1 to bring the FUV illuminating direction in the horizontal direction (from the right). The circle shows the DF position targeted with SOFIA in SH (20″ beam) and with the IRAM 30 m telescope in H2S and H3S+. |
2.3 ALMA imaging of Orion Bar edge in SH+ emission
We carried out mosaics of a small field of the Orion Bar using twenty-seven ALMA 12 m antennas in band 7 (at ~346 GHz). These unpublished observations belong to project 2012.1.00352.S (PI: J. R. Goicoechea) and consisted of a 27-pointing mosaic centered at α(2000) = 5h35m20.6s; δ(2000) = −05°25′20″. The total field-of-view (FoV) is 58″ × 52″ (shown in Fig. 1). The two hyperfine line components of the SH+ NJ = 10−01 transition were observed with correlators providing ~500 kHz resolution (0.4 km s−1) over a 937.5 MHz bandwidth. The total observation time with the ALMA 12 m array was ~2h. In order to recover the large-scale extended emission filtered out by the interferometer, we used deep and fully sampled single-dish maps, obtained with the total-power (TP) antennas at 19″ resolution, as zero- and short-spacings. Data calibration procedures and image synthesis steps are described in Goicoechea et al. (2016). The synthesized beam is ~1″. This is a factor of ~4 better than previous interferometric SH+ observations (Goicoechea et al. 2017). Figure 3 shows the resulting image of the SH+ 10 − 01 F = 1/2–3/2 hyperfine emission line at 345.944 GHz. We rotated this image 37.5° clockwise to bring the FUV illumination in the horizontal direction. The typical rms noise of the final cube is ~ 80 mK per velocity channel and 1″-beam. As expected from their Einstein coefficients, the other F = 1/2–1/2 hyperfine line component at 345.858 GHz is a factor of ~2 fainter (see Table E.2) and the resulting image has low signal-to-noise (S/N).
We complemented the SH+ dataset with the higher frequency lines observed by HIFI (Nagy et al. 2013, 2017) at ~526 and ~683 GHz (upper limit). These pointed observations have HPBWs of ~41″ and ~32″ respectively, thus they do not spatially resolve the SH+ emission. To determine their beam coupling factors (fb), we smoothed the bigger 4″-resolution ACA + TP SH+ image shown in Goicoechea et al. (2017) to the different HIFI’s HPBWs. We obtain fb ≃ 0.4 at ~526 GHz and fb ≃ 0.6 at ~683 GHz. The corrected intensities are computed as Wcorr = WHIFI / fb. These correction factors are only a factor of ≲2 lower than simply assuming uniform SH+ emission from a 10″ width filament.
2.4 SOFIA/GREAT search for SH emission
We finally used the GREAT receiver (Heyminck et al. 2012) on board the Stratospheric Observatory For Infrared Astronomy (SOFIA; Young et al. 2012) to search for the lowest-energy rotational lines of SH (2 Π3∕2 J = 3/2–1/2) at 1382.910 and 1383.241 GHz (e.g., Klisch et al. 1996; Martin-Drumel et al. 2012). These lines lie in a frequency gap that Herschel/HIFI could not observe from space. These SOFIA observations belong to project 07_ 0115 (PI: J. R. Goicoechea). The SH lines were searched on the lower side band of 4GREAT band 3. We employed the 4GREAT/HFA frontends and 4GFFT spectrometers as backends. The HPBW of SOFIA at 1.3 THz is ~20″, thus comparable with IRAM 30 m/EMIR and Herschel/HIFI observations. We also employed the total power mode with a reference position at (−600″,0″). The originalplan was to observe during two flights in November 2019 but due to bad weather conditions, only ~70 min of observationswere carried out in a single flight.
After calibration, data reduction included: removal of a first order spectral baseline, dropping scans with problematic receiver response, rms weighted average of the spectral scans, and calibration to Tmb intensity scale (ηmb = 0.71). The final spectrum, smoothed to a velocity-resolution of 1 km s−1 has a rms noise of ~50 mK (shown in Fig. 4). Two emission peaks are seen at the frequencies of the Λ-doublet lines. Unfortunately, the achieved rms is not enough to assure the unambiguous detection of each component of the doublet. Although the stacked spectrum does display a single line (suggesting a tentative detection) the resulting line-width (Δv ≃ 7 km s−1) is a factor of ~3 broader than expected in the Orion Bar (see Table E.3). Hence, this spectrum provides stringent upper limits to the SH column density but deeper integrations would be needed to confirm the detection.
 |
Fig. 4 Search for the SH 2 Π3∕2 J = 5/2–3/2 doublet (at ~1383 GHz) toward the DF position with SOFIA/GREAT. Vertical magenta lines indicate the position of hyperfine splittings taken from CDMS. |
3 Observational results
3.1 H232 S, H234 S, and H233 S across the PDR
Figure 1 shows an expanded view of the Orion Bar in the 13 CO (J = 3–2) emission. FUV radiation from the Trapezium stars comes from the upper-right corner of the image. The FUV radiation field is attenuated in the direction perpendicular to the Bar. The infrared H2 v = 1–0 S(1) line emission (white contours) delineates the position of the H-to-H2 transition, the DF. Many molecular species, such as SO, specifically emit from deeper inside the PDR where the flux of FUV photons has considerably decreased. In contrast, H2 S, and even its isotopologueH S, show bright 11,0 –10,1 line emission toward the DF (right panels in Fig. 1; see also Jansen et al. 1995). Rotationally excited H2 S lines have been also detected toward this position (Nagy et al. 2017), implying the presence of warm H2 S close to the irradiated cloud surface (i.e., at relatively low extinctions). The presence of moderately large H2 S column densities in the PDR is also demonstrated by the unexpected detection of the rare isotopologue H
S, show bright 11,0 –10,1 line emission toward the DF (right panels in Fig. 1; see also Jansen et al. 1995). Rotationally excited H2 S lines have been also detected toward this position (Nagy et al. 2017), implying the presence of warm H2 S close to the irradiated cloud surface (i.e., at relatively low extinctions). The presence of moderately large H2 S column densities in the PDR is also demonstrated by the unexpected detection of the rare isotopologue H S toward the DF (at the correct LSR velocity of the PDR: vLSR ≃ 10.5 km s−1). Figure 2 shows the H
S toward the DF (at the correct LSR velocity of the PDR: vLSR ≃ 10.5 km s−1). Figure 2 shows the H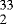 S 11,0 –10,1 line and its hyperfine splittings (produced by the 33S nuclear spin). To our knowledge, H
S 11,0 –10,1 line and its hyperfine splittings (produced by the 33S nuclear spin). To our knowledge, H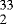 S lines had only been reported toward the hot cores in Sgr B2 and Orion KL before (Crockett et al. 2014).
S lines had only been reported toward the hot cores in Sgr B2 and Orion KL before (Crockett et al. 2014).
The observed o-H2S/o–H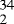 S 11,0 -10,1 line intensity ratio toward the DF is 15 ± 2, below the solar isotopic ratio of 32 S/34S = 23 (e.g., Anders & Grevesse 1989). The observed ratio thus implies optically thick o-H2S line emission at ~168 GHz. However, the observed o-H
S 11,0 -10,1 line intensity ratio toward the DF is 15 ± 2, below the solar isotopic ratio of 32 S/34S = 23 (e.g., Anders & Grevesse 1989). The observed ratio thus implies optically thick o-H2S line emission at ~168 GHz. However, the observed o-H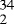 S/o-H
S/o-H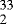 S 11,0 –10,1 intensity ratio is 6 ± 1, thus compatible with the solar isotopic ratio (34 S/33S = 5.5) and with H
S 11,0 –10,1 intensity ratio is 6 ± 1, thus compatible with the solar isotopic ratio (34 S/33S = 5.5) and with H S and H
S and H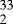 S optically thin emission.
S optically thin emission.
 |
Fig. 5 Search for H3S+ toward the Orion Bar with the IRAM 30 m telescope. The blue curve shows the expected position of the line. |
3.2 SH+ emission from the PDR edge
Figure 3 zooms into a small field of the Bar edge. The ALMA image of the CO J = 3–2 line peak temperature was first presented by Goicoechea et al. (2016). Because the CO J = 3–2 emission is nearly thermalized and optically thick from the DF to the molecular cloud interior, the line peak temperature scale (Tpeak) is a good proxy of the gas temperature (Tk ≃Tex ≃Tpeak). The CO image implies small temperature variations around Tk ≃ 200 K. The middle panel in Fig. 3 shows the ALMA image of the SH+ NJ = 10–01 F = 1/2–3/2 hyperfine line at 345.944 GHz. Compared to CO, the SH+ emission follows the edge of the molecular PDR, akin to a filament of ~10″ width (for the spatial distribution of other molecular ions, see, Goicoechea et al. 2017). The SH+ emission shows localized small-scale emission peaks (density or column density enhancements) that match, or are very close to, the vibrationally excited H2 (v = 1–0) emission (Fig. 3). We note that while some H2 (v = 1–0) emission peaks likely coincide with gas density enhancements (e.g., Burton et al. 1990), the region also shows extended emission from FUV-pumped H2 (v = 2–1) (van der Werf et al. 1996) that does not necessarily coincide with the H2 (v = 1–0) emission peaks.
3.3 Search for SH, H3 S+, and H2 S ν2 = 1 emission
We used SOFIA/GREAT to search for SH 2 Π3∕2 J = 5/2–3/2 lines toward the DF (Fig. 4). This would have been the first time that interstellar SH rotational lines were seen in emission. Unfortunately, the achieved rms of the observation does not allow a definitive confirmation of these lines, so here we will only discuss upper limitsto the SH column density. The red, green, and blue curves in Fig. 4 show radiative transfer models for nH = 106 cm−3, Tk = 200 K, and different SH column densities (see Sect. 4 for more details).
Our IRAM 30 m observations toward the DF neither resulted in a detection of H3 S+, a key gas-phase precursor of H2S. The ~293.4 GHz spectrum around the targeted H3 S+ 10–00 line is shown in Fig. 5. Again, the achieved low rms allows us to provide a sensitive upper limit to the H3 S+ column density. This results in N(H3S+) = (5.5–7.5) × 1010 cm−2 (5σ) assuming an excitation temperature range Tex = 10–30 K and extended emission. Given the bright H2S emission close to the edge of the Orion Bar, and because H2S formation at the DF might be driven by very exoergic processes, we also searched for the 11,0 –10,1 line of vibrationally excited H2S (in the bending mode ν2). The frequency of this line lies at ~181.4 GHz (Azzam et al. 2013), thus at the end of our 2 mm-band observations of the DF (rms ≃ 16 mK). However, we do not detect this line either.
4 Coupled nonlocal excitation and chemistry
In this section we study the rotational excitation of the observed S-bearing hydrides3. We determine the SH+, SH (upper limit), and H2S column densities in the Orion Bar, and the “average” gas physical conditions in the sense that we search for the combination of single Tk, nH, and N that better reproduces the observed line intensities (so-called “single-slab” approach). In Sect. 6 we expand these excitation models to multi-slab calculations that take into account the expected steep gradients in a PDR.
In the ISM, rotationally excited levels are typically populated by inelastic collisions. However, the lifetime of very reactive molecules can be so short that the details of their formation and destruction need to be taken into account when determining how these levels are actually populated (Black 1998). Reactive collisions (collisions that lead to a reaction and thus to molecule destruction)influence the excitation of these species when their timescales become comparable to those of nonreactive collisions. The lifetimeof reactive molecular ions observed in PDRs (e.g., Fuente et al. 2003; Nagy et al. 2013; van der Tak et al. 2013; Goicoechea et al. 2017, 2019) can be so short that they do not get thermalized by nonreactive collisions or by absorption of the background radiation field (Black 1998). In these cases, a proper treatment of the molecule excitation requires including chemical formation and destruction rates in the statistical equilibrium equations (dni / dt = 0) that determine the level populations:
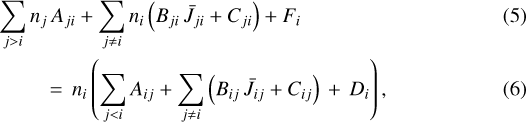
where ni [cm −3] is the population of rotational level i, Aij and Bij are the Einstein coefficients for spontaneous and induced emission, Cij [s −1 ] is the rate of inelastic collisions4 (Cij = ∑kγij, k nk, where γij, k(T) [cm 3s−1] are the collisional rate coefficients and k stands for H2, H, and e−), and 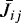 is the mean intensity of the total radiation field over the line profile. In these equations, ni Di is the destruction rate per unit volume of the molecule in level i, and Fi its formation rate per unit volume (both in cm−3s−1). When state-to-state formation rates are not available, and assuming that the destruction rate is the same in every level (Di = D), one can use the total destruction rate D [s−1] (= ∑knk kk(T) + photodestruction rate, where kk [cm 3s−1] is the state-averaged rate of the two-body chemical reaction with species k) and consider that the level populations of the nascent molecule follow a Boltzmann distribution at an effective formation temperature Tform:
is the mean intensity of the total radiation field over the line profile. In these equations, ni Di is the destruction rate per unit volume of the molecule in level i, and Fi its formation rate per unit volume (both in cm−3s−1). When state-to-state formation rates are not available, and assuming that the destruction rate is the same in every level (Di = D), one can use the total destruction rate D [s−1] (= ∑knk kk(T) + photodestruction rate, where kk [cm 3s−1] is the state-averaged rate of the two-body chemical reaction with species k) and consider that the level populations of the nascent molecule follow a Boltzmann distribution at an effective formation temperature Tform:
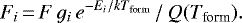 (7)
(7)
In this formalism, F [cm −3 s−1] is the state-averaged formation rate per unit volume, gi the degeneracy of level i, and Q(Tform) is the partition function at Tform (van der Tak et al. 2007).
This “formation pumping” formalism has been previously implemented in large velocity gradient codes to treat, for example, the local excitation of the very reactive ion CH+ (Nagy et al. 2013; Godard & Cernicharo 2013; Zanchet et al. 2013b; Faure et al. 2017). However, interstellar clouds are inhomogeneous and gas velocity gradients are typically modest at small spatial scales. This means that line photons can be absorbed and reemitted several times before leaving the cloud. Here we implemented this formalism in a Monte Carlo code that explicitly models the nonlocal behavior of the excitation and radiative transfer problem (see Appendix of Goicoechea et al. 2006).
Although radiative pumping by dust continuum photons does not generally dominate in PDRs, for completeness we also included radiative excitation by a modified blackbody at a dust temperature of ~50 K and a dust opacity τλ = 0.03 (150/λ[μm])1.6 (which reproduces the observed intensity and wavelength dependence of the dust emission in the Bar; Arab et al. 2012). The molecular gas fraction, f(H2) = 2n(H2)/nH, is set to 2/3, where nH = n(H) + 2n(H2) is the total density of H nuclei. This choice is appropriate for the dissociation front and implies n(H2) = n(H). As most electrons in the DF come from the ionization of carbon atoms, the electron density ne is set to ne ≃n(C+) = 1.4 × 10−4 nH (e.g., Cuadrado et al. 2019). For the inelastic collisions with o-H2 and p-H2, we assumed that the H2 ortho-to-para (OTP) ratio is thermalized to the gas temperature.
4.1 SH+ excitation and column density
We start by assuming that the main destruction pathway of SH+ are reactions with H atoms and recombinations with electrons (see Sect. 6.1). Hence, the SH+ destruction rate is D ≃ne ke(T) + n(H) kH(T) (see Table 1 for the relevant chemical destruction rates). For Tk = Te = 200 K and nH = 106 cm−3 (e.g., Goicoechea et al. 2016) this implies D ≃ 10−4 s−1 (i.e., the lifetime of an SH+ molecule in the Bar is less than 3 h). At these temperatures and densities, D is about ten times smaller than the rate of radiative and inelastic collisional transitions that depopulate the lowest-energy rotational levels of SH+. Hence, formation pumping does not significantly alter the excitation of the observed SH+ lines, but it does influence the population of higher-energy levels. Formation pumping effects have been readily seen in CH+ because this species is more reactive5 and its rotationally excited levels lie at higher-energy (i.e., their inelastic collision pumping rates are slower, e.g., Zanchet et al. 2013b)
Figure 6 shows results of several models: without formation pumping (dotted curves for model “F = D = 0”), adding formation pumping with SH+ destruction by H and e− (continuous curves for model “F, D”), and using a factor of ten higher SH+ destruction rates (simulating a dominant role of SH+ photodissociation or destruction by reactions with vibrationally excited H2 ; dashed curves for model “F, D × 10”). Since the formation of SH+ is driven by reaction (1) when H2 molecules are in v ≥ 2, here we adopted Tform ≃E(v = 2, J = 0) / k− 9860 K ≈ 2000 K. Because these are constant column density N(SH+) excitation and radiative transfer models, we used a normalized formation rate F = ∑ Fi that assumes steady-state SH+ abundances consistent with the varying gas density in each model. That is, F = ∑ Fi = x(SH+) nH D [cm −3s−1], where x refers to the abundance with respect to H nuclei.
The detected SH+ rotational lines connect the fine-structure levels NJ = 10–01 (345 GHz) and 12 –01 (526 GHz). Upper limits also exist for the 11 -01 (683 GHz) lines. SH+ critical densities (ncr = Aij / γij) for inelastic collisions with H or H2 are of the same order and equal to several 106 cm−3. As for many molecular ions (e.g., Desrousseaux et al. 2021), SH+–H2 (and SH+–H) inelastic collisional rate coefficients4 are large (γij ≳ 10−10 cm3 s−1). Thus, collisions with H (at low AV) and H2 (at higher AV) generally dominate over collisions with electrons (γij of a few 10−7 cm3 s−1). At low densities (meaning nH <ncr) formation pumping increases the population of the higher-energy levels (and their Tex), but there are only minor effects in the low-energy submillimeter lines. At high densities, nH > 107 cm−3, formation pumping with Tform = 2000 K produces lower intensities in these lines because the lowest-energy levels (Eu ∕k <Tk <Tform) are less populated.
The best fit to the observed lines in model F, D is for N(SH+) ≃ 1.1 × 1013 cm−3, nH ≃ 3 × 105 cm−3, and Tk ≃ 200 K. This is shown by the vertical dotted line in Fig. 6. This model is consistent with the upper limit intensity of the 683 GHz line (Nagy et al. 2013). In this comparison, and following the morphology of the SH+ emission revealed by ALMA (Fig. 3), we corrected the line intensities of the SH+ lines detected by Herschel/HIFI with the beam coupling factors discussed in Sect. 2.3, The observed 12 –01/10–01 line ratio (R = W(526.048)/W(345.944) ≃ 2) is sensitive to the gas density. In these models, R is 1.1 for nH = 105 cm−3 and 3.0 for nH = 106 cm−3. We note that nH could be lower if SH+ formation/destruction rates were faster, as in the F, D × 10 model. This could happen if SH+ photodissociation or destruction reactions with H2 (v ≥ 2) were faster than reactions of SH+ with H atoms or with electrons. In Sect. 6 we show that this is not the case.
 |
Fig. 6 Non-LTE excitation models of SH+. The horizontal lines mark the observed line intensities in the Orion Bar. Dotted curves are for a standard model (F = D = 0). Continuous curves are for a model that includes chemical destruction by H atoms and e− (model F, D). Dashed lines are for a model in which destruction rates are multiplied by ten (model F, D × 10). The verticalblack line marks the best model. |
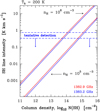 |
Fig. 7 Non-LTE excitation models of SH emission lines targeted with SOFIA/GREAT. Horizontal dashed lines refer to observational limits, assuming extended emission (lower intensities) and for a 10″ width emission filament at the PDR surface (higher intensities). |
4.2 SH excitation and column density
SH is a 2Π open-shell radical with fine-structure, Λ-doubling, and hyperfine splittings (e.g., Martin-Drumel et al. 2012). However, the frequency separation of the SH 2 Π3∕2 J = 5/2–3/2 hyperfine components is too small to be spectrally resolved in observations of the Orion Bar (see Fig. 4).The available rate coefficients for inelastic collisions of SH with helium atoms do not resolve the hyperfine splittings. Hence, we first determined line frequencies, level degeneracies, and Einstein coefficients of an SH molecule without hyperfine structure. To do this, we took the complete set of hyperfine levels tabulated in CDMS. Lacking specific inelastic collision rate coefficients, we scaled the available SH– He rates of Kłos et al. (2009) by the square root of the reduced mass ratios and estimated the SH– H and SH– H2 collisional rates.
The scaled rate coefficients are about an order of magnitude smaller than those of SH+. However, the chemical destruction rate of SH at the PDR edge (reactions with H, photodissociation, and photoionization, see Sect. 6.1) is also slower (we take the rates of SH–H reactive collisions from Zanchet et al. 2019). We determine D ≃ 3 × 10−6 s−1 for nH = 106 cm−3, Tk = 200 K, and AV ≃ 0.7 mag. Models in Fig. 7 include these chemical rates for Tform = Tk (a lower limit to the unknown formation temperature). Formation pumping enhances the intensity of the 2 Π3∕2 J = 5/2–3/2 ground-state lines by a few percent only.
To estimate the SH column density in the Orion Bar we compare with the upper limit intensities of the SH lines targeted by SOFIA. If SH and SH+ arise from roughly the same gas at similar physical conditions (nH ≃ 106 cm−3 and Tk ≃ 200 K) the best model column density is for N(SH) ≤ (0.6–1.6) × 1014 cm−2. If densities were lower, around nH ≃ 105 cm−3, the upper limit N(SH) column densities will be a factor ten higher.
 |
Fig. 8 Non-LTE excitation models for o-H2S and p-H2S. Thin horizontal lines show the observed intensities assuming either extended emission (lower limit) or emission that fills the 15″ beam at168.7 GHz. The vertical line marks the best model, resulting in an OTP ratio of 2.9 ± 0.3. |
4.3 H2S excitation and column density
H2 S has a X2 A ground electronic state and two nuclear spin symmetries that we treat separately, o-H2S and p-H2S. Previous studies of the H2S line excitation have used collisional rates coefficients scaled from those of the H2 O – H2 system. Dagdigian (2020) recently carried out specific calculations of the cross sections of o-H2S and p-H2S inelastic collisions with o–H2 and p-H2 at different temperatures. The behavior of the new and the scaled rates is different and it depends on the H2 OTP ratio (e.g., on gas temperature) because the collisional cross sections are different for o-H2–H2S and p-H2–H2S systems. At the warm temperatures of the PDR, collisions with o-H2 dominate, resulting in rate coefficients for the ~168 GHz o-H2S line that are a factor up to ~2.5 smaller than those scaled from H2 O–H2.
H2S is not a reactive molecule. At the edge of the PDR its destruction is driven by photodissociation. We determine that the radiative and collisional pumping rates are typically a factor of ~100 higher than D ≈ 2 × 10−6 s−1 (for nH = 106 cm−3, Tk = 200 K, G0 ≃104, and AV ≃ 0.7 mag). Figure 8 shows non-LTE o-H2S and p-H2S excitation and radiative transfer models. As H2S may have its abundance peak deeper inside the PDR and display more extended emission than SH+ (e.g., Sternberg & Dalgarno 1995), we show results for Tk = 200 and 100 K. When comparing with the observed line intensities, we considered either emission that fills all beams, or a correction that assumes that the H2S emission only fills the15″ beam of the IRAM 30 m telescope at 168 GHz. The vertical dotted lines in Fig. 8 show the best model, N(H2S) = N(o-H2S)+N(p-H2S) = 2.5 × 1014 cm−2, with an OTP ratio of 2.9 ± 0.3, thus consistent with the high-temperature statistical ratio of 3/1 (see discussion at the end of Sect. 6.4). Models with lower densities, nH ≃ 105 cm−3, show worse agreement, and would translate into even higher N(H2S) of ≳ 1015 cm−2. In either case, these calculations imply large columns of warm H2 S toward the PDR. They result in a limit to the SH to H2S column density ratio of ≤0.2–0.6. This upper limit is already lower than the N(SH)/N(H2S) = 1.1–3.0 ratios observed in diffuse clouds (Neufeld et al. 2015). This difference suggests an enhanced H2 S formation mechanism in FUV-illuminated dense gas.
5 New results on sulfur-hydride reactions
In this section we summarize the ab initio quantum calculations we carried out to determine the vibrationally-state-dependent rates of gas-phase reactions of H2 (v > 0) with several S-bearing species. We recall that all hydrogen abstraction reactions, ![$\textrm{S}^+ \xrightarrow[(1)]{{{+{\textrm{H}_2}}}} \textrm{SH}^+ \xrightarrow[(2)]{{+{\textrm{H}_2}}} {\textrm{H}_2S^+} \xrightarrow[(3)]{{+{\textrm{H}_2}}} {\textrm{H}_3\textrm{S}^+,}\hspace{1cm}\textrm{S} \xrightarrow[(4)]{{{+{\textrm{H}_2}}}} \textrm{SH,}\vspace{0.1cm}$](/articles/aa/full_html/2021/03/aa39756-20/aa39756-20-eq25.png) are very endoergic for H2 (v = 0), with endothermicities in Kelvin units that are significantly higher than Tk even in PDRs. This is markedly different to O+ chemistry, for which all hydrogen abstraction reactions leading to H3 O+ are exothermic and fast (Gerin et al. 2010; Neufeld et al. 2010; Hollenbach et al. 2012).
are very endoergic for H2 (v = 0), with endothermicities in Kelvin units that are significantly higher than Tk even in PDRs. This is markedly different to O+ chemistry, for which all hydrogen abstraction reactions leading to H3 O+ are exothermic and fast (Gerin et al. 2010; Neufeld et al. 2010; Hollenbach et al. 2012).
The endothermicity of reactions involving Hn S+ ions decreases as the number of hydrogen atoms increases. The potential energy surfaces (PES) of these reactions possess shallow wells at the entrance and products channels (shown in Fig. 9). In addition, these PESs show saddle points between the energy walls of reactants and products whose heights increase with the number of H atoms. For reaction (2), the saddle point has an energy of 0.6 eV (≃7000 K) and is slightly below the energy of the products. However, for reaction (3), the saddle point is above the energy of the products and is a reaction barrier. These saddle points act as a bottleneck in the gas-phase hydrogenation of S+.
If one considers the state dependent reactivity of vibrationally excited H2, the formation of SH+ through reaction (1) becomes exoergic6 when v ≥ 2 (Zanchet et al. 2019). The detection of bright H2S emission in the Orion Bar (Figs. 1 and 4) might suggest that subsequent hydrogen abstraction reactions with H2 (v ≥ 2) proceed as well. Motivated by these findings, and before carrying out any PDR model, we studied reaction (2) and the reverse process in detail. This required to build a full dimensional quantum PES of the H3 S+ (X1A1) system (see Appendix A).
In addition, we studied reaction (4) (and its reverse) through quantum calculations. Details of these ab initio calculations and of the resulting reactive cross sections are given in Appendix B. Table 1 summarizes the updated reaction rate coefficients that we will include later in our PDR models.
The H2 S+ formation rate through reaction (2) with H2 (v = 0) is very slow. For H2 (v = 1), the rate constant increases at ≈500 K, corresponding to the opening of the H2 S+ + H threshold. For H2 (v = 2) and H2 (v = 3), the reactionrate is much faster, close to the Langevin limit (see Appendix A.2). However, our estimated vibrational-state specific rates for SH formation through reaction (4) (S + H2) are considerably smaller than for reactions (1) and (2), and show an energy barrier even for H2 (v = 2) and H2 (v = 3). We anticipate that this reaction is not a relevant formation route for SH.
In FUV-illuminated environments, collisions with H atoms are very important because they compete with electron recombinations in destroying molecular ions, and also they contribute to their excitation. An important result of our calculations is that the destruction rate of H2 S+ (SH+) in reactions with H atoms are a factor of ≥3.5 (≥1.7) faster (at Tk ≤ 200 K) than those previously used in astrochemical models (Millar et al. 1986). Conversely, we find that destruction of SH in reactions with H atoms (Appendix B) is slower than previously assumed.
 |
Fig. 9 Minimum energy paths for reactions (1), (2), and (3). Points correspond to RCCSD(T)-F12a calculations and lines to fits (Appendix A). The reaction coordinate, s, is defined independently for each path. The geometries of each species at s = 0 are different. |
Relevant rate coefficients from a fit of the Arrhenius-like form k (T) = 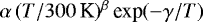 to the calculated reaction rates.
to the calculated reaction rates.
6 PDR models of S-bearing hydrides
We now investigate the chemistry of S-bearing hydrides and the effect of the new reaction rates in PDR models adapted to the Orion Bar conditions. In this analysis we used version 1.5.4. of the Meudon PDR code (Le Petit et al. 2006; Bron et al. 2014). Following our previous studies, we model the Orion Bar as a stationary PDR at constant thermal-pressure (i.e., with density and temperature gradients). When compared to time-dependent hydrodynamic PDR models (e.g., Hosokawa & Inutsuka 2006; Bron et al. 2018; Kirsanova & Wiebe 2019), stationary isobaric models seem a good description of the most exposed and compressed gas layers of the PDR, from AV ≈ 0.5 to ≈ 5 mag (Goicoechea et al. 2016; Joblin et al. 2018).
In our models, the FUV radiation field incident at the PDR edge is G0 = 2 × 104 (e.g., Marconi et al. 1998). We adopted an extinction to color-index ratio, RV = AV/EB−V, of 5.5 (Joblin et al. 2018), consistent with the flatter extinction curve observed in Orion (Lee 1968; Cardelli et al. 1989). This choice implies slightly more penetration of FUV radiation into the cloud (e.g., Goicoechea & Le Bourlot 2007). The main input parameters and elemental abundances of these PDR models are summarized in Table 2. Figure 10 shows the resulting H2, H, and electron density profiles, as well as the Tk and Td gradients.
Our chemical network is that of the Meudon code updated with the new reaction rates listed in Table 1. This network includes updated photoreaction rates from Heays et al. (2017). To increase the accuracy of our abundance predictions, we included the explicit integration of wavelength-dependent SH, SH+, and H2 S photodissociation cross sections (σdiss), as well as SH and H2S photoionization cross sections (σion). These cross sections are shown in Fig. C.1. The integration is performed over the specific FUV radiation field at each position of the PDR. In particular, we took σion(SH) from Hrodmarsson et al. (2019) and σdiss(H2S) from Zhou et al. (2020), both determined in laboratory experiments. Figure 11 summarizes the relevant chemical network that leads to the formation of S-bearing hydrides and that we discuss in the following sections.
Main parameters used in the PDR models of the Orion Bar.
6.1 Pure gas-phase PDR model results
Figure 12 shows results of the “new gas-phase” model using the reaction rates in Table 1. The continuous curves display the predicted fractional abundance profiles as a function of cloud depth in magnitudes of visual extinction (AV). The dashed curves are for a model that uses the standard thermal rates previously adopted in the literature (see, e.g., Neufeld et al. 2015). As noted by Zanchet et al. (2013a, 2019), the inclusion of H2 (v ≥ 2) state-dependent quantum rates for reaction (1) enhances the formation of SH+ in a narrow layer at the edge of the PDR (AV ≃ 0 to 2 mag). This agrees with the morphology of the SH+ emission revealed by ALMA images (Fig. 3). For H2 (v = 2), the reaction rate enhancement with respect to the thermal rate Δk = k2(T)∕k0(T) (see discussion by Agúndez et al. 2010) is about 4 × 108 at Tk = 500 K (Millar et al. 1986). Indeed, when the fractional abundance of H2 (v = 2) with respect to H2 (v = 0), defined as f2 = n (H2 v = 2)/n (H2 v = 0), exceeds a few times 10−9, meaning Δk ⋅f2 > 1, reaction (1) with H2 (v ≥ 2) dominates SH+ formation. This reaction enhancement takes place only at the edge of the PDR, where FUV-pumped H2 (v ≥ 2) molecules are abundant enough (gray dashed curves in Fig. 12) and drive the formation of SH+. The resulting SH+ column density increases by an order of magnitude compared to models that use the thermal rate.
In this isobaric model, the SH+ abundance peak occurs at AV ≃ 0.7 mag, where the gas density has increased from nH ≃ 6 × 104 cm−3 at the PDR edge (the IF) to ~5 × 105 cm−3 (at the DF). At this point, SH+ destruction is dominated by recombination with electrons and by reactive collisions with H atoms. This implies D(SH+) [s−1 ] ~ne ke ≃ nH kH ≫n(H2 v ≥ 2) k2, as we assumed in the single-slab SH+ excitation models (Sect. 4.1). Therefore, only a small fraction of SH+ molecules further react with H2 (v ≥ 2) to form H2 S+. The resulting low H2 S+ abundances limit the formation of abundant SH from dissociative recombinations of H2 S+ (recall that we estimated that reaction S + H2 (v ≥2) → SH + H is very slow). The SH abundance peak is shifted deeper inside the cloud, at about AV ≃ 1.8 mag, where SH forms by dissociative recombination of H2 S+ and it is destroyed by FUV photons and reactions with H atoms. In these gas-phase models the H2 S abundance peaks even deeper inside the PDR, at AV ≃ 5 mag, where it forms by recombinations of H2 S+ and H3 S+ with electrons as well as by charge exchange S + H2S+. However, the new rate of reaction H2 S+ + H is higher than assumed in the past, so the new models predict lower H2 S+ abundances at intermediate PDR depths (thus, less H3 S+ and H2S; see Fig. 12).
The SH column density predicted by the new gas-phase model is below the upper limit determined from SOFIA. However, the predicted H2 S column density is much lower than the value we derive from observations (Table 3) and the predicted H2 S line intensities are too faint (see Sect. 6.4).
Because the cross sections of the different H2S photodissociation channels have different wavelength dependences (Zhou et al. 2020), the H2 S and SH abundances between AV ≈ 2 and 6 mag are sensitive to the specific shape of the FUV radiation field (determined by line blanketing, dust absorption, and grain scattering; e.g., Goicoechea & Le Bourlot 2007). Still, we checked that using steeper extinction curves does not increase H2 S column density any closer to the observed levels. This disagreement between the observationally inferred N(H2S) column density and the predictions of gas-phase PDR models is even worse7 if one considers the uncertain rates of radiative association reactions S+ + H2 → H2S+ + hν and SH+ + H2 → H3S+ + hν included in the new gas-phase model. For the latter reaction, the main problem is that the electronic states of the reactants do not correlate with the 1 A1 ground electronic state of the activated complex H3 S+ * (denoted by *). Instead, H3 S+ * forms in an excited triplet state (3A). Herbst et al. (1989) proposed that a spin-flip followed by a radiative association can occur in interstellar conditions and form H3 S+ * (X1A1) (Millar & Herbst 1990). In Appendix A.3, we give arguments against this mechanism. For similar reasons, Prasad & Huntress (1982) avoided to include the S+ + H2 radiative association in their models. Removing these reactions in pure gas-phase models drastically decreases the H2 S+ and H3 S+ abundances, and thus those of SH and H2S (by a factor of ~100 in these models). The alternative H2 S+ formation route through reaction SH+ + H2(v = 2) is only efficient at the PDR surface (AV < 1 mag). This is due to the large H2 (v = 2) fractional abundances, f2 > 10−6 at Tk > 500 K, required to enhance the H2 S+ production. Therefore, and contrary to S+ destruction, reaction of SH+ with H2 is not the dominant destruction pathway for SH+. Only deeper inside the PDR, reactions of S with H produce small abundances of SH+ and H2 S+, but the hydrogenation of Hn S+ ions is not efficient and limits the gas-phase production H2 S.
produce small abundances of SH+ and H2 S+, but the hydrogenation of Hn S+ ions is not efficient and limits the gas-phase production H2 S.
 |
Fig. 10 Structure of an isobaric PDR representing the most FUV-irradiated gas layers of the Orion Bar (see Table 2 for the adopted parameters). This plot shows the H2, H, and electron density profiles (left axis scale), and the gas and dust temperatures (right axis scale). |
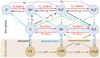 |
Fig. 11 Main gas and grain reactions leading to the formation of sulfur hydrides. Red arrows represent endoergic reactions (endothermicity given in units of K). Dashed arrows are uncertain radiative associations (see Appendix A.3), γ stands for a FUV photon, and “s-” for solid. |
Column density predictions from different PDR models (up to AV = 10 mag) and estimated values from observations (single-slab approach).
 |
Fig. 12 Pure gas-phase PDR models of the Orion Bar. Continuous curves show fractional abundances as a function of cloud depth, in logarithm scale to better display the irradiated edge of the PDR, using the new reaction rates listed in Table 1. The gray dotted curve shows f2, the fraction of H2 that is in vibrationally excited levels v ≥ 2 (right axis scale). Dashed curves are for a model using standard reaction rates. |
6.2 Grain surface formation of solid H2S
Similarly to the formation of water ice (s-H2O) on grains (e.g., Hollenbach et al. 2009, 2012), the formation of H2 S may be dominated by grain surface reactions followed by desorption back to the gas (e.g., Charnley 1997). Indeed, water vapor is relatively abundant in the Bar (N(H2O) ≈ 1015 cm−2; Choi et al. 2014; Putaud et al. 2019) and large-scale maps show that the H2 O abundance peaks close to cloud surfaces (Melnick et al. 2020).
To investigate the s-H2S formation on grains, we updated the chemical model by allowing S atoms to deplete onto grains as the gas temperature drops inside the molecular cloud (for the basic grain chemistry formalism, see, Hollenbach et al. 2009). The timescale of this process (τgr, S) goes as 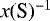
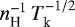 , where x(S) is the abundance of neutral sulfur atoms with respect to H nuclei. In a PDR, the abundance of H atoms is typically higher than that of S atoms8 and H atoms stick on grains more frequently than S atoms unless x(H) <x(S)⋅0.18. An adsorbed H atom (s-H) is weakly bound, mobile, and can diffuse throughout the grain surface until it finds an adsorbed S atom (s-S). If the timescale for a grain to be hit by a H atom (τgr, H) is shorter that the timescale for a s-S atom to photodesorb (τphotdes, S) or sublimate(τsubl, S) then reaction of s-H with s-S will proceed and form a s-SH radical roughly upon “collision” and without energy barriers (e.g., Tielens & Hagen 1982; Tielens 2010). Likewise, if τgr, H <τphotdes, SH and τgr, H <τsubl, SH, a newly adsorbed s-H atom can diffuse, find a grain site with an s-SH radical and react without barriers to form s-H2S. In these surface processes, a significant amount of S is ultimately transferred to s-H2S (e.g., Vidal et al. 2017), which can subsequently desorb: thermally, by FUV photons, or by cosmic rays. In addition, laboratory experiments show that the excess energy of certain exothermic surface reactions can promote the direct desorption of the product (Minissale et al. 2016). In particular, reaction s-H + s-SH directly desorbs H2 S with a maximum efficiency of ~60% (as observed in experiments, Oba et al. 2018). Due to the high flux of FUV photons in PDRs, chemical desorption may not always compete with photodesorption. However, it can be a dominant process inside molecular clouds (Garrod et al. 2007; Esplugues et al. 2016; Vidal et al. 2017; Navarro-Almaida et al. 2020).
, where x(S) is the abundance of neutral sulfur atoms with respect to H nuclei. In a PDR, the abundance of H atoms is typically higher than that of S atoms8 and H atoms stick on grains more frequently than S atoms unless x(H) <x(S)⋅0.18. An adsorbed H atom (s-H) is weakly bound, mobile, and can diffuse throughout the grain surface until it finds an adsorbed S atom (s-S). If the timescale for a grain to be hit by a H atom (τgr, H) is shorter that the timescale for a s-S atom to photodesorb (τphotdes, S) or sublimate(τsubl, S) then reaction of s-H with s-S will proceed and form a s-SH radical roughly upon “collision” and without energy barriers (e.g., Tielens & Hagen 1982; Tielens 2010). Likewise, if τgr, H <τphotdes, SH and τgr, H <τsubl, SH, a newly adsorbed s-H atom can diffuse, find a grain site with an s-SH radical and react without barriers to form s-H2S. In these surface processes, a significant amount of S is ultimately transferred to s-H2S (e.g., Vidal et al. 2017), which can subsequently desorb: thermally, by FUV photons, or by cosmic rays. In addition, laboratory experiments show that the excess energy of certain exothermic surface reactions can promote the direct desorption of the product (Minissale et al. 2016). In particular, reaction s-H + s-SH directly desorbs H2 S with a maximum efficiency of ~60% (as observed in experiments, Oba et al. 2018). Due to the high flux of FUV photons in PDRs, chemical desorption may not always compete with photodesorption. However, it can be a dominant process inside molecular clouds (Garrod et al. 2007; Esplugues et al. 2016; Vidal et al. 2017; Navarro-Almaida et al. 2020).
The photodesorption timescale of an ice mantle is proportional to Y −1 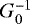 exp (+bAV), where Y is the photodesorption yield (the number of desorbed atoms or molecules per incident photon) and b is a dust-related FUV field absorption factor. The timescale for mantle sublimation (thermal desorption) goes as
exp (+bAV), where Y is the photodesorption yield (the number of desorbed atoms or molecules per incident photon) and b is a dust-related FUV field absorption factor. The timescale for mantle sublimation (thermal desorption) goes as 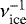 exp (+Eb / k Td), where νice is the characteristic vibrational frequency of the solid lattice, Td is the dust grain temperature, and Eb∕k is the adsorption binding energy of the species (in K). Binding energies play a crucial role in model predictions because they determine the freezing temperatures and sublimation timescales. Table 4 lists the Eb ∕k and Y values considered here.
exp (+Eb / k Td), where νice is the characteristic vibrational frequency of the solid lattice, Td is the dust grain temperature, and Eb∕k is the adsorption binding energy of the species (in K). Binding energies play a crucial role in model predictions because they determine the freezing temperatures and sublimation timescales. Table 4 lists the Eb ∕k and Y values considered here.
Representative timescales of the basic grain processes described above are summarized in the upper panel of Fig. 13. In this plot, Td is a characteristic dust temperature inside the PDR, Td = (3 × 104 + 2 × 103 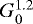 )0.2, taken from Hollenbach et al. (2009). In the upper panel, the continuous black curve is the timescale for a grain to be hit by an H atom (τgr, H). The dashed magenta curves show the timescale for thermal desorption of an s-S atom (τsubl, S) (left curve for Eb ∕k (S) = 1100 K and right curve for Eb∕k (S) = 2600 K), and the same for an s-O atom (blue curve). The gray dotted curve is the timescale for s-S atom photodesorption (τphotodes, S) at AV = 5 mag. At G0 strengths where the continuous line is below the dashed and dotted lines, an adsorbed s-S atom remains on the grain surface sufficiently long to react with a diffusing s-H atom, form s-SH, and ultimately s-H2S.
)0.2, taken from Hollenbach et al. (2009). In the upper panel, the continuous black curve is the timescale for a grain to be hit by an H atom (τgr, H). The dashed magenta curves show the timescale for thermal desorption of an s-S atom (τsubl, S) (left curve for Eb ∕k (S) = 1100 K and right curve for Eb∕k (S) = 2600 K), and the same for an s-O atom (blue curve). The gray dotted curve is the timescale for s-S atom photodesorption (τphotodes, S) at AV = 5 mag. At G0 strengths where the continuous line is below the dashed and dotted lines, an adsorbed s-S atom remains on the grain surface sufficiently long to react with a diffusing s-H atom, form s-SH, and ultimately s-H2S.
Figure 13 shows that, if one takes Eb ∕k (S) = 1100 K (the most common value in the literature; Hasegawa & Herbst 1993), the formation of s-H2S is possible inside clouds illuminated by modest FUV fields, when grains are sufficiently cold (Td < 22 K). However, recent calculations of s-S atoms adsorbed on water ice surfaces suggest higher binding energies (~ 2600 K; Wakelam et al. 2017). This would imply that S atoms freeze at higher Td (≲ 50 K) and that s-H2S mantles form in more strongly illuminated PDRs (the observed Td at the edge of the Bar is ≃50 K and decreases to ≃35 K behind the PDR; see, Arab et al. 2012).
The freeze-out depth for sulfur in a PDR, the AV at which most sulfur is incorporated as S-bearing solids (s-H2S in our simple model) can be estimated by equating τgr, S and  . This implicitly assumes the H2S chemical desorption does not dominate in FUV-irradiated regions, which is in line with the particularly large FUV absorption cross section of s-H2S measured in laboratory experiments (Cruz-Diaz et al. 2014). With these assumptions, the lower panel of Fig. 13 shows the predicted s-H2S and s-H2O freeze-out depths. Owing to the lower abundance and higher atomic mass of sulfur atoms (i.e., grains are hit slower by S atoms than by O atoms), the H2 S freeze-out depth appears slightly deeper than that of water ice. For the FUV-illumination conditions in the Bar, the freeze-out depth of sulfur is expected at AV ≳ 6 mag. This implies that photodesorption of s-H2S can produce enhanced abundances of gaseous H2S at AV < 6 mag.
. This implicitly assumes the H2S chemical desorption does not dominate in FUV-irradiated regions, which is in line with the particularly large FUV absorption cross section of s-H2S measured in laboratory experiments (Cruz-Diaz et al. 2014). With these assumptions, the lower panel of Fig. 13 shows the predicted s-H2S and s-H2O freeze-out depths. Owing to the lower abundance and higher atomic mass of sulfur atoms (i.e., grains are hit slower by S atoms than by O atoms), the H2 S freeze-out depth appears slightly deeper than that of water ice. For the FUV-illumination conditions in the Bar, the freeze-out depth of sulfur is expected at AV ≳ 6 mag. This implies that photodesorption of s-H2S can produce enhanced abundances of gaseous H2S at AV < 6 mag.
FUV-irradiation and thermal desorption of H2S ice mantles have been studied in the laboratory (e.g., Cruz-Diaz et al. 2014; Jiménez-Escobar & Muñoz Caro 2011). These experiments show that pure s-H2S ices thermally desorb around 82 K, and at higher temperatures for H2 S–H2O ice mixtures. These experiments determine a photodesorption yield of 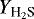 ~ 1.2 × 10−3 molecules per FUV photon (see also Fuente et al. 2017). Regarding surface grain chemistry, experiments show that reaction s-H + s-SH → s-H2S is exothermic (Oba et al. 2018), whereas reaction s-H + s-H2S, although it has an activation energy barrier of ~1500 K, it may directly desorb gaseous SH. Finally, reaction s-SH + s-SH → s-H2S2 may trigger the formation of doubly sulfuretted species, but it requires mobile s-SH radicals (e.g., Jiménez-Escobar & Muñoz Caro 2011; Fuente et al. 2017). Here we will only consider surface reactions with mobile s-H.
~ 1.2 × 10−3 molecules per FUV photon (see also Fuente et al. 2017). Regarding surface grain chemistry, experiments show that reaction s-H + s-SH → s-H2S is exothermic (Oba et al. 2018), whereas reaction s-H + s-H2S, although it has an activation energy barrier of ~1500 K, it may directly desorb gaseous SH. Finally, reaction s-SH + s-SH → s-H2S2 may trigger the formation of doubly sulfuretted species, but it requires mobile s-SH radicals (e.g., Jiménez-Escobar & Muñoz Caro 2011; Fuente et al. 2017). Here we will only consider surface reactions with mobile s-H.
Adopted binding energies and photodesorption yields.
 |
Fig. 13 Representative timescales relevant to the formation of s-H2S and s-H2O as well as their freeze-out depths. Upper panel: the continuous black curve is the timescale for a grain to be hit by an H atom. Once in the grain surface, the H atom diffuses and can react with an adsorbed S atom to form s-SH. The dashed magenta curves show the timescale for thermal desorption of an s-S atom (Eb ∕k (S) = 1100 K left curve, and 2600 K right curve) and of an s-O atom (blue curve; Eb∕k (O) = 1800 K). The gray dotted curve is the photodesorption timescale of s-S. At G0 values where the continuous line is below the dashed and dotted lines, s-O and s-S atoms remain on grain surfaces sufficiently long to combine with an adsorbed H atom and form s-OH and s-SH (and then s-H2 O and s-H2S). These timescales are for nH = 105 cm−3 and n(H) = 100 cm−3. Bottom panel: freeze-out depth at which most O and S are incorporated as s-H2 O and s-H2S (assuming no chemical desorption and Tk = Td). |
 |
Fig. 14 Gas-grain PDR models leading to the formation of s-H2S (shown as black curves). Continuous colored curves show gas-phase fractional abundances as a function of depth into the cloud. ϵ refers to the efficiency of the chemical desorption reaction s-H + s-H2S → SH + H2 (see text). Left panel: gas-grain high Eb model (high adsorption binding energies for S and SH, see Table 4). Right panel: low Eb model. |
6.3 Gas-grain PDR model results
Here we show PDR model results in which we add a simple network of gas-grain reactions for a small number of S-bearing (S, SH, and H2 S) and O-bearing (O, OH, H2O, O2, and CO) species. These species can adsorb on grains as temperatures drop, photodesorb by FUV photons (stellar and secondary), desorb by direct impact of cosmic-rays, or sublimate at a given PDR depth (depending on Td and on their Eb). Grain size distributions (ngr ∝a−3.5, where a is the grain radius) and gas-grain reactions are treated within the Meudon code formalism (see, Le Petit et al. 2006; Goicoechea & Le Bourlot 2007; Le Bourlot et al. 2012; Bron et al. 2014). As grain surface chemistry reactions we include s-H + s-X → s-XH and s-H + s-XH → s-H2X, where s-X refers to s-S and s-O. In addition, we add the direct chemical desorption reaction s-H + s-SH → H2S with an efficiency of 50% per reactive event, and also tested different efficiencies (ϵ) for the chemical desorption process s-H + s-H2S → SH + H2.
In our models we compute the relevant gas-grain timescales and atomic abundances at every depth AV of the PDR. If the timescale for a grain to be struck by an H atom (τgr, H) is shorter than the timescales to sublimate or to photodesorb an s-X atom or a s-XH molecule; and if H atoms stick on grains more frequently than X atoms, we simply assume these surface reactions proceed instantaneously. At large AV, larger than the freeze-out depth, this grain chemistry builds abundant s-H2O and s-H2S ice mantles.
Figure 14 shows results of two types of gas-grain models. The only difference between them is the adopted adsorption binding energies for s-S and s-SH. Left panel is for a “high Eb ” model and right panel is for a “low Eb” model (see Table 4). We note that these models do not include the gas-phase radiative association reactions S+ + H2 → H2S+ + hν and SH+ + H2 → H3S+ + hν; although their effect is smaller than in pure gas-phase models.
The chemistry of the most exposed PDR surface layers (AV ≲ 2 mag) is the same to that of the gas-phase models discussed in Sect. 6.1. Photodesorption keeps dust grains free of ice mantles, and fast gas-phase ion-neutral reactions, photoreactions, and reactions with FUV-pumped H2 drive the chemistry. The resulting SH+ abundance profile is nearly identical and there is no need to invoke depletion of elemental sulfur from the gas-phase to explain the observed SH+ emission (see Fig. 15). Beyond these first PDR irradiated layers, the chemistry does change because the formation of s-H2S on grains and subsequent desorption alters the chemistry of the other S-bearing hydrides.
In model high Eb, S atoms start to freeze out closer to the PDR edge (Td < 50 K). Because of the increasing densities and decreasing temperatures, the s-H2S abundance with respect to H nuclei reaches ~10−6 at AV ≃ 4 mag. In model low Eb, this level of s-H2S abundance is only reachedbeyond an AV of 7 mag. At lower AV, the formation of s-H2S on bare grains and subsequent photodesorption produces more H2 S than pure-gas phase models independently of whether H2S chemical desorption is included or not. In these intermediate PDR layers, at AV ≃ 2–7 mag for the strong irradiation conditions in the Bar, the flux of FUV photons drives much of the chemistry, desorbing grain mantles, preventing complete freeze out, and dissociating the gas-phase products.
There are two H2S abundance peaks at AV ≃ 4 and 7 mag. The H2S abundance in these “photodesorption peaks” depends on the amount of s-H2S mantles formed on grains and on the balance between s-H2S photodesorption and H2S photodissociation (which now becomes the major source of SH). The enhanced H2 S abundance modifies the chemistry of H2 S+ and H3 S+ as well: H2S photoionization (with a threshold at ~10.4 eV) becomes the dominant source of H2 S+ at AV ≃ 4 mag because the H2 (v ≥2) abundance is too low to make reaction (2) competitive. Besides, reactions of H2 S with abundant molecular ions such as HCO+, H , and H3 O+ dominate the H3 S+ production.
, and H3 O+ dominate the H3 S+ production.
Our gas-grain models predict that other S-bearing molecules, such as SO2 and SO, can be the major sulfur reservoirs at these intermediate PDR depths. However, their abundances strongly depend on those of O2 and OH through reactions S + O2 → SO + O and SO + OH → SO2 + H (see e.g., Sternberg & Dalgarno 1995; Fuente et al. 2016, 2019). These reactions link the chemistry of S- and O-bearing neutral molecules (Prasad & Huntress 1982) and are an important sink of S atoms at AV ≳ 5 mag. However, while large column densities of OH have been detected in the Orion Bar (≳ 1015 cm−2; Goicoechea et al. 2011), O2 remains undetected despite deep searches (Melnick et al. 2012). Furthermore, the inferred upper limit N(O2) columns arebelow the expectations of PDR models (Hollenbach et al. 2009). This discrepancy likely implies that these gas-grain models miss details of the grain surface chemistry leading to O2 (for other environments and modeling approaches see, e.g., Ioppolo et al. 2008; Taquet et al. 2016). Here we will not discuss SO2, SO, or O2 further.
At large cloud depths, AV ≳ 8 mag, the FUV flux is largely attenuated, temperatures drop, the chemistry becomes slower, and other chemical processes dominate. The H2 S abundance is controlled by the chemical desorption reaction s-H + s-SH → H2S. This process keeps a floor of detectable H2S abundances (>10−9) in regions shielded from stellar FUV radiation. In addition, and although not energetically favorable, the chemical desorption s-H + s-H2S → SH + H2 enhances the SH production at large AV (the enhancement depends on the desorption efficiency ϵ), which in turn boosts the abundances of other S-bearing species, including that of neutral S atoms.
The H2S abundances predicted bythe high Eb model reproduce the H2S line intensities observed in the Bar (Sect. 6.4). In this model s-H2S becomes the main sulfur reservoir. However, we stress that here we do not consider the formation of more complex S-bearing ices such as s-OCS, s-H2S2, s-Sn, s-SO2 or s-HSO (Jiménez-Escobar & Muñoz Caro 2011; Vidal et al. 2017; Laas & Caselli 2019). Together with our steady-state solution of the chemistry, this implies that our predictions are not precise deep inside the PDR. However, we recall that our observations refer to the edge of the Bar, so it is not plausible that the model conditions at AV ≳ 8 mag represent the line of sight we observe.
Model low Eb produces less H2S in the PDR layers below AV ≲ 8 mag because S atoms do not freeze until the dust temperature drops deep inside the PDR. Even beyond these layers, thermal desorption of s-S maintains higher abundances of S atoms at large depths. Indeed, model low Eb predicts that the major sulfur reservoir deep inside the cloud are gas-phase S atoms. This agrees with recent chemical models of cold dark clouds (Vidal et al. 2017; Navarro-Almaida et al. 2020).
6.4 Line intensity comparison and H2S ortho-to-para ratio
We now specifically compare the SH+, SH, and H2S line intensities implied by the different PDR models with the intensities observed toward the DF position of the Bar. We used the output of the PDR models – Tk, Td, n(H2), n(H), ne, n(SH+), n(SH), and n(H2S) profiles fromAV = 0 to 10 mag – as input for a multi-slab Monte Carlo model of their line excitation, including formation pumping (formalism presented in Sect. 4) and radiative transfer. As the Orion Bar is not a perfectly edge-on, this comparison requires a knowledge of the tilt angle (α) with respect to a pure edge-on PDR. Different studies suggest α of ≈5° (e.g., Jansen et al. 1995; Melnick et al. 2012; Andree-Labsch et al. 2017). This inclination implies an increase in line-of-sight column density, compared to a face-on PDR, by a geometrical factor (sin α)−1. It also means that optically thin lines are limb-brightened.
The left panel of Fig. 15 shows SH+ line intensity predictions for isobaric PDR models of different Pth values (leading to different Tk and nH profiles). Since the bulk of the SH+ emission arises from the PDR edge (AV ≃ 0 to 2 mag) all models (gas-phase or gas-grain) give similar results. The best fit is for Pth ≃ (1–2) × 108 cm−3 K and α ≃ 5°. These high pressures, at least close to the DF, agree with those inferred from ALMA images of HCO+ (J = 4–3) emission (Goicoechea et al. 2016), Herschel observations of high-J CO lines (Joblinet al. 2018), and IRAM 30 m detections of carbon recombination lines (Cuadrado et al. 2019).
Right panel of Fig. 15 shows SH and H2 S line emission predictions for the high Eb gas-grain model (magenta squares), low Eb gas-grain model (gray triangles), and a pure gas-phase model (cyan circles). For each model, the upper limit intensities refer to radiative transfer calculations with an inclination angle α = 5°. The lower intensity limits refer to a face-on PDR. Gas-phase models largely underestimate the observed H2 S intensities. Model low Eb produces higher H2S columns and brighter H2S lines, but still below the observed levels (by up to a factor of ten). Model high Eb provides a good agreement with observations; the two possible inclinations bracket the observed intensities, and it should be considered as the reference model of the Bar. It is also consistent with the observational SH upper limits.
Our observations and models provide a (line-of-sight) N(o-H2S)/N(p-H2S) OTP ratio of 2.9 ± 0.3, consistent with the (gas-phase) high-temperature statistical equilibrium value. However, the cold “nuclear-spin-temperatures” (Tspin ≪Tk; see definition in Eq. (D.1)) implied by the low water vapor OTP ratios observed in some sources (< 2.5) have been associated with the temperature of the ice mantles where H2 O molecules might have formed (i.e., Tspin ≃ Td; Mumma et al. 1987; Lis et al. 2013). In the case of H2S, our derived OTP ratio toward the DF position implies any Tspin above 30 ±10 K (see Fig. D.1). Hence, this temperature might be also compatible with s-H2S formation9 in warm grains if Tspin ≃ Td upon formation is preserved in the gas-phase after photodesorption (e.g., Guzmán et al. 2013). Interestingly, the H2 O OTP ratio derived from observations of the Orion Bar is 2.8 ± 0.1 (Putaud et al. 2019) and implies Tspin(H2O) = 35 ± 2 K. This value is compatible with Tspin(H2S) and might reflect the similar Td of the PDR layers where most s-H2O and s-H2S form and photodesorb. Nevertheless, laboratory experiments have challenged this Tspin ≃Td association, at least for s-H2O: cold water ice surfaces, at 10 K, photodesorb H2O molecules with an OTP ratio of ~3 (Hama et al. 2016). Follow up observations of p-H2S lines across the Bar will allow us to study possible variations of the OTP ratio as G0 diminishes and grains get colder.
 |
Fig. 15 Line intensity predictions for different isobaric PDR models. Calculations were carried out in a multi-slab Monte Carlo code (Sect. 4) that uses the output of the PDR model. Blue stars show the line intensities observed toward the Bar (corrected by beam dilution). Left panel: SH+ emission models for PDRs of different Pth values and α = 5°. Right panel: SH and H2S (adopting anOTP ratio of 3) emission from: high Eb (magenta squares), low Eb (gray triangles), and gas-phase (cyan circles) PDR models, all with Pth / k = 2 × 108 K cm−3. Upper limit intensity predictions are for a PDR with an inclination angle of α = 5° with respectto a edge-on geometry. Lower limit intensities refer to a face-on PDR model. |
6.5 Generalization to different G0 and nH conditions
In this section we generalize our results to a broader range of gas densities and FUV illumination conditions (i.e., to clouds with different G0 / nH ratios). We run several PDR models using the high Eb gas-grain chemistry. The main difference compared to the Orion Bar models is that here we model constant density clouds with standard interstellar grain properties (RV = 3.1). Figure 16 (left panel) shows models of clouds with constant nH = 104 cm−3 and varying FUV radiation fields, while Fig. 16 (right panel) show models of constant FUV illumination (G0 = 100) and varying gas densities10. The main result of this study is the similar gas-phase H2S column density (a few 1014 cm−2 up to AV = 10) and H2 S abundance peak (a few 10−8 close to the FUV-irradiated cloud edge) predicted by these models nearly irrespective of G0 and nH. A similar conclusion was reached previously for water vapor in FUV-illuminated clouds (Hollenbach et al. 2009, 2012). Increasing G0 shifts the position of the H2 S abundance peak to larger AV until the rate of S atoms sticking on grains balances the H2S photodissociation rate (the dominant H2S destruction mechanism except in shielded gas; see also Fig. 13). Since s-H2S photodesorption and H2S photodissociation rates depend on G0, the peak H2S abundance in the PDR is roughly the same independently of G0. On the other hand, the formation rate of s-H2S mantles depends on the product n(S) ngr ∝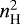 , whereas the H2S photodesorption rate depends on ngr ∝nH. Hence, the H2S abundance peak moves toward the cloud surface for denser PDRs (like the Orion Bar). The exact abundance value depends on the adopted grain-size distribution and on the H2S photodesorption yield (which is well constrained by experiments; see, Cruz-Diaz et al. 2014; Fuente et al. 2017).
, whereas the H2S photodesorption rate depends on ngr ∝nH. Hence, the H2S abundance peak moves toward the cloud surface for denser PDRs (like the Orion Bar). The exact abundance value depends on the adopted grain-size distribution and on the H2S photodesorption yield (which is well constrained by experiments; see, Cruz-Diaz et al. 2014; Fuente et al. 2017).
The roleof chemical desorption increases and can dominate beyond the photodesorption peak as the flux of stellar FUV photons is attenuated. Here we do not carry out an exhaustive study of this mechanism, which is hard to model in full detail because its efficiency decreases considerably with the properties of grain surfaces (bare vs. icy; see e.g., Minissale & Dulieu 2014). In our models, and depending on ζCR, photodesorption by secondary FUV photons can also be important in cloud interiors. These processes limit the conversion of most of the sulfur reservoir into S-bearing ices and increase the abundance of other gas-phase species deep inside clouds, notably S atoms and H2 S molecules.
The H2S abundance in shielded gas depends on the destruction rate by gas-phase reactions different than photodissociation, in particular H2 S reactions with H . The H
. The H abundance increases with ζCR and decreases with the electron density. Figure 16 (right) shows models of constant G0 and constant ζCR in which the H2S abundance at large depths increases with decreasing density (more penetration of FUV photons, more ionization, more electrons, less H
abundance increases with ζCR and decreases with the electron density. Figure 16 (right) shows models of constant G0 and constant ζCR in which the H2S abundance at large depths increases with decreasing density (more penetration of FUV photons, more ionization, more electrons, less H ). The lowest gas density model, nH = 103 cm−3, shows the highest H2S abundance at large AV. Because S freeze-out is less efficient at low densities, the low-density model shows higher gas-phase S abundances at large depths, making atomic S a dominant gas-phase sulfur reservoir. Unfortunately, direct observation of atomic S in cold gas is complicated, which makes it difficult to benchmark this prediction.
). The lowest gas density model, nH = 103 cm−3, shows the highest H2S abundance at large AV. Because S freeze-out is less efficient at low densities, the low-density model shows higher gas-phase S abundances at large depths, making atomic S a dominant gas-phase sulfur reservoir. Unfortunately, direct observation of atomic S in cold gas is complicated, which makes it difficult to benchmark this prediction.
In warm PDRs, in addition to S radio recombination lines (e.g., Smirnov et al. 1995), the 3 P fine-structure lines of atomic sulfur, the [S I] 25, 56 μm lines, can be interesting diagnostics of gas physical conditions and of [S/H] abundances. Unfortunately, the low sensitivity of previous infrared telescopes was not sufficient to detect the [S I] 25 μm line (ΔE12 = 570 K) in the Orion Bar (Rosenthal et al. 2000); although it is detected in protostellar outflows (e.g., Neufeld et al. 2009; Goicoechea et al. 2012). Moreover, the 3 P2-1D2 forbidden line of atomic sulfur at 1.082 μm can be an interesting tracer of the ionization and dissociation fronts in PDRs. Some of these lines will be accesible to high-angular-resolution and high sensitivity observations with JWST.
 |
Fig. 16 Constant density gas-grain PDR models using the high Eb chemical network and undepleted sulfur elemental abundances. Left panel: effects of changing the FUV radiation field. Right panel:effects of varying the gas density. |
6.6 The origin of H2S emission in other environments
Irrespective of nH and G0, grain surface formation of s-H2S and photodesorption back to the gas-phase lead to H2 S column densities of a few 1014 cm−2 in PDRs. This is in agrement with the observed column in the Bar (G0 ≈ 104) as well as at the mildly illuminated rims of TMC-1 and Barnard 1b clouds (G0 ≈ 10; Navarro-Almaida et al. 2020). The inferred H2 S abundance in the interior of these dark clouds (AV > 10 mag) drops to a few 10−9, but the species clearly does not disappear from the gas (N(H2S) of a few 1013 cm−2; Navarro-Almaida et al. 2020). Interestingly, neither in the Bar the H2S line emission at ~168 GHz decreases much behind the PDR (Fig. 1) even if the flux of FUV photons is largely attenuated compared to the irradiated PDR edge.
Despite oxygen is ~25 times more abundant than sulfur, the H2O to H2S column density ratio in the Orion Bar PDR is only about ~5. This similarity must also reflect the higher abundances of CO compared to CS. Furthermore, the H2 S column density in cold cores is strikingly similar to that of water vapor (Caselli et al. 2010, 2012). This coincidence points to a more efficient desorption mechanism of s-H2S compared tos-H2O in gas shielded from stellar FUV photons. Navarro-Almaida et al. (2020) argues that chemical desorption is able to reproduce the observed H2S abundance floor if the efficiency of this process diminishes as ice grain mantles get thicker inside cold dense cores.
Turning back to warmer star-forming environments, our predicted H2 S abundance in FUV-illuminated gas is comparable to that observed toward many hot cores (~10−9–10−8; van der Tak et al. 2003; Herpin et al. 2009). In these massive protostellar environments, thermal desorption of icy mantles, suddenly heated to Td ≳ 100 K by the luminosity of the embedded massive protostar, drives the H2 S production. Early in their evolution, young hot cores (≲104 yr) can show even higher abundances of recently desorbed H2 S (before further chemical processing takes place in the gas-phase; e.g., Charnley 1997; Hatchell et al. 1998; Jiménez-Serra et al. 2012; Esplugues et al. 2014). Indeed, Crockett et al. (2014) reports a gas-phase H2 S abundance of several 10−6 toward the hot core in Orion KL. This high value likely reflects the minimum s-H2S abundance locked as s-H2S mantles just before thermal desorption. In addition, the H2S abundance in the Orion Bar is only slightly lower than that inferred in protostellar outflows (several 10−8). In these regions, fast shocks erode and sputter the grain mantles, releasing a large fraction of their molecular content and activating a high-temperature gas-phase chemistry that quickly reprocesses the gas (e.g., Holdship et al. 2019). All in all, it seems reasonable to conclude that everywhere s-H2S grain mantles form, or already formed in a previous evolutionary stage, emission lines from gas-phase H2 S will be detectable.
In terms of its detectability with single-dish telescopes, H2 S rotational lines are bright in hot cores (Tpeak, 168 GHz ≃ 30 K in Orion KL but ≃ 1–3 K toward most hot cores; Tercero et al. 2010; van der Tak et al. 2003; Herpin et al. 2009), in strongly irradiated PDRs (≃6 K, this work), and in lower-illumination PDRs such as the Horsehead (≃1 K; Rivière-Marichalar et al. 2019). The H2S emission is fainter toward cold dark clouds (≃ 0.2 K in TMC-1; Navarro-Almaida et al. 2020) and protostellar outflows (≃ 0.6 K in L1157; Holdship et al. 2019). These line intensity differences are mostly produced by different gas physical conditions and not by enormous changes of the H2S abundance.
Finally, H2S is also detected outside the Milky Way (firstly by Heikkilä et al. 1999). Lacking enough spatial-resolution it is more difficult to determine the origin of the extragalactic H2S emission. The derived abundances in starburst galaxies such as NGC 253 (~10−9; Martín et al. 2006)might be interpreted as arising from a collection of spatially unresolved hot cores (Martín et al. 2011). However, hot cores have low filling factors at star-forming cloud scales. Our study suggests that much of this emission can arise from (the most common) extended molecular gas illuminated by stellar FUV radiation (e.g., Goicoechea et al. 2019).
7 Summary and conclusions
We carried out a self-consistent observational and modeling study of the chemistry of S-bearing hydrides in FUV-illuminated gas. We obtained the following results:
ALMAimages of the Orion Bar show that SH+ is confined to narrow gas layers of the PDR edge, close to the H2 dissociation front. Pointed observations carried out with the IRAM 30 m telescope show bright H
 S, H
S, H S, H
S, H S emission toward the PDR (but no H3 S+, a key gas precursor of H2S) as well as behind the Bar, where the flux of FUV photons is largely attenuated. SOFIA observations provide tight limits to the SH emission.
S emission toward the PDR (but no H3 S+, a key gas precursor of H2S) as well as behind the Bar, where the flux of FUV photons is largely attenuated. SOFIA observations provide tight limits to the SH emission.The SH+ line emission arises from a high-pressure gas component, Pth ≃ (1–2) × 108 cm−3 K, where SH+ ions are destroyed by reactive collisions with H atoms and electrons (as most Hn S+ ions do). We derive N(SH+) ≃ 1013 cm−2 and an abundance peak of several ~10−9. H2 S shows larger column densities toward the PDR, N(H2S) = N(o-H2S) + N(p-H2S) ≃ 2.5 × 1014 cm−2. Our tentative detection of SH translates into an upper limit column density ratio N(SH)/N(H2S) of < 0.2–0.6, already lower than the ratio of 1.1–3.0 observed in low-density diffuse molecular clouds (Neufeld et al. 2015). This implies an enhanced H2 S production mechanism in FUV-illuminated dense gas.
All gas-phase reactions X + H2 (v = 0) → XH + H (with X = S+, S, SH+, or H2 S+) are highly endoergic. While reaction of FUV-pumped H2 (v ≥ 2) molecules with S+ ions becomes exoergic and explains the observed levels of SH+, further reactions of H2 (v ≥ 2) with SH+ or with neutral S atoms, both reactions studied here through ab initio quantum calculations, do not form enough H2 S+ or H3 S+ to ultimately produce abundant H2S. In particular, pure gas-phase models underestimate the H2S column density observed in the Orion Bar by more than two orders of magnitude. This implies that these models miss the main H2 S formation route. The disagreement is even worse as we favor, after considering the potential energy surfaces of the H2 S+* and H3 S+* complexes, that the radiative associations S+ + H2 → H2S+ + hν and SH+ + H2 → H3S+ + hν may actually not occur or possess slower rates than considered in the literature.
To overcome these bottlenecks, we built PDR models that include a simple network of gas-grain and grain surface reactions. The higher binding energies of S and SH suggested by recent studies imply that bare grains start to grow s-H2S mantles notfar from the illuminated edges of molecular clouds. Indeed, the observed N(H2S) in the Orion Bar can only be explained by the freeze-out of S atoms, grain surface formation of s-H2S mantles, and subsequent photodesorption back to the gas phase. The inferred H2 S OTP ratio of 2.9 ± 0.3 (equivalent to Tspin ≥ 30 K) is compatible with the high-temperature statistical ratio as well as with warm grain surface formation if Tspin ≃Td and if Tspin is preserved inthe gas-phase after desorption.
Comparing observations with chemical and excitation models, we conclude that the SH+-emitting layers at the edge of the Orion Bar (AV < 2 mag) are charaterized by no or very little depletion of sulfur from the gas-phase. At intermediate PDR depths (AV < 8 mag) the observedH2S column densities do not require depletion of elemental (cosmic) sulfur abundances either.
We conclude that everywhere s-H2S grain mantles form (or formed) gas-phase H2S will be present in detectable amounts. Independently of nH and G0, FUV-illuminated clouds produce roughly the same H2S column density (a few 1014 cm−2) and H2S peak abundances (a few 10−8). This agrees with the H2S column densities derived in the Orion Bar and at the edges of mildly illuminated clouds. Deep inside molecular clouds (AV > 8 mag), H2 S still forms by direct chemical desorption and photodesorption by secondary FUV photons. These processes alter the abundances of other S-bearing species and makes difficult to predict the dominant sulfur reservoir in cloud interiors.
In this study we focused on S-bearing hydrides. Still, many subtle details remain to be fully understood: radiative associations, electron recombinations, and formation of multiply sulfuretted molecules. For example, the low-temperature (Tk < 1000 K) rates of the radiative and dielectronic recombination of S+ used in PDR models may still be not accurate enough (Badnell 1991). In addition, the main ice-mantle sulfur reservoirs are not fully constrained observationally. Thus, some of the narrative may be subject to speculation. Similarly, reactions of S+ with abundant organic molecules desorbed from grains (such as s-H2CO, not considered in our study) may contribute to enhance the H2 S+ abundance through gas-phase reactions (e.g., S+ +H2CO → H2S++CO; Prasad & Huntress 1982). Future observations of the abundance and freeze out depths of the key ice carriers with JWST will clearly help in these fronts.
Acknowledgements
We warmly thank Prof. György Lendvay for interesting discussions and for sharing the codes related to their S(3 P) + H2 (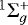 ,v) PES. We thank Paul Dagdigian, François Lique, and Alexandre Faure for sharing their H2 S–H2, SH+–H, and SH+–e− inelastic collisional rate coefficients and for interesting discussions in Grenoble and Salamanca. We thank Helgi Hrodmarsson for sending his experimental SH photoionization cross section in tabulated format. We finally thank our referee, John H. Black, for encouraging and insightful suggestions. This paper makes use of the ALMA data ADS/JAO.ALMA#2012.1.00352.S. ALMA is a partnership of ESO (representing its member states), NSF (USA), and NINS (Japan), together with NRC (Canada), and NSC and ASIAA (Taiwan), in cooperation with the Republic of Chile. The Joint ALMA Observatory is operated by ESO, AUI/NRAO, and NAOJ. It also includes IRAM 30 m telescope observations. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain). We thank the staff at the IRAM 30 m telescope and the work of the USRA and NASA staff of the Armstrong Flight Research Center in Palmdale and of the Ames Research Center in Mountain View (California), and the Deutsches SOFIA Institut. Wethank the Spanish MICIU for funding support under grants AYA2016-75066-C2-2-P, AYA2017-85111-P, FIS2017-83473-C2 PID2019-106110GB-I00, and PID2019-106235GB-I00 and the French-Spanish collaborative project PICS (PIC2017FR). We finally acknowledge computing time at Finisterrae (CESGA) under RES grant ACCT-2019-3-0004.
,v) PES. We thank Paul Dagdigian, François Lique, and Alexandre Faure for sharing their H2 S–H2, SH+–H, and SH+–e− inelastic collisional rate coefficients and for interesting discussions in Grenoble and Salamanca. We thank Helgi Hrodmarsson for sending his experimental SH photoionization cross section in tabulated format. We finally thank our referee, John H. Black, for encouraging and insightful suggestions. This paper makes use of the ALMA data ADS/JAO.ALMA#2012.1.00352.S. ALMA is a partnership of ESO (representing its member states), NSF (USA), and NINS (Japan), together with NRC (Canada), and NSC and ASIAA (Taiwan), in cooperation with the Republic of Chile. The Joint ALMA Observatory is operated by ESO, AUI/NRAO, and NAOJ. It also includes IRAM 30 m telescope observations. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain). We thank the staff at the IRAM 30 m telescope and the work of the USRA and NASA staff of the Armstrong Flight Research Center in Palmdale and of the Ames Research Center in Mountain View (California), and the Deutsches SOFIA Institut. Wethank the Spanish MICIU for funding support under grants AYA2016-75066-C2-2-P, AYA2017-85111-P, FIS2017-83473-C2 PID2019-106110GB-I00, and PID2019-106235GB-I00 and the French-Spanish collaborative project PICS (PIC2017FR). We finally acknowledge computing time at Finisterrae (CESGA) under RES grant ACCT-2019-3-0004.
Appendix A H2S+ formation and destruction
In this appendix we give details about how we calculated the H2 vibrational-state-dependent rates of reaction (2) and of the reverse reaction, the destruction of H2 S+ (2A′) by reactive collisons with H (2S) atoms (summarized in Fig. A.1).
We first built a full dimensional potential energy surface (PES) of the triplet H3 S+ (3A) system by fitting more than 150 000 ab initio points, including the long range interactions in the reactants and products channels. The main topological features of the PES are summarized in the minimum energy path between reactants and products (see middle panel of Fig. 9). These ab initio points were calculated with an explicitly correlated restricted coupled cluster including a single, double, and (perturbatively) triple excitations (RCCSD(T)-F12a) method (Knizia et al. 2009). The analytical fit has a overall rms error of ≃ 0.01 eV (Fig. A.2). Appendix A.1 provides more details.
Reaction (2) is endothermic by 0.672 eV, and the PES of the triplet state shows two shallow wells in the H2 + SH+ entrance channel (named 3W1a and 3 W1b, with a depth of ≃0.118 eV) and another one near the H + H2 S+ products (named 3W2, with a depth of 0.08 eV). Between the reactants and products wells there is a saddle point, with an energy of 0.601 eV. This saddle point, slightly below the products, has a geometry similar to 3 W2 in which the H–H distance is strongly elongated compared to that of H2. These features are also present in the maximum multiplicity PES of reactions H2 + S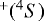 and H2 + H2 S+(2A) (see Fig. 9). We determine the state-dependent rates of reaction (2) and of the reverse reaction using a quasi-classical trajectory (QCT) method on our ground triplet PES. We provide more details on how the reactive cross sections for fixed collision energies were calculated in Appendix A.2.
and H2 + H2 S+(2A) (see Fig. 9). We determine the state-dependent rates of reaction (2) and of the reverse reaction using a quasi-classical trajectory (QCT) method on our ground triplet PES. We provide more details on how the reactive cross sections for fixed collision energies were calculated in Appendix A.2.
The formation rate of H2 S+ from H2 (v = 0) is very slow. For H2 (v = 1), the rate constant significantly increases at ≈500 K, corresponding with the opening of the H2 S+ + H threshold. At this point, it is important to consider the zero-point energy (ZPE) of the products (see next section for details). For H2 (v = 2) and H2 (v = 3), reaction rates are faster, close to the Langevin limit. Finally, the H2 S+ destruction rate constant is very similar to that of its formation from H2 (v = 2). In Appendix A.3 we provide more information about the destruction of Hn S+ ions through radiative association and spin flip mechanisms.
A.1 Ab initio calculations and PES
Dagdigian (2019b) presented a PES for the SH+-H2 system that includes 4-dimensions and is based on RCCSD(T)-F12a ab initio calculations. This PES was used to study SH+–H2 inelastic collisions using a rigid rotor approach in which the two diatomic molecules are kept fixed at their equilibrium distances. However, in order to study the reactivity of the collision, the two diatomic distances have to be included to account for the breaking and formation of new bonds.
Reaction (2) corresponds to a triplet state H3S+ (3A). The H2S+ (2A′) + H (2S) products can form a triplet and a singlet state. The triplet state can lead to the destruction of H2S+ through reaction with H atoms. The singlet state, however, produces very excited states of the reactants. Thus, it only leads to inelastic collisions but not not to the destruction of H2S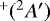 . In consequence, here we only consider the ground triplet electronic state of the system. In addition, the H
. In consequence, here we only consider the ground triplet electronic state of the system. In addition, the H + S (3P) channel is about 2.4 eV above the H2 + SH+ asymptote, and will not be included in the present study.
+ S (3P) channel is about 2.4 eV above the H2 + SH+ asymptote, and will not be included in the present study.
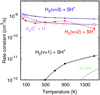 |
Fig. A.1 Calculated rate constants as a function of temperature (for translation and rotation) for SH+ (v = 0, j = 0) + H2 (v = 1, 2, 3, j = 0) and H2S+ (v = 0, j = 0) + H reactions (lavender) using ZPE corrected QCT method. Dotted curves are fits of the form k(T) = α (T/300)β exp(−γ∕T). Rate coefficients are listed in Table 1. |
 |
Fig. A.2 Rms error as a function of total energy, showing the number of ab initio points used to evaluate the error in the PES calculation. Arrows indicate selected critical points in the PES and provide an estimate of the error in each region. TS means transition state. |
In order to study the regions where several electronic states intersect, we performed a explicitly correlated internally contracted multireference configuration interaction (ic-MRCI-F12) calculation (Shiozaki & Werner 2013; Werner & Knowles 1988a,b) including the Davidson correction (icMRCI-F12+Q; Davidson 1975). The ic-MRCI-F12 calculations were carried out using state-averaged complete active space self-consistent field (SA-CASSCF) orbitals with all the CAS configurations as the reference configuration state functions. We used a triple zeta correlation consistent basis set for explicitly correlated wave functions (cc-pVTZ-F12; Peterson et al. 2008). In order to avoid orbital flipping between core and valence orbitals. SA-CASSCF calculations with three lowest triplet states were carried out including the core and valence orbitals as active space (18 electrons in 11 orbitals). For the ic-MRCI-F12 calculation, the core orbitals was kept doubly occupied, resulting in about 2.5 × 106 (9 × 107) contracted (uncontracted) configurations. All ab initio calculations were performed with MOLPRO (Werner et al. 2012).
Our ic-MRCI-F12 calculations show that the crossings with electronic excited states are 2 eV above the energy of the reactants. The energy interval below 2 eV is enough to study reaction (2). In these low-energy regions, RCCSD(T)-F12a calculations were also performed. They are in good agreement with the ic-MRCI-F12 results and the t1 diagnostic is always below 0.03. This allows us to conclude that for energies below 2 eV, the RCCSD(T)-F12a method performs well, presents a simple convergence, and being size consistent, is well adapted to the present case. This method is the same one employed in the inelastic collision calculations by Dagdigian (2019b).
We performed extensive RCCSD(T)-F12a calculations in all accessible regions to properly describe the six-dimensional phase space. 150 000 ab initio points were fitted to a multidimensional analytic function, that generates the six-dimensional PES represented as
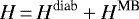 (A.1)
(A.1)
(Aguado et al. 2010; Sanz-Sanz et al. 2013; Zanchet et al. 2018; Roncero et al. 2018), where Hdiab is an electronic diabatic matrix in which each diagonal matrix element describes a rearrangement channel – six in this case, three equivalent for SH+ + H2 channels, and three equivalent for H2 S+ + H fragments (we omitted the H + S channel) – as an extension of the reactive force field approach (Farah et al. 2012). In each diagonal term, the molecular fragments (SH+, H2 and H2 S+) are described by 2 or 3 body fits (Aguado & Paniagua 1992), and the interaction among them is described by a sum of atom-atom terms plus the long range interaction. The non diagonal terms of Hdiab are described aspreviously (Zanchet et al. 2018; Roncero et al. 2018) and the parameters are fitted to approximately describe the saddle points along the minimum energy path in the right geometry.
+ S channel) – as an extension of the reactive force field approach (Farah et al. 2012). In each diagonal term, the molecular fragments (SH+, H2 and H2 S+) are described by 2 or 3 body fits (Aguado & Paniagua 1992), and the interaction among them is described by a sum of atom-atom terms plus the long range interaction. The non diagonal terms of Hdiab are described aspreviously (Zanchet et al. 2018; Roncero et al. 2018) and the parameters are fitted to approximately describe the saddle points along the minimum energy path in the right geometry.
In the reactants channel, the leading long range interaction 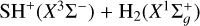 corresponds to charge-quadrupole and charge-induced dipole interactions (Buckinghan 1967):
corresponds to charge-quadrupole and charge-induced dipole interactions (Buckinghan 1967):
![\begin{eqnarray*} && \hspace*{-6pt} V_{{charge}}(\mathbf{r}_{\textrm{HH}},\mathbf{R})\,{=}\,\Theta_2 (r_{\textrm{HH}}) P_2(\cos \theta_2) R^{-3} \nonumber\\ &&\hspace*{-6pt} \quad - \left[ \frac12 \alpha_0(r_{\textrm{HH}}) + \frac{1}{3} \left(\alpha_{\parallel}(r_{\textrm{HH}})-\alpha_{\perp}(r_{\textrm{HH}}) \right)P_2(\cos \theta_2) \right] R^{-4} \end{eqnarray*}](/articles/aa/full_html/2021/03/aa39756-20/aa39756-20-eq49.png) (A.2)
(A.2)
and the dipole-quadrupole interactions (Buckinghan 1967):
![\begin{eqnarray*} && \hspace*{-6pt}V_{{dipole}}(\mathbf{r}_{\textrm{SH}}, \mathbf{r}_{\textrm{HH}},\mathbf{R})\,{=}\,3 \mu_1 (r_{\textrm{SH}}) \Theta_2 (r_{\textrm{HH}}) \nonumber\\ && \hspace*{-6pt} \quad {\times}\, \left[ \cos \theta_1 P_2(\cos \theta_2) + \sin \theta_1 \sin \theta_2 \cos \theta_2 \cos \phi \right] R^{-4}, \end{eqnarray*}](/articles/aa/full_html/2021/03/aa39756-20/aa39756-20-eq50.png) (A.3)
(A.3)
where Θ2(rHH) is the cuadrupole moment of 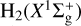 , α0 (rHH), α∥ (rHH), and α⊥ (rHH) are the average, parallel, and perpendicular polarizabilities of
, α0 (rHH), α∥ (rHH), and α⊥ (rHH) are the average, parallel, and perpendicular polarizabilities of 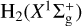 , respectively,and μ1(rSH) is the dipole moment of SH+(X3Σ−). P2 (cosθ) represents the Legendre polynomial of degree 2. The dependence of the molecular properties of H2 with the interatomic distance rHH is obtained from Velilla et al. (2008). The dipole moment of SH+ depends on the origin of coordinates. Since SH+(X3Σ−) dissociates in
, respectively,and μ1(rSH) is the dipole moment of SH+(X3Σ−). P2 (cosθ) represents the Legendre polynomial of degree 2. The dependence of the molecular properties of H2 with the interatomic distance rHH is obtained from Velilla et al. (2008). The dipole moment of SH+ depends on the origin of coordinates. Since SH+(X3Σ−) dissociates in 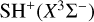 , we select the origin of coordinates in the S atom, so that the dipole moment tends to zero when R goes to infinity.
, we select the origin of coordinates in the S atom, so that the dipole moment tends to zero when R goes to infinity.
RCCSD(T)-F12a and fit stationary points on the PES.
Ev of reactants and products, and adiabatic switching energies for the QCT initial conditions.
In the products channel, the long range interaction 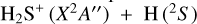 corresponds to the isotropic charge-induced dipole and charge-induced quadrupole dispersion terms
corresponds to the isotropic charge-induced dipole and charge-induced quadrupole dispersion terms
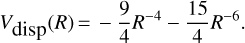
These long range terms diverge at R = 0. To avoid this behavior, we replace R by 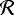 :
:

In Eq. (A.1), HMB is the many-body term, which is described by permutationaly invariant polynomials following the method of Aguado an collaborators (Aguado & Paniagua 1992; Tablero et al. 2001; Aguado et al. 2001). This many-body term improves the accuracy of the PES, especially in the region of the reaction barriers (as shown in Fig. 9). Features of the stationary points are listed in Table A.1.
A.2 Determination of reactive collision rates
We studied the reaction dynamics using a quasi-classical trajectory (QCT) method with the code miQCT (Zanchet et al. 2018; Roncero et al. 2018). In this method, the initial vibrational energy of the reactants is included using the adiabatic switching method (AS) (Grozdanov & Solov’ev 1982; Johnson 1987; Qu & Bowman 2016; Nagy & Lendvay 2017). Energies arelisted in Table A.2. The initial distance between the center-of-mass of the reactants (H2 + SH+ or H2 S+ + H) is set to 85 bohr, and the initial impact parameter is set randomly within a disk, the radius of which is set according to a capture model (Levine & Bernstein 1987) using the corresponding long-range interaction. The orientation among the two reactants is set randomly.
A first exploration of the reaction dynamics is done at fixed collision energy, for H2 (v = 0, 1, 2, 3) + SH+ (v = 0) and H + H2 S+(v = 0), and the reactive cross section is calculated as in Karplus et al. (1965)
 (A.4)
(A.4)
where Nt is the maximum number of trajectories with initial impact parameter lower than bmax, the maximum impact parameter for which the reaction takes place, and Nr is the number of trajectories leading to products. Figure A.2 shows results for Nt > 20 000 and all energies and initial reactant and vibrational states.
 |
Fig. A.3 Reaction cross section (in bohr2) as a function of collision energy (in meV) for the SH+ (v = 0, j = 0) + H2 (v = 1, 2, 3, j = 0) and H2S+ (v = 0, j = 0) + H collisions. Filled symbols are obtained counting all trajectories leading to products, while open symbols correspond to the ZPE corrected ones. |
For the SH+ (v = 0, j = 0) + H2 (v, j = 0) reaction there is a strong dependence on the initial vibrational state. For H2 (v = 0), there is nearly no reactive event, and only at 1 eV there are some reactive trajectories. For H2 (v = 2 and 3), however, the reaction shows a relatively large cross section, that decreases with increasing collision energy, as expected for exoergic reactions. Energies below 10–100 meV are dominated by long range interactions, leading to an increase in the maximum impact parameter, bmax, consistent with the variation of the cross section.
Reaction SH+ (v = 0, j = 0) + H2 (v = 1, j = 0) shows an unexpected behavior that deserves some discussion. At energies below 40 meV, the cross section is large and decreases with increasing energy. In the 40–200 meV that is consistent with the endothermicity of the reaction.
In order to analyze the reaction mechanism for H2 (v = 1) below 40 meV, we carried out an extensive analysis of the trajectories. A typical one is presented in Fig. A.4 for 10 meV. The H2 and SH+ reactants are attracted to each other by long range interactions, until they get trapped in the 3 W1 wells, as it is shown by the evolution of R, the distancebetween center-of-mass of the two molecules. The trapping lasts for 8 ps, thus allowing several collisions between H2 and SH+ and permitting the energy transfer between them. The H2 molecule ultimately breaks, and leaves SH+ with less vibrational energy. This can be inferred from the decrease in the amplitudes of the SH+ distance. The energy of the H2 S+ product is below the ZPE (see Table A.2). This is a clear indication of ZPE leakage in the QCT method, due to the energy transfer promoted by the long-lived collision complex.
 |
Fig. A.4 H–H, SH+ and R distances(in bohr) versus time (in ps), for a typical reactive trajectory for the SH+ (v = 0, j = 0) + H2 (v = 1, j = 0) collision at 10 meV. |
Several methods exist that correct the ZPE leakage. One is the Gaussian binning (Bonnet & Rayez 1997, 2004; Bañares et al. 2003, 2004). Here we have applied a simplification of this method, which assigns a weight (w) for each trajectory as
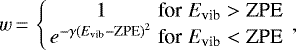 (A.5)
(A.5)
where Evib is the vibrational energy of reactants (adding those of H2 and SH+) or H2 S+ products at the end of each trajectory. These new weights are used to calculate Nr and Ntot in Eq. (tabfloatlinking A.4). ZPE-corrected results are shown in Fig. A.3 with open symbols. This plot shows that all values are nearly the same as those calculated simply by counting trajectories as an integer (as done in the normal binning method; see filled symbols in Fig. A.3). The only exception is the case of SH+ + H2 (v = 1) below 400 meV, which becomes zero when considering the ZPE of fragments at the end of the trajectories.
The reaction thermal rate in specific initial vibrational state of reactants are calculated running a minimum of 105 trajectories per temperature, with fixed vibrational states of reactants, assuming a Boltzmann distribution over translational and rotational degrees of freedom, and following the ZPE-corrected method as:
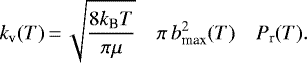 (A.6)
(A.6)
The results of these calculations are shown in Fig. A.1. A.3:
A.3 On the radiative associations of Hn S+
Herbst et al. (1989) and Millar & Herbst (1990) proposed that the radiative association HnS+ + H2 → Hn+1S+ + hν is viable process at low gas temperatures. Although this chemical route is widely used in astrochemical models, here we question the viability of this process. The lower multiplicity (L) PESs of H2 S+ (2A″) and H3 S+ (1A) are L = 1/2 and 0 respectively. These are shown in Fig. 9, together with the minimum multiplicity electronic state of H4 S+ (bottom panel). This state does not have a deep well or any higher multiplicity state that could connect to higher states of reactants and products.
For of H3 S+ formation through radiative association, this process assumes that a H3 S * complex forms in a triplet state, the high spin state H considered here. According to our calculations, such a complex is formed after low-energy H2 (v = 0, 1) + SH+ reactions (below 40 meV). The complex is formed in the 3W1 well, corresponding to geometries very far from those of the low spin well, the 1 W well. Therefore, a radiative spin flip and decay through phosphorescence is not possible. Herbst et al. (1989) proposed a second step, in which the spin flips from the triplet to the singlet state, followed by a radiative association, finally leading to the H3 S
* complex forms in a triplet state, the high spin state H considered here. According to our calculations, such a complex is formed after low-energy H2 (v = 0, 1) + SH+ reactions (below 40 meV). The complex is formed in the 3W1 well, corresponding to geometries very far from those of the low spin well, the 1 W well. Therefore, a radiative spin flip and decay through phosphorescence is not possible. Herbst et al. (1989) proposed a second step, in which the spin flips from the triplet to the singlet state, followed by a radiative association, finally leading to the H3 S product.
product.
The origin of the spin flip must be the spin-orbit couplings, very relevant for S-bearing species, that favor the spin transition when singlet and triplet states are close in energy. Using the PESs calculated here, the lowest crossing region is at ≃ 0.25 eV, very close to that of H2 (v = 0). At low temperatures, the H3 S * complex formed by H2 (v = 0) + SH+ reactions might allow a transition between the two electronic states with different spin. However, the spin flip probability is proportional to the square of the overlap
* complex formed by H2 (v = 0) + SH+ reactions might allow a transition between the two electronic states with different spin. However, the spin flip probability is proportional to the square of the overlap 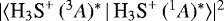 . This probability is very small because the two wells, 3W1 and 1W1, correspond to very different geometries. In consequence, we conclude that this radiative association mechanism must be negligible, especially at the high gas temperatures of PDR edges where the H3 S+(3A)* complex is not formed.
. This probability is very small because the two wells, 3W1 and 1W1, correspond to very different geometries. In consequence, we conclude that this radiative association mechanism must be negligible, especially at the high gas temperatures of PDR edges where the H3 S+(3A)* complex is not formed.
As an alternative, a spin flip in a direct collision (not forming a H3 S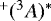 complex) may be more efficient and should be further investigated. Indeed, experimental measurements of the S
complex) may be more efficient and should be further investigated. Indeed, experimental measurements of the S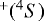 + H2 (v = 0) cross section show a maximum at about 1 eV of collisional energy attributed to spin-orbit transitions leading to spin flip (Stowe et al. 1990).
+ H2 (v = 0) cross section show a maximum at about 1 eV of collisional energy attributed to spin-orbit transitions leading to spin flip (Stowe et al. 1990).
Appendix B Reaction S (3P) + H2 (v) ⇄ SH + H
This reaction involves open shell reactants, S (3P), and products, SH (2Π). Neglecting spin flipping, there are three states that correlate to S(3 P), two of them connect to the SH (2Π). These two electronic states are of 3A′ and 3A″ symmetry, and have been studied in detail by Maiti et al. (2004). Here we use the adiabatic PES calculated by Maiti et al. (2004). Reaction S + H2 → SH + H is endothermic by ≃1.02 eV (without zero-point energy corrections), very similar to the endothermicity of reaction S+ + H2 → SH+ + H (Zanchet et al. 2013a, 2019). The main difference is the presence of a barrier, of ≃78 meV (≃905 K) with respect to the SH + H asymptote.
We performed quantum wave packet calculations for the reactions S + H2 (v = 2, 3, j = 0) and SH (v = 0, j = 0) + H. We used MADWAVE3 (Gómez-Carrasco & Roncero 2006; Zanchet et al. 2009) to calculate the reaction probabilities for the initial vibrational state of the diatomic reactant (in the ground state rotational state, j = 0). We employed the usual partial wave expansion to calculate the reaction cross section. We calculated only few total angular momenta of the triatomic system, J = 0, 10 and 20. The other J needed in the partial wave expansion were obtained using the J-shifting-interpolation method (see Zanchet et al. 2013a). The initial-state-specific rate constants are obtained by numerical integration of the cross section using a Boltzmann distribution (Zanchet et al. 2013a). The resulting reaction rate constants are shown in Figs. B.1 and B.2. The numerical values of the rate constants are fitted to the usual analytical Arrhenius-like expresion (shown as dotted curves). We note that the shoulder in the rate constants of reaction SH (v = 0) + H requires two functions in the temperature range of 200–800 K. Rate coefficients are tabulated in Table 1.
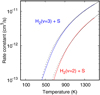 |
Fig. B.1 Calculated rate constants as a function of temperature for reaction S(3 P) + H2(v) → SH + H. Dotted curves are fits of the form k(T) = α (T/300)β exp(−γ∕T). Rate coefficients are listed in Table 1. |
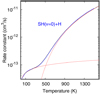 |
Fig. B.2 Calculated rate constants as a function of temperature for reaction SH (v = 0) + H → S + H2. The best fitto the calculated rate requires two Arrhenius-like expressions (one for low temperatures and one for high temperatures). Rate coefficients of these fits are listed in Table 1. |
Appendix C SH and H2S photoionization and photodissociation cross sections
Figure C.1 shows the experimental SH and H2 S photoionization and photodissociation cross sections (cm−2) used in our PDR models. We integrate these cross sections over the specific FUV radiation field at each AV depth of the PDR to obtain the specific photoionization and photodissociation rates (s−1).
 |
Fig. C.1 Photoionization and photodissociation cross sections. Top panel: σion(SH) (blue curve from laboratory experiments by Hrodmarsson et al. 2019). The pink curve is σdiss (SH) (Heays et al. 2017, and references therein). Bottom panel: σion(H2S) (blue curve) and σdiss(H2S) (gray and pink curves; from Zhou et al. 2020). |
Appendix D H2S ortho-to-para ratio and Tspin
The OTP ratio is sometimes related to a nuclear-spin-temperature (Tspin, e.g., Mumma et al. 1987)defined, for H2O or H2S, as:
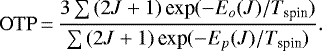 (D.1)
(D.1)
Here, Eo(J) and Ep (J) are the energies (in Kelvin) of o-H2S and p-H2S rotational levels (with the two ground rotational states separated by ΔE = 19.8 K). Figure D.1shows the OTP ratio of the two H2S nuclear spin isomers as a function of Tspin. The OTP ratio we infer toward the DF position of the Bar, 2.9 ± 0.3, is consistent with the statistical ratio of 3/1, and implies Tspin ≥ 30 ± 10 K.
Appendix E Line parameters of IRAM 30 m, ALMA, and SOFIA observations
Parameters of H2S and H S lines detected with the IRAM 30 m telescope toward three positions of the Orion Bar.
S lines detected with the IRAM 30 m telescope toward three positions of the Orion Bar.
Parameters of SH+ targeted with ALMA toward the DF position.
Parameters of SH lines (neglecting HFS) targeted with SOFIA toward the DF position.
References
- Aguado, A., & Paniagua, M. 1992, J. Chem. Phys., 96, 1265 [NASA ADS] [CrossRef] [Google Scholar]
- Aguado, A., Tablero, C., & Paniagua, M. 2001, Comput. Phys. Comm., 134, 97 [NASA ADS] [CrossRef] [Google Scholar]
- Aguado, A., Barragan, P., Prosmiti, R., et al. 2010, J. Chem. Phys., 133, 024306 [NASA ADS] [CrossRef] [Google Scholar]
- Agúndez, M., & Wakelam, V. 2013, Chemical Reviews, 113, 8710 [Google Scholar]
- Agúndez, M., Goicoechea, J. R., Cernicharo, J., Faure, A., & Roueff, E. 2010, ApJ, 713, 662 [NASA ADS] [CrossRef] [Google Scholar]
- Allers, K. N., Jaffe, D. T., Lacy, J. H., Draine, B. T., & Richter, M. J. 2005, ApJ, 630, 368 [NASA ADS] [CrossRef] [Google Scholar]
- Anders, E., & Grevesse, N. 1989, Geochim. Cosmochim. Acta, 53, 197 [Google Scholar]
- Andree-Labsch, S., Ossenkopf-Okada, V., & Röllig, M. 2017, A&A, 598, A2 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Anicich, V. G. 2003, An Index Of The Literature For Bimolecular Gas Phase Cation-Molecule Reaction Kinetics (California: JPL Publication), 1 [Google Scholar]
- Arab, H., Abergel, A., Habart, E., et al. 2012, A&A, 541, A19 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Asplund, M., Grevesse, N., Sauval, A. J., & Scott, P. 2009, ARA&A, 47, 481 [NASA ADS] [CrossRef] [Google Scholar]
- Azzam, A. A. A., Yurchenko, S. N., Tennyson, J., Martin-Drumel, M.-A., & Pirali, O. 2013, J. Quant. Spectr. Rad. Transf., 130, 341 [NASA ADS] [CrossRef] [Google Scholar]
- Badnell, N. R. 1991, ApJ, 379, 356 [NASA ADS] [CrossRef] [Google Scholar]
- Bally, J. 2008, Overview of the Orion Complex, ed. B. Reipurth (USA: Nasa), 459 [Google Scholar]
- Bañares, L., Aoiz, F. J., Honvault, P., Bussery-Honvault, B., & Launay, J.-M. 2003, J. Chem. Phys., 118, 565 [Google Scholar]
- Bañares, L., Aoiz, F. J., Honvault, P., & Launay, J.-M. 2004, J. Phys. Chem., 108, 1616 [Google Scholar]
- Black, J. H. 1998, Faraday Discuss., 109, 257 [NASA ADS] [CrossRef] [Google Scholar]
- Bonnet, L., & Rayez, J.-C. 1997, Chem. Phys. Lett., 277, 183 [NASA ADS] [CrossRef] [Google Scholar]
- Bonnet, L., & Rayez, J.-C. 2004, Chem. Phys. Lett., 397, 106 [NASA ADS] [CrossRef] [Google Scholar]
- Brittain, A., Coolbroth, K., & Boogert, A. 2020, AAS Meeting Abstracts, 236, 247.08 [NASA ADS] [Google Scholar]
- Bron, E., Le Bourlot, J., & Le Petit, F. 2014, A&A, 569, A100 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bron, E., Agúndez, M., Goicoechea, J. R., & Cernicharo, J. 2018, ArXiv eprints [arXiv:1801.01547] [Google Scholar]
- Buckinghan, A. D. 1967, Adv. Chem. Phys., 12, 107 [CrossRef] [Google Scholar]
- Burton, M. G., Hollenbach, D. J., & Tielens, A. G. G. M. 1990, ApJ, 365, 620 [NASA ADS] [CrossRef] [Google Scholar]
- Calmonte, U., Altwegg, K., Balsiger, H., et al. 2016, MNRAS, 462, S253 [CrossRef] [Google Scholar]
- Cardelli, J. A., Clayton, G. C., & Mathis, J. S. 1989, ApJ, 345, 245 [NASA ADS] [CrossRef] [Google Scholar]
- Caselli, P., Keto, E., Pagani, L., et al. 2010, A&A, 521, L29 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Caselli, P., Keto, E., Bergin, E. A., et al. 2012, ApJ, 759, L37 [Google Scholar]
- Charnley, S. B. 1997, ApJ, 481, 396 [NASA ADS] [CrossRef] [Google Scholar]
- Choi, Y., van der Tak, F. F. S., Bergin, E. A., & Plume, R. 2014, A&A, 572, L10 [Google Scholar]
- Collings, M. P., Anderson, M. A., Chen, R., et al. 2004, MNRAS, 354, 1133 [NASA ADS] [CrossRef] [Google Scholar]
- Crockett, N. R., Bergin, E. A., Neill, J. L., et al. 2014, ApJ, 781, 114 [NASA ADS] [CrossRef] [Google Scholar]
- Cruz-Diaz, G. A., Muñoz Caro, G. M., Chen, Y. J., & Yih, T. S. 2014, A&A, 562, A119 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cuadrado, S., Goicoechea, J. R., Pilleri, P., et al. 2015, A&A, 575, A82 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cuadrado, S., Goicoechea, J. R., Roncero, O., et al. 2016, A&A, 596, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cuadrado, S., Goicoechea, J. R., Cernicharo, J., et al. 2017, A&A, 603, A124 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cuadrado, S., Salas, P., Goicoechea, J. R., et al. 2019, A&A, 625, L3 [CrossRef] [EDP Sciences] [Google Scholar]
- Dagdigian, P. J. 2019a, MNRAS, 487, 3427 [CrossRef] [Google Scholar]
- Dagdigian, P. J. 2019b, J. Chem. Phys., 150, 084308 [CrossRef] [Google Scholar]
- Dagdigian, P. J. 2020, MNRAS, 494, 5239 [NASA ADS] [CrossRef] [Google Scholar]
- Dartois, E. 2005, Space Sci. Rev., 119, 293 [NASA ADS] [CrossRef] [Google Scholar]
- Davidson, E. R. 1975, J. Comp. Phys., 17, 87 [NASA ADS] [CrossRef] [MathSciNet] [Google Scholar]
- de Graauw, T., Helmich, F. P., Phillips, T. G., et al. 2010, A&A, 518, L6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Desrousseaux, B., Lique, F., Goicoechea, J. R., Quintas-Sánchez, E., & Dawes, R. 2021, A&A, 645, A8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Endres, C. P., Schlemmer, S., Schilke, P., Stutzki, J., & Müller, H. S. P. 2016, J. Mol. Spectr., 327, 95 [NASA ADS] [CrossRef] [Google Scholar]
- Esplugues, G. B., Viti, S., Goicoechea, J. R., & Cernicharo, J. 2014, A&A, 567, A95 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Esplugues, G. B., Cazaux, S., Meijerink, R., Spaans, M., & Caselli, P. 2016, A&A, 591, A52 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Farah, K., Muller-Plathe, F., & Bohm, M. C. 2012, Chem. Phys. Chem., 13, 1127 [Google Scholar]
- Faure, A., Halvick, P., Stoecklin, T., et al. 2017, MNRAS, 469, 612 [NASA ADS] [CrossRef] [Google Scholar]
- Freeman, A., & Williams, D. A. 1982, Ap&SS, 83, 417 [CrossRef] [Google Scholar]
- Fuente, A., Rodrıguez-Franco, A., Garcıa-Burillo, S., Martın-Pintado, J., & Black, J. H. 2003, A&A, 406, 899 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fuente, A., Cernicharo, J., Roueff, E., et al. 2016, A&A, 593, A94 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fuente, A., Goicoechea, J. R., Pety, J., et al. 2017, ApJ, 851, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Fuente, A., Navarro, D. G., Caselli, P., et al. 2019, A&A, 624, A105 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., Wakelam, V., & Herbst, E. 2007, A&A, 467, 1103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Genzel, R., & Stutzki, J. 1989, ARA&A, 27, 41 [NASA ADS] [CrossRef] [Google Scholar]
- Gerin, M., de Luca, M., Black, J., et al. 2010, A&A, 518, L110 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Gerin, M., Neufeld, D. A., & Goicoechea, J. R. 2016, ARA&A, 54, 181 [NASA ADS] [CrossRef] [Google Scholar]
- Gibb, E. L., Whittet, D. C. B., Boogert, A. C. A., & Tielens, A. G. G. M. 2004, ApJS, 151, 35 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Godard, B., & Cernicharo, J. 2013, A&A, 550, A8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Godard, B., Falgarone, E., Gerin, M., et al. 2012, A&A, 540, A87 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Godard, B., Falgarone, E., & Pineau des Forêts, G. 2014, A&A, 570, A27 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., & Le Bourlot, J. 2007, A&A, 467, 1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Pety, J., Gerin, M., et al. 2006, A&A, 456, 565 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Pety, J., Gerin, M., Hily-Blant, P., & Le Bourlot, J. 2009, A&A, 498, 771 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Joblin, C., Contursi, A., et al. 2011, A&A, 530, L16 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Cernicharo, J., Karska, A., et al. 2012, A&A, 548, A77 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Pety, J., Cuadrado, S., et al. 2016, Nature, 537, 207 [NASA ADS] [CrossRef] [Google Scholar]
- Goicoechea, J. R., Cuadrado, S., Pety, J., et al. 2017, A&A, 601, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Santa-Maria, M. G., Bron, E., et al. 2019, A&A, 622, A91 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goicoechea, J. R., Pabst, C. H. M., Kabanovic, S., et al. 2020, A&A, 639, A1 [CrossRef] [EDP Sciences] [Google Scholar]
- Gómez-Carrasco, S., & Roncero, O. 2006, J. Chem. Phys., 125, 054102 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Graedel, T. E., Langer, W. D., & Frerking, M. A. 1982, ApJS, 48, 321 [NASA ADS] [CrossRef] [Google Scholar]
- Grozdanov, T. P., & Solov’ev, E. A. 1982, J. Phys. B, 15, 1195 [CrossRef] [Google Scholar]
- Guzmán, V. V., Goicoechea, J. R., Pety, J., et al. 2013, A&A, 560, A73 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Habart, E., Dartois, E., Abergel, A., et al. 2010, A&A, 518, L116 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Habing, H. J. 1968, Bull. Astron. Inst. Netherlands, 19, 421 [Google Scholar]
- Hama, T., Kouchi, A., & Watanabe, N. 2016, Science, 351, 65 [NASA ADS] [CrossRef] [Google Scholar]
- Hamilton, J. R., Faure, A., & Tennyson, J. 2018, MNRAS, 476, 2931 [NASA ADS] [CrossRef] [Google Scholar]
- Hasegawa, T. I., & Herbst, E. 1993, MNRAS, 261, 83 [NASA ADS] [CrossRef] [Google Scholar]
- Hatchell, J., Thompson, M. A., Millar, T. J., & MacDonald, G. H. 1998, A&A, 338, 713 [Google Scholar]
- He, J., Shi, J., Hopkins, T., Vidali, G., & Kaufman, M. J. 2015, ApJ, 801, 120 [NASA ADS] [CrossRef] [Google Scholar]
- Heays, A. N., Bosman, A. D., & van Dishoeck, E. F. 2017, A&A, 602, A105 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Heikkilä, A., Johansson, L. E. B., & Olofsson, H. 1999, A&A, 344, 817 [NASA ADS] [Google Scholar]
- Herbst, E., DeFrees, D. J., & Koch, W. 1989, MNRAS, 237, 1057 [NASA ADS] [CrossRef] [Google Scholar]
- Herpin, F., Marseille, M., Wakelam, V., Bontemps, S., & Lis, D. C. 2009, A&A, 504, 853 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Heyminck, S., Graf, U. U., Güsten, R., et al. 2012, A&A, 542, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hogerheijde, M. R., Jansen, D. J., & van Dishoeck, E. F. 1995, A&A, 294, 792 [NASA ADS] [Google Scholar]
- Holdship, J., Jimenez-Serra, I., Viti, S., et al. 2019, ApJ, 878, 64 [NASA ADS] [CrossRef] [Google Scholar]
- Hollenbach, D. J., & Tielens, A. G. G. M. 1997, ARA&A, 35, 179 [NASA ADS] [CrossRef] [Google Scholar]
- Hollenbach, D., Kaufman, M. J., Bergin, E. A., & Melnick, G. J. 2009, ApJ, 690, 1497 [CrossRef] [Google Scholar]
- Hollenbach, D., Kaufman, M. J., Neufeld, D., Wolfire, M., & Goicoechea, J. R. 2012, ApJ, 754, 105 [NASA ADS] [CrossRef] [Google Scholar]
- Hosokawa, T., & Inutsuka, S.-i. 2006, ApJ, 646, 240 [NASA ADS] [CrossRef] [Google Scholar]
- Howk, J. C., Sembach, K. R., & Savage, B. D. 2006, ApJ, 637, 333 [NASA ADS] [CrossRef] [Google Scholar]
- Hrodmarsson, H. R., Garcia, G. A., Nahon, L., Loison, J.-C., & Gans, B. 2019, Physi. Chem. Chem. Phys., 21, 25907 [NASA ADS] [CrossRef] [Google Scholar]
- Indriolo, N., Neufeld, D. A., Gerin, M., et al. 2015, ApJ, 800, 40 [NASA ADS] [CrossRef] [Google Scholar]
- Ioppolo, S., Cuppen, H. M., Romanzin, C., van Dishoeck, E. F., & Linnartz, H. 2008, ApJ, 686, 1474 [NASA ADS] [CrossRef] [Google Scholar]
- Jansen, D. J., Spaans, M., Hogerheijde, M. R., & van Dishoeck, E. F. 1995, A&A, 303, 541 [NASA ADS] [Google Scholar]
- Jiménez-Escobar, A., & Muñoz Caro, G. M. 2011, A&A, 536, A91 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Jiménez-Serra, I., Zhang, Q., Viti, S., Martín-Pintado, J., & de Wit, W. J. 2012, ApJ, 753, 34 [NASA ADS] [CrossRef] [Google Scholar]
- Joblin, C., Bron, E., Pinto, C., et al. 2018, A&A, 615, A129 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Johnson, B. R. 1987, J. Chem. Phys., 86, 1445 [NASA ADS] [CrossRef] [Google Scholar]
- Kaplan, K. F., Dinerstein, H. L., Oh, H., et al. 2017, ApJ, 838, 152 [NASA ADS] [CrossRef] [Google Scholar]
- Karplus, M., Porter, R. N., & Sharma, R. D. 1965, J. Chem. Phys., 43, 3259 [NASA ADS] [CrossRef] [Google Scholar]
- Kirsanova, M. S., & Wiebe, D. S. 2019, MNRAS, 486, 2525 [CrossRef] [Google Scholar]
- Klisch, E., Klaus, T., Belov, S. P., et al. 1996, ApJ, 473, 1118 [NASA ADS] [CrossRef] [Google Scholar]
- Kłos, J., Lique, F., & Alexander, M. H. 2009, Chem. Phys. Lett., 476, 135 [NASA ADS] [CrossRef] [Google Scholar]
- Knizia, G., Adler, T. B., & Werner, H. J. 2009, J. Chem. Phys., 130, 054104 [NASA ADS] [CrossRef] [Google Scholar]
- Laas, J. C., & Caselli, P. 2019, A&A, 624, A108 [CrossRef] [EDP Sciences] [Google Scholar]
- Le Bourlot, J., Le Petit, F., Pinto, C., Roueff, E., & Roy, F. 2012, A&A, 541, A76 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Lee, T. A. 1968, ApJ, 152, 913 [NASA ADS] [CrossRef] [Google Scholar]
- Le Petit, F., Nehmé, C., Le Bourlot, J., & Roueff, E. 2006, ApJS, 164, 506 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Leurini, S., Rolffs, R., Thorwirth, S., et al. 2006, A&A, 454, L47 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Levine, R. D., & Bernstein, R. B. 1987, Mol Reaction Dynamics and Chemical Reactivity (Oxford: Oxford University Press) [Google Scholar]
- Lique, F., Zanchet, A., Bulut, N., Goicoechea, J. R., & Roncero, O. 2020, A&A, 638, A72 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Lis, D. C., Bergin, E. A., Schilke, P., & van Dishoeck, E. F. 2013, J. Phys. Chem. A, 117, 9661 [CrossRef] [Google Scholar]
- Lucas, R., & Liszt, H. S. 2002, A&A, 384, 1054 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Maiti, B., Schatz, G. C., & Lendvay, G. 2004, J. Phys. Chem. A, 108, 8772 [NASA ADS] [CrossRef] [Google Scholar]
- Marconi, A., Testi, L., Natta, A., & Walmsley, C. M. 1998, A&A, 330, 696 [NASA ADS] [Google Scholar]
- Martín, S., Mauersberger, R., Martín-Pintado, J., Henkel, C., & García-Burillo, S. 2006, ApJS, 164, 450 [NASA ADS] [CrossRef] [Google Scholar]
- Martín, S., Krips, M., Martín-Pintado, J., et al. 2011, A&A, 527, A36 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Martin-Drumel, M. A., Eliet, S., Pirali, O., et al. 2012, Chem. Phys. Lett., 550, 8 [CrossRef] [Google Scholar]
- Melnick, G. J., Tolls, V., Goldsmith, P. F., et al. 2012, ApJ, 752, 26 [NASA ADS] [CrossRef] [Google Scholar]
- Melnick, G. J., Tolls, V., Snell, R. L., et al. 2020, ApJ, 892, 22 [CrossRef] [Google Scholar]
- Menten, K. M., Wyrowski, F., Belloche, A., et al. 2011, A&A, 525, A77 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Millar, T. J., & Herbst, E. 1990, A&A, 231, 466 [NASA ADS] [Google Scholar]
- Millar, T. J., Adams, N. G., Smith, D., Lindinger, W., & Villinger, H. 1986, MNRAS, 221, 673 [NASA ADS] [CrossRef] [Google Scholar]
- Minissale, M., & Dulieu, F. 2014, J. Chem. Phys., 141, 014304 [NASA ADS] [CrossRef] [Google Scholar]
- Minissale, M., Dulieu, F., Cazaux, S., & Hocuk, S. 2016, A&A, 585, A24 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Mumma, M. J., Weaver, H. A., & Larson, H. P. 1987, A&A, 187, 419 [NASA ADS] [Google Scholar]
- Nagy, T., & Lendvay, G. 2017, J. Phys. Chem. Lett., 8, 4621 [CrossRef] [Google Scholar]
- Nagy, Z., Van der Tak, F. F. S., Ossenkopf, V., et al. 2013, A&A, 550, A96 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Nagy, Z., Choi, Y., Ossenkopf-Okada, V., et al. 2017, A&A, 599, A22 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Navarro-Almaida, D., Le Gal, R., Fuente, A., et al. 2020, A&A, 637, A39 [CrossRef] [EDP Sciences] [Google Scholar]
- Neufeld, D. A., Nisini, B., Giannini, T., et al. 2009, ApJ, 706, 170 [NASA ADS] [CrossRef] [Google Scholar]
- Neufeld, D. A., Goicoechea, J. R., Sonnentrucker, P., et al. 2010, A&A, 521, L10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Neufeld, D. A., Falgarone, E., Gerin, M., et al. 2012, A&A, 542, L6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Neufeld, D. A., Godard, B., Gerin, M., et al. 2015, A&A, 577, A49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Oba, Y., Tomaru, T., Lamberts, T., Kouchi, A., & Watanabe, N. 2018, Nat. Astron., 2, 228 [NASA ADS] [CrossRef] [Google Scholar]
- O’Dell, C. R. 2001, ARA&A, 39, 99 [NASA ADS] [CrossRef] [Google Scholar]
- Pabst, C., Higgins, R., Goicoechea, J. R., et al. 2019, Nature, 565, 618 [NASA ADS] [CrossRef] [Google Scholar]
- Pabst, C. H. M., Goicoechea, J. R., Teyssier, D., et al. 2020, A&A, 639, A2 [CrossRef] [EDP Sciences] [Google Scholar]
- Palumbo, M. E., Geballe, T. R., & Tielens, A. G. G. M. 1997, ApJ, 479, 839 [NASA ADS] [CrossRef] [Google Scholar]
- Pankonin, V., & Walmsley, C. M. 1978, A&A, 64, 333 [NASA ADS] [Google Scholar]
- Parikka, A., Habart, E., Bernard-Salas, J., et al. 2017, A&A, 599, A20 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pellegrini, E. W., Baldwin, J. A., Ferland, G. J., Shaw, G., & Heathcote, S. 2009, ApJ, 693, 285 [NASA ADS] [CrossRef] [Google Scholar]
- Peterson, K. A., Adler, T. B., & Werner, H. J. 2008, J. Chem. Phys., 128, 084102 [NASA ADS] [CrossRef] [Google Scholar]
- Pineau des Forets, G., Flower, D. R., Hartquist, T. W., & Dalgarno, A. 1986, MNRAS, 220, 801 [NASA ADS] [CrossRef] [Google Scholar]
- Prasad, S. S., & Huntress, W. T., J. 1980, ApJS, 43, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Prasad, S. S., & Huntress, W. T., J. 1982, ApJ, 260, 590 [NASA ADS] [CrossRef] [Google Scholar]
- Putaud, T., Michaut, X., Le Petit, F., Roueff, E., & Lis, D. C. 2019, A&A, 632, A8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Qu, C., & Bowman, J. M. 2016, J. Phys. Chem. A, 120, 4988 [CrossRef] [Google Scholar]
- Rivière-Marichalar, P., Fuente, A., Goicoechea, J. R., et al. 2019, A&A, 628, A16 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Roelfsema, P. R., Helmich, F. P., Teyssier, D., et al. 2012, A&A, 537, A17 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Roncero, O., Zanchet, A., & Aguado, A. 2018, Phys. Chem. Chem. Phys., 20, 25951 [NASA ADS] [CrossRef] [Google Scholar]
- Rosenthal, D., Bertoldi, F., & Drapatz, S. 2000, A&A, 356, 705 [NASA ADS] [Google Scholar]
- Sandford, S. A., & Allamandola, L. J. 1988, Icarus, 76, 201 [NASA ADS] [CrossRef] [Google Scholar]
- Sanz-Sanz, C., Roncero, O., Paniagua, M., & Aguado, A. 2013, J. Chem. Phys., 139, 184302 [NASA ADS] [CrossRef] [Google Scholar]
- Shiozaki, T., & Werner, H.-J. 2013, Mol. Phys., 111, 607 [NASA ADS] [CrossRef] [Google Scholar]
- Smirnov, G. T., Sorochenko, R. L., & Walmsley, C. M. 1995, A&A, 300, 923 [NASA ADS] [Google Scholar]
- Smith, R. G. 1991, MNRAS, 249, 172 [NASA ADS] [CrossRef] [Google Scholar]
- Sofia, U. J., Lauroesch, J. T., Meyer, D. M., & Cartledge, S. I. B. 2004, ApJ, 605, 272 [NASA ADS] [CrossRef] [Google Scholar]
- Stecher, T. P., & Williams, D. A. 1972, ApJ, 177, L141 [NASA ADS] [CrossRef] [Google Scholar]
- Sternberg, A., & Dalgarno, A. 1995, ApJS, 99, 565 [NASA ADS] [CrossRef] [Google Scholar]
- Stoerzer, H., Stutzki, J., & Sternberg, A. 1995, A&A, 296, L9 [NASA ADS] [Google Scholar]
- Stowe, G. F., Schultz, R. H., Wright, C. A., & Armentrout, P. B. 1990, Int. J. Mass Spectr. Ion Proc., 100, 377 [Google Scholar]
- Tablero, C., Aguado, A., & Paniagua, M. 2001, Comput. Phys. Comm., 140, 412 [NASA ADS] [CrossRef] [Google Scholar]
- Taquet, V., Furuya, K., Walsh, C., & van Dishoeck, E. F. 2016, MNRAS, 462, S99 [Google Scholar]
- Tercero, B., Cernicharo, J., Pardo, J. R., & Goicoechea, J. R. 2010, A&A, 517, A96 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Tieftrunk, A., Pineau des Forets, G., Schilke, P., & Walmsley, C. M. 1994, A&A, 289, 579 [Google Scholar]
- Tielens, A. G. G. M. 2010, The Physics and Chemistry of the Interstellar Medium (Cambridge: Cambridge University Press) [Google Scholar]
- Tielens, A. G. G. M., & Hagen, W. 1982, A&A, 114, 245 [Google Scholar]
- Tielens, A. G. G. M., & Hollenbach, D. 1985, ApJ, 291, 722 [Google Scholar]
- Tielens, A. G. G. M., Meixner, M. M., van der Werf, P. P., et al. 1993, Science, 262, 86 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Turner, B. E. 1996, ApJ, 468, 694 [NASA ADS] [CrossRef] [Google Scholar]
- van der Tak, F. F. S., Boonman, A. M. S., Braakman, R., & van Dishoeck, E. F. 2003, A&A, 412, 133 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- van der Tak, F. F. S., Black, J. H., Schöier, F. L., Jansen, D. J., & van Dishoeck, E. F. 2007, A&A, 468, 627 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- van der Tak, F. F. S., Nagy, Z., Ossenkopf, V., et al. 2013, A&A, 560, A95 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- van der Werf, P. P., Stutzki, J., Sternberg, A., & Krabbe, A. 1996, A&A, 313, 633 [NASA ADS] [Google Scholar]
- van der Werf, P. P., Goss, W. M., & O’Dell, C. R. 2013, ApJ, 762, 101 [NASA ADS] [CrossRef] [Google Scholar]
- van der Wiel, M. H. D., van der Tak, F. F. S., Ossenkopf, V., et al. 2009, A&A, 498, 161 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- van Dishoeck, E. F. 2004, ARA&A, 42, 119 [NASA ADS] [CrossRef] [Google Scholar]
- Velilla, L., Lepetit, B., Aguado, A., Beswick, J., & Paniagua, M. 2008, J. Chem. Phys., 129, 084307 [CrossRef] [PubMed] [Google Scholar]
- Vidal, T. H. G., Loison, J.-C., Jaziri, A. Y., et al. 2017, MNRAS, 469, 435 [Google Scholar]
- Wakelam, V., Loison, J. C., Mereau, R., & Ruaud, M. 2017, Mol. Astrophys., 6, 22 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Walmsley, C. M., Natta, A., Oliva, E., & Testi, L. 2000, A&A, 364, 301 [NASA ADS] [Google Scholar]
- Werner, H. J., & Knowles, P. J. 1988a, J. Chem. Phys., 89, 5803 [NASA ADS] [CrossRef] [Google Scholar]
- Werner, H. J., & Knowles, P. J. 1988b, Chem. Phys. Lett., 145, 514 [NASA ADS] [CrossRef] [Google Scholar]
- Werner, H.-J., Knowles, P. J., Knizia, G., Manby, F. R., & Schütz, M. 2012, WIREs Comput. Mol. Sci., 2, 242 [CrossRef] [Google Scholar]
- Wyrowski, F., Schilke, P., Hofner, P., & Walmsley, C. M. 1997, ApJ, 487, L171 [NASA ADS] [CrossRef] [Google Scholar]
- Yamamura, I., Kawaguchi, K., & Ridgway, S. T. 2000, ApJ, 528, L33 [NASA ADS] [CrossRef] [Google Scholar]
- Young, E. T., Becklin, E. E., Marcum, P. M., et al. 2012, ApJ, 749, L17 [NASA ADS] [CrossRef] [Google Scholar]
- Zanchet, A., Roncero, O., González-Lezana, T., et al. 2009, J. Phys. Chem. A, 113, 14488 [CrossRef] [PubMed] [Google Scholar]
- Zanchet, A., Agúndez, M., Herrero, V. J., Aguado, A., & Roncero, O. 2013a, AJ, 146, 125 [NASA ADS] [CrossRef] [Google Scholar]
- Zanchet, A., Godard, B., Bulut, N., et al. 2013b, ApJ, 766, 80 [NASA ADS] [CrossRef] [Google Scholar]
- Zanchet, A., del Mazo, P., Aguado, A., et al. 2018, PCCP, 20, 5415 [NASA ADS] [CrossRef] [Google Scholar]
- Zanchet, A., Lique, F., Roncero, O., Goicoechea, J. R., & Bulut, N. 2019, A&A, 626, A103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Zhou, J., Zhao, Y., Hansen, C. S., et al. 2020, Nat. Commun., 11, 1547 [NASA ADS] [CrossRef] [Google Scholar]
SH was first reported by IR spectroscopy toward the cirumstellar envelope around the evolved star R Andromedae (Yamamura et al. 2000).
Readers interested only in the chemistry of these species and in depth-dependent PDR models could directly jump to Sect. 6.
We use the following inelastic collision rate coefficients γij: • SH+– e−, including hyperfine splittings (Hamilton et al. 2018). • SH+– o-H2 and p-H2, including hyperfine splittings (Dagdigian 2019a). • SH+– H, including hyperfine splittings (Lique et al. 2020). • o-H2S and p-H2S with o-H2 and p-H2 (Dagdigian 2020). • SH– He, including fine-structure splittings (Kłos et al. 2009).
CH+ is more reactive than SH+ because CH+ does react with H2 (v = 0) exothermically producing CH at
k = 1.2 × 10−9 cm3 s−1
(Anicich 2003) and also because reaction of CH+ with H is faster,
k = 7.5 × 10−10 cm3 s−1.
at
k = 1.2 × 10−9 cm3 s−1
(Anicich 2003) and also because reaction of CH+ with H is faster,
k = 7.5 × 10−10 cm3 s−1.
If one considers H2 rovibrational levels, reaction (1) becomes exoergic for v = 0, J ≥ 11 and for v = 1, J ≥ 7 (Zanchet et al. 2019).
Older gas-phase PDR models previously predicted low H2 S column densities (Jansen et al. 1995; Sternberg & Dalgarno 1995).
Crockett et al. (2014) inferred N(o-H2S)/N(p-H2S) = 2.5 ± 0.8 in the hot core of Orion KL using LTE rotational digrams. However, they favored an OTP ratio of 1.7 ± 0.8 based on the column density ratio of selected pairs of rotational levels with similar energies. This latter OTP ratio implies Tspin(H2S) ≃ 12 K (Fig. D.1), perhaps related to much colder dust grains than in PDRs or to colder gas conditions just before the hot core phase; so that reactive collisions did not have time to establish the statistical equilibrium value. The observed OTP ratios of H2 CO, H2 CS, and H2 CCO in the Bar are also ~3 (Cuadrado et al. 2017).
All Tables
Relevant rate coefficients from a fit of the Arrhenius-like form k (T) = 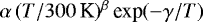 to the calculated reaction rates.
to the calculated reaction rates.
Column density predictions from different PDR models (up to AV = 10 mag) and estimated values from observations (single-slab approach).
Ev of reactants and products, and adiabatic switching energies for the QCT initial conditions.
Parameters of H2S and H S lines detected with the IRAM 30 m telescope toward three positions of the Orion Bar.
S lines detected with the IRAM 30 m telescope toward three positions of the Orion Bar.
Parameters of SH lines (neglecting HFS) targeted with SOFIA toward the DF position.
All Figures
 |
Fig. 1 Overview of the Orion Bar. The (0″, 0″) position corresponds to α2000 = 05h 35m 20.1s ; δ2000 = -05°25′07.0″. Left panel: integrated line intensity maps in the 13CO J = 3–2 (color scale) and SO 89–78 emission (gray contours; from 6 to 23.5 K km s−1 in steps of 2.5 K km s−1) obtained with the IRAM 30 m telescope at 8″ resolution. The white dotted contours delineate the position of the H2 dissociation front as traced by the infrared H2 v = 1–0 S(1) line (from 1.5 to 4.0 × 10−4 erg s−1 cm−2 sr−1 in steps of 0.5 × 10−4 erg s−1 cm−2 sr−1; from Walmsley et al. 2000). The black-dashed rectangle shows the smaller FoV imaged with ALMA (Fig. 3). The DF position has been observed with SOFIA, IRAM 30 m, and Herschel. Cyan circles represent the ~15″ beam at 168 GHz. Right panel: H2S lines lines detected toward three positions of the Orion Bar. |
| In the text | |
 |
Fig. 2 Detection of H2 33 S (at ~168.3 GHz) toward the DF position of the Orion Bar. Red lines indicate hyperfine components. Blue lines show interloping lines from 13CCH. The length of each line is proportional to the transition line strength (taken from the Cologne Database for Molecular Spectroscopy, CDMS; Endres et al. 2016). |
| In the text | |
 |
Fig. 3 ALMA 1″-resolution images zooming into the edge of the Orion Bar in 12CO 3–2 (left panel, Goicoechea et al. 2016) and SH+ 10 –01 F = 1/2–3/2 line (middle panel, integrated line intensity). The right panel shows the H2 v = 1–0 S(1) line (Walmsley et al. 2000). We rotated these images (all showing the same FoV) with respect to Fig. 1 to bring the FUV illuminating direction in the horizontal direction (from the right). The circle shows the DF position targeted with SOFIA in SH (20″ beam) and with the IRAM 30 m telescope in H2S and H3S+. |
| In the text | |
 |
Fig. 4 Search for the SH 2 Π3∕2 J = 5/2–3/2 doublet (at ~1383 GHz) toward the DF position with SOFIA/GREAT. Vertical magenta lines indicate the position of hyperfine splittings taken from CDMS. |
| In the text | |
 |
Fig. 5 Search for H3S+ toward the Orion Bar with the IRAM 30 m telescope. The blue curve shows the expected position of the line. |
| In the text | |
 |
Fig. 6 Non-LTE excitation models of SH+. The horizontal lines mark the observed line intensities in the Orion Bar. Dotted curves are for a standard model (F = D = 0). Continuous curves are for a model that includes chemical destruction by H atoms and e− (model F, D). Dashed lines are for a model in which destruction rates are multiplied by ten (model F, D × 10). The verticalblack line marks the best model. |
| In the text | |
 |
Fig. 7 Non-LTE excitation models of SH emission lines targeted with SOFIA/GREAT. Horizontal dashed lines refer to observational limits, assuming extended emission (lower intensities) and for a 10″ width emission filament at the PDR surface (higher intensities). |
| In the text | |
 |
Fig. 8 Non-LTE excitation models for o-H2S and p-H2S. Thin horizontal lines show the observed intensities assuming either extended emission (lower limit) or emission that fills the 15″ beam at168.7 GHz. The vertical line marks the best model, resulting in an OTP ratio of 2.9 ± 0.3. |
| In the text | |
 |
Fig. 9 Minimum energy paths for reactions (1), (2), and (3). Points correspond to RCCSD(T)-F12a calculations and lines to fits (Appendix A). The reaction coordinate, s, is defined independently for each path. The geometries of each species at s = 0 are different. |
| In the text | |
 |
Fig. 10 Structure of an isobaric PDR representing the most FUV-irradiated gas layers of the Orion Bar (see Table 2 for the adopted parameters). This plot shows the H2, H, and electron density profiles (left axis scale), and the gas and dust temperatures (right axis scale). |
| In the text | |
 |
Fig. 11 Main gas and grain reactions leading to the formation of sulfur hydrides. Red arrows represent endoergic reactions (endothermicity given in units of K). Dashed arrows are uncertain radiative associations (see Appendix A.3), γ stands for a FUV photon, and “s-” for solid. |
| In the text | |
 |
Fig. 12 Pure gas-phase PDR models of the Orion Bar. Continuous curves show fractional abundances as a function of cloud depth, in logarithm scale to better display the irradiated edge of the PDR, using the new reaction rates listed in Table 1. The gray dotted curve shows f2, the fraction of H2 that is in vibrationally excited levels v ≥ 2 (right axis scale). Dashed curves are for a model using standard reaction rates. |
| In the text | |
 |
Fig. 13 Representative timescales relevant to the formation of s-H2S and s-H2O as well as their freeze-out depths. Upper panel: the continuous black curve is the timescale for a grain to be hit by an H atom. Once in the grain surface, the H atom diffuses and can react with an adsorbed S atom to form s-SH. The dashed magenta curves show the timescale for thermal desorption of an s-S atom (Eb ∕k (S) = 1100 K left curve, and 2600 K right curve) and of an s-O atom (blue curve; Eb∕k (O) = 1800 K). The gray dotted curve is the photodesorption timescale of s-S. At G0 values where the continuous line is below the dashed and dotted lines, s-O and s-S atoms remain on grain surfaces sufficiently long to combine with an adsorbed H atom and form s-OH and s-SH (and then s-H2 O and s-H2S). These timescales are for nH = 105 cm−3 and n(H) = 100 cm−3. Bottom panel: freeze-out depth at which most O and S are incorporated as s-H2 O and s-H2S (assuming no chemical desorption and Tk = Td). |
| In the text | |
 |
Fig. 14 Gas-grain PDR models leading to the formation of s-H2S (shown as black curves). Continuous colored curves show gas-phase fractional abundances as a function of depth into the cloud. ϵ refers to the efficiency of the chemical desorption reaction s-H + s-H2S → SH + H2 (see text). Left panel: gas-grain high Eb model (high adsorption binding energies for S and SH, see Table 4). Right panel: low Eb model. |
| In the text | |
 |
Fig. 15 Line intensity predictions for different isobaric PDR models. Calculations were carried out in a multi-slab Monte Carlo code (Sect. 4) that uses the output of the PDR model. Blue stars show the line intensities observed toward the Bar (corrected by beam dilution). Left panel: SH+ emission models for PDRs of different Pth values and α = 5°. Right panel: SH and H2S (adopting anOTP ratio of 3) emission from: high Eb (magenta squares), low Eb (gray triangles), and gas-phase (cyan circles) PDR models, all with Pth / k = 2 × 108 K cm−3. Upper limit intensity predictions are for a PDR with an inclination angle of α = 5° with respectto a edge-on geometry. Lower limit intensities refer to a face-on PDR model. |
| In the text | |
 |
Fig. 16 Constant density gas-grain PDR models using the high Eb chemical network and undepleted sulfur elemental abundances. Left panel: effects of changing the FUV radiation field. Right panel:effects of varying the gas density. |
| In the text | |
 |
Fig. A.1 Calculated rate constants as a function of temperature (for translation and rotation) for SH+ (v = 0, j = 0) + H2 (v = 1, 2, 3, j = 0) and H2S+ (v = 0, j = 0) + H reactions (lavender) using ZPE corrected QCT method. Dotted curves are fits of the form k(T) = α (T/300)β exp(−γ∕T). Rate coefficients are listed in Table 1. |
| In the text | |
 |
Fig. A.2 Rms error as a function of total energy, showing the number of ab initio points used to evaluate the error in the PES calculation. Arrows indicate selected critical points in the PES and provide an estimate of the error in each region. TS means transition state. |
| In the text | |
 |
Fig. A.3 Reaction cross section (in bohr2) as a function of collision energy (in meV) for the SH+ (v = 0, j = 0) + H2 (v = 1, 2, 3, j = 0) and H2S+ (v = 0, j = 0) + H collisions. Filled symbols are obtained counting all trajectories leading to products, while open symbols correspond to the ZPE corrected ones. |
| In the text | |
 |
Fig. A.4 H–H, SH+ and R distances(in bohr) versus time (in ps), for a typical reactive trajectory for the SH+ (v = 0, j = 0) + H2 (v = 1, j = 0) collision at 10 meV. |
| In the text | |
 |
Fig. B.1 Calculated rate constants as a function of temperature for reaction S(3 P) + H2(v) → SH + H. Dotted curves are fits of the form k(T) = α (T/300)β exp(−γ∕T). Rate coefficients are listed in Table 1. |
| In the text | |
 |
Fig. B.2 Calculated rate constants as a function of temperature for reaction SH (v = 0) + H → S + H2. The best fitto the calculated rate requires two Arrhenius-like expressions (one for low temperatures and one for high temperatures). Rate coefficients of these fits are listed in Table 1. |
| In the text | |
 |
Fig. C.1 Photoionization and photodissociation cross sections. Top panel: σion(SH) (blue curve from laboratory experiments by Hrodmarsson et al. 2019). The pink curve is σdiss (SH) (Heays et al. 2017, and references therein). Bottom panel: σion(H2S) (blue curve) and σdiss(H2S) (gray and pink curves; from Zhou et al. 2020). |
| In the text | |
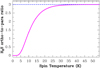 |
Fig. D.1 OTP ratio of H2S as a function of spin temperature (Eq. (D.1)). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


