| Issue |
A&A
Volume 693, January 2025
|
|
|---|---|---|
| Article Number | A20 | |
| Number of page(s) | 14 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/202451933 | |
| Published online | 23 December 2024 | |
A chemical link between saturated and unsaturated aldehydes and ketenes in the interstellar medium
Laboratory experiments on the H-atom reactions of propenal (CH2CHCHO) and propanal (CH3CH2CHO) at low temperatures
1
MTA-ELTE Lendület Laboratory Astrochemistry Research Group, Institute of Chemistry, ELTE Eötvös Loránd University,
PO Box 32,
1518
Budapest,
Hungary
2
Freie Universität Berlin, Institut für Chemie und Biochemie-Anorganische Chemie,
Fabeckstrasse 34/36,
14195
Berlin,
Germany
3
Laboratory of Molecular Spectroscopy, Institute of Chemistry, ELTE Eötvös Loránd University,
PO Box 32,
1518
Budapest,
Hungary
4
Hevesy György PhD School of Chemistry, Institute of Chemistry, ELTE Eötvös Loránd University,
PO Box 32,
1518
Budapest,
Hungary
5
National Synchrotron Radiation Laboratory, University of Science and Technology of China,
Hefei
230029,
PR China
6
Centre for Astrophysics and Space Science, ELTE Eötvös Loránd University,
PO Box 32,
1518
Budapest,
Hungary
★ Corresponding author; gyorgy.tarczay@ttk.elte.hu
Received:
20
August
2024
Accepted:
8
November
2024
Context. Propenal (CH2CHCHO) and propanal (CH3CH2CHO) have been detected in various regions of the interstellar medium (ISM), from star-forming regions to a comet’s dusty coma. These molecules attract considerable attention due to their structural similarity to aldose sugars and their potential role in prebiotic astrochemistry. Their reactions with H atoms may significantly contribute to the chemical diversity in the ISM and link these molecules with each other and other isomers.
Aims. In this study, we aimed to investigate the astrophysically relevant low-temperature reactions of propenal and propanal molecules with H atoms to explore possible reaction pathways between these molecules and their isomers.
Methods. Propenal and propanal were isolated in solid para-H2 at 3.1 K. This medium, with its weak interactions, provides spec-troscopic data close to gas-phase values and allows for studying highly reactive short-lived species. Additionally, H atoms can be conveniently generated, they diffuse, and they react with the isolated molecules. The reactions were monitored using infrared (IR) spectroscopy. Quantum-chemical computations were employed to determine possible reaction paths and aid in spectral assignments.
Results. The reaction of CH2CHCHO and CH3CH2CHO with H atoms in the first step results in the production of CH2CH •CO/•CH2CHCO, CH3CH2•CO, and CH3•CHCHO radicals. Further H-atom reactions of CH3•CHCHO and R•CO radicals lead to the formation of methylketene (CH3CHCO) as the product of both the reaction of propenal and propanal. The two-step addition of H atoms to CH2CHCHO was found tentatively to produce CH3CH2CHO.
Conclusions. The radicals observed in the experiments are likely produced in dark molecular clouds on icy grains, increasing interstellar chemical complexity. The experiments suggest that H-atom reactions with propanal and propenal are important channels for methylketene production. The observed reactions imply that consecutive H-atom addition and H-atom abstraction reactions of propenal and propanal can catalyze interstellar H2 formation.
Key words: astrochemistry / molecular processes / methods: laboratory: molecular / ISM: molecules
© The Authors 2024
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1 Introduction
In recent decades, aldehydes have attracted significant attention from astrochemists and astrobiologists due to their supposed key role in the interstellar synthesis of amino acids and their structural similarity to aldose sugars, often referred to as “sugars of space” (Hollis et al. 2000; Cleaves II 2003; Hollis et al. 2004; Bennett & Kaiser 2007; de Marcellus et al. 2015; Abplanalp et al. 2015; Singh et al. 2022; Field-Theodore & Taylor 2022). In this regard, propenal (CH2CHCHO) and propanal (CH3CH2CHO) appear especially interesting because both have been discovered in various regions of the interstellar medium (ISM; Dickens et al. 2001; Hollis et al. 2004; Requena-Torres et al. 2008; Lykke et al. 2017; Manigand et al. 2021; Agúndez et al. 2021; Agúndez et al. 2023; Fuentetaja et al. 2023; Hänni et al. 2023). In particular, these aldehydes were observed in spectral line surveys toward the massive star-forming regions in the Galactic center (Dickens et al. 2001; Hollis et al. 2004; Requena-Torres et al. 2008), the hot corino IRAS 16293-2422 B (Lykke et al. 2017; Manigand et al. 2021), the cold starless core TMC-1 (Agúndez et al. 2021; Agúndez et al. 2023; Fuentetaja et al. 2023), and comet 67P’s dusty coma (Hänni et al. 2023). This has stimulated research on the possible formation pathways of these species in the ISM and their subsequent astrochemical evolution (Abplanalp et al. 2015; Jonusas et al. 2017; Krim et al. 2018; Qasim et al. 2019; Singh et al. 2022). In addition, CH2 CHCHO and CH3CH2CHO are of particular interest in combustion chemistry because they are supposed to be significant intermediates in low-temperature combustion processes (Cavaliere et al. 1993) and important species in the development of sustainable fuels from biomass (Burluka et al. 2010). They also play roles in atmospheric chemistry (Grosjean & Williams II 1994; Le Crâne et al. 2005), physical chemistry (da Silva & Bozzelli 2006; Lewars et al. 2013), and organic chemistry (Clayden et al. 2012; Folliard et al. 2021).
In dark molecular clouds, atomic hydrogen plays a crucial role in chemical transformations through H-atom abstraction from molecules or by forming bonds with molecules. These reactions are usually exoergic and often occur without a significant energy barrier or with a barrier small enough to be crossed by hydrogen atom tunneling. The primary products of these reactions are radicals, which are highly reactive species. In the ISM, these radicals can further recombine, leading to the formation of larger complex organic molecules (i-COMs; Haupa et al. 2022). The participation of H atoms in both H-atom abstraction and addition reactions can establish a quasi-equilibrium between saturated and unsaturated species (for example, Haupa et al. 2022; Schneiker et al. 2022; Schneiker et al. 2024), or lead to the isomerization of molecules (for instance, Schneiker et al. 2022; Tsai et al. 2022). In the case of saturated aldehydes, H-atom mediated isomerization could result in the synthesis of enols (>C=CH–O– H), while for unsaturated aldehydes, it may also produce ketenes (>C=C=O). Thus, a particularly intriguing question for astrochemists is the chemistry that occurs during the reaction of CH2CHCHO and CH3CH2CHO with H atoms, potentially significantly diversifying the chemical composition of the specific region of the ISM where these reactions can take place (Jonusas et al. 2017; Krim et al. 2018; Qasim et al. 2019).
Fourier transform infrared (FTIR) studies on the H-atom reactions of propenal ices at 10 K have demonstrated its reduction to propanal, whereas solid propanal appears stable against H-atom bombardment at low temperatures, with no reduction products (alcohols) detected (Jonusas et al. 2017; Krim et al. 2018). In contrast, mass spectrometry of sublimed propanal ice that had been bombarded with H atoms at low temperatures showed the signals of 1-propanol (CH3CH2CH2OH; Qasim et al. 2019). Therefore, the reactions of H atoms with propenal and propanal are a curious issue that necessitates advanced methods for investigation.
Para-H2 matrix isolation is an effective method for studying the reactions of molecular species with H atoms at low temperatures (Tsuge et al. 2018; Tarczay et al. 2019; Haupa et al. 2022). This approach offers several advantages. Firstly, the interactions between this quantum host and isolated species are typically minimal, leaving the electronic and molecular structures of the guest molecules largely unperturbed. Consequently, one can observe sharp spectral bands of the isolated species, making spectral assignment relatively straightforward when compared to gas-phase values and results from quantum-chemical computations (Momose & Shida 1998; Yoshioka et al. 2006; Bahou et al. 2014; Tsuge et al. 2018; Tarczay et al. 2019; Tsuge & Lee 2020; Haupa et al. 2022). Secondly, this host medium allows for a rather straightforward and convenient generation of H atoms through a two-step photolysis process (Haupa et al. 2022; see Sect. 2.2). In contrast to other isolated species, H atoms can move quite efficiently in the solid para-H2 medium by quantum diffusion (Fushitani & Momose 2003; Ruzi & Anderson 2015; Balabanoff et al. 2018). These phenomena collectively contribute to the greater sensitivity of this method for detecting the products of H-atom reactions, compared to the conventional method of H-atom bombardment on astrophysical ice analogs. Although solid para-H2 is not a typical astrophysical environment, it can be assumed that if an H-atom reaction occurs in a solid para-H2 matrix, it may also occur under astrophysical conditions, such as the gas phase or in (more polar) solid environments and at higher temperatures. This makes matrix isolation in para-H2 a very useful pre-screening technique for studying H-atom reactions (Keresztes et al. 2023).
This technique has been previously applied to study H-atom reactions of astrochemically relevant molecular species, such as methanol (CH3OH; Haupa et al. 2017), formamide (H2NCHO; Haupa et al. 2019b), methyl formate (HC(O)OCH3; Haupa et al. 2019a), acetamide (CH3CONH2; Haupa et al. 2020), glycine (H2NCH2COOH; Schneiker et al. 2021), acetic acid (CH3 COOH; Joshi et al. 2021), N-methylformamide (HC(O)NH(CH3); Tsai et al. 2022), methylamine (CH3NH2; Joshi & Lee 2022), furan (c-C4H4O; Schneiker et al. 2022), fulminic acid (HCNO) and formaldoxime (H2CNOH; Keresztes et al. 2023), indene (C9H8) and indane (C9H10; Schneiker et al. 2024), ethanol (C2H5OH; Zasimov et al. 2024), different [H,C,N,S] isomers (Keresztes et al. 2024b), glyoxal (CHOCHO), glycoladehyde (HOCH2CHO), and ethylene glycol (HOCH2CH2OH; Keresztes et al. 2024a). Furthermore, acetaldehyde (CH3CHO) was also recently studied (Zasimov et al. 2024). It was found that its reactions with H atoms mainly lead to the formation of dehydrogenation products – acetyl radical (CH3•CO) and ketene (CH2CO).
In this work, we applied this approach to more complex, astrochemically important aldehydes, investigating the H-atom reactions with propenal and propanal in solid para-H2 at 3.1 K. Quantum-chemical computations were also performed to verify the experimental assignments and analyze the energetics and the feasibility of the proposed reactions. The objective of this combined experimental and theoretical research is to investigate the efficiency of H-atom reactions with CH2CHCHO and CH3CH2CHO molecules. Specifically, the study aims to identify the reaction products, particularly radicals; determine if both hydrogen addition and abstraction reactions occur; assess the directionality of these reactions; and understand their impact on the equilibrium between the investigated molecules. Ultimately, the goal is to comprehend how H-atom reactions contribute to establishing chemical connections between saturated and unsaturated aldehydes, their isomers, and hydrogenation products (such as enols, ketenes, and alcohols) under astrophysically relevant conditions.
2 Methods
2.1 Computational details
Quantum-chemical computations at the B3LYP/cc-pVTZ level of theory were performed by means of the Gaussian 16 (Rev. A.03) program package (Frisch et al. 2016). The unrestricted version of the method was used to compute the electronic structure of the open-shell species. The geometries corresponding to the minimum energy points and transition states were optimized, followed by the computation of harmonic and anhar- monic frequencies, IR intensities, and zero-point vibration energies (ZPVE) at these optimized structures. The harmonic ZPVEs were used for energy comparisons. Vibrational analysis at the optimized geometries verified the nature of points on the potential energy surface (PES). Anharmonic vibrational frequencies were computed using vibrational perturbation theory (VPT2; Barone 2005). Intrinsic reaction coordinate (IRC) scans were conducted to analyze the reaction pathways. Regarding the second reaction with an H atom, both triplet and singlet products of the radical–radical recombination reactions are expected, ideally in a statistical three-to-one ratio. Since the possible triplet products have a much higher energy, they rapidly relax to the singlet species both in para-H2 and in an astrophysical environment. Therefore, we computed the structure and energy of the singlet products only. Radical–radical recombination reactions are generally barrierless, with some exceptions (for example, steric hindrance), so we assumed that these reactions occur without a barrier on the singlet PES and did not check for barriers. Additionally, we did not analyze which conformation of the initial radical leads to a specific conformation of the product. We did not theoretically inspect the possibility of carbene and diradical production either because they are high-energy products, and we are unaware of any reports on the observation of these species in solid para-H2 matrices.
2.2 Experimental details
Propenal (CH2CHCHO, 90%, stabilized by hydroquinone (C6H4(OH)2), Sigma-Aldrich) and propanal (CH3CH2CHO, 97%, Sigma-Aldrich) were placed into a glass vial connected to the premixing vacuum line. Furthermore, these substances were additionally purified with five freeze-pump-thaw cycles before the experiment. The experiments were conducted in the ultrahigh-vacuum compatible stainless steel chamber of the Versatile ice zigzag sublimation setup for laboratory astrochemistry (VIZSLA) setup (Bazsó et al. 2021), which can be evacuated to a base pressure of a few × 10−9 mbar when cooled. The deposition was performed on a gold-plated silver wafer mounted on the cold finger of an RDK-415D2 cryostat (Sumitomo Heavy Industries Inc.), which allows the substrate to be cooled to 3.1 K. Para-H2 was prepared by flowing normal-H2 through porous Fe2O3 catalyst (Sigma-Aldrich, hydrated, catalyst grade, 30–50 mesh) at 13.9 K. The prepared para-H2 was collected in a 1 L glass bulb. The substance (CH2CHCHO or CH3CH2CHO) was mixed with the para-H2 collected in a 0.5 L flask on the vacuum line beforehand in two steps, with a homogenization time of approximately 45 min applied after each step, resulting in a final CH2CHCHO/para-H2 or CH3CH2CHO/para-H2 ratio of approximately 1 : 750–1 : 1100.
The matrix was prepared by co-depositing of CH2CHCHO/para-H2 or CH3CH2CHO/para-H2 gaseous mixture and Cl2 molecules onto the substrate. The mixture and the Cl2 were introduced by two separate stainless steel capillary arrays. The mixture inlet rate was regulated by a leak valve to keep the pressure inside the chamber at around 6 × 10−5 mbar while ensuring that the Cl2/para-H2 ratio was around 1 : 250– 1 : 400. For blank experiments, the Cl2 -free samples were investigated. After the matrix deposition, the purity of para-H2 was checked by recording the near-IR (NIR) spectrum of the non-irradiated matrix and measuring the absorption of the band belonging to ortho-H2 at 4740 cm−1 (Q1(0) + S0(1) transition) relative to that of para-H2 at 4855–4825 cm−1 (S1(0) + S0(0) transition).
The Cl2 molecules in the studied matrices were dissociated to produce Cl atoms upon exposing the sample to 365 nm ultraviolet (UV) photons. The total UV photolysis time was ca. 1 h. The IR source of the spectrometer was diverted during the 365 nm photolysis. The amount of Cl atoms was monitored by the absorption bands of atomic chlorine in the NIR region (5094.9 cm−1). The reaction of Cl atoms with para-H2 was initiated by exciting the Q1(1) + S0(0) and Q1(0) + S0(0) combinational vibrations of para-H2 molecules via irradiating the matrix at 2217 nm for ca. 90–100 min using the same laser setup as described above. The H-atom generation was monitored in situ by measuring the HCl absorption in the mid-IR (MIR) region (2894.5 cm−1). After finishing the NIR laser irradiation, the samples were kept in darkness overnight, and MIR spectra were collected every 30 min. During this time, H atoms can diffuse in the matrix and further react with the sample molecules. The second cycle of H-atom generation and/or a set of secondary photolysis (in order to affect the products of hydrogen atom reactions) was carried out on the next day. No significant consumption of CH2CHCHO or CH3CH2CHO molecules was observed as a result of the chlorine-free sample UV and NIR photolyses.
The sample analysis was performed by recording the MIR spectra of the matrix after each step and regularly during the night process. The FTIR spectra were recorded by a Bruker Inve- nio FTIR spectrometer working in reflection–absorption mode and using a liquid N2 -cooled mid-band mercury cadmium telluride (MCT) detector. For each NIR (9000–600 cm−1) and MIR (4000–600 cm−1) spectrum, 32–128 scans were collected with a resolution of 0.5 cm−1 (128 and 256 scans were collected in the case of the NIR and MIR background spectra, respectively). A long-wave pass (LPW) 3860 low-pass filter (with a cutoff wavelength of 3860 cm−1) was placed in front of the detector in the process of the spectral collection during the NIR photolysis of the sample.
The NIR irradiations were carried out by an optical parametric oscillator (OPO; GWU/Spectra-Physics VersaScan MB 240, FWHM ≈ 5 cm−1) equipped with a frequency-doubling unit (Spectra-Physics uvScan) pumped by a pulsed Nd:YAG laser (Spectra-Physics Quanta Ray Lab 150, λ = 355 nm, f = 10 Hz, pulse duration = 2–3 ns); the photon flux varied from (3 ± 1) × 1014 to (7.9 ± 0.2) × 1014 photons mm−2 s−1. For UV irradiation, the same Nd:YAG–OPO system was used – photon fluxes were between (3.0 ± 0.2) × 1013 and (5.5 ± 0.6) × 1013 for the wavelength of 400 nm, between (1.2 ± 0.1) × 1013 and (2.8 ± 0.6) × 1013 (365 nm), between (4.9 ± 0.1) × 1012 and (1.3 ± 0.3) × 1013 (330 nm), between (7.0 ± 0.6) × 1012 and (1.7 ± 0.3) × 1013 (295 nm), between (2.0 ± 0.1) × 1013 and (2.9 ± 0.3) × 1013 (260 nm) and between (5.7 ± 0.4) × 1012 and (1.5 ± 0.2) × 1013 photons mm−2 s−1 (225 nm). For the 365 nm irradiation, an M365L3 light-emitting diode (LED) was used (Thorlabs, λmax = 365 nm, photon flux measured at a distance of 200 mm from the LED at I = 0.33 A is 8.8 × 1012 photons mm−2 s−1, respectively).
3 Results
3.1 Computational results
3.1.1 Reactions of H atoms with CH2CHCHO
The computational results at the B3LYP/cc-pVTZ level reveal two structures of CH2CHCHO: trans-CH2CHCHO (E-CH2CHCHO) and cis-CH2CHCHO (Z-CH2CHCHO). The trans-CH2CHCHO is thermodynamically more stable by 8.8 kJ mol−1, which is in agreement with the previously published theoretical data (Bokareva et al. 2009; Field-Theodore & Taylor 2022) and experimental value of 6.8 ± 0.7 kJ mol−1 (Blom et al. 1980). The reaction of H atoms with CH2CHCHO molecules in principle may result in the abstraction of an H atom from one of four possible positions (Reactions (1)–(4); XH2 + H• → XH• + H2 process in Fig. 1):
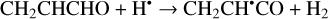 (1)
(1)
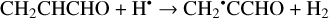 (2)
(2)
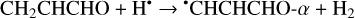 (3)
(3)
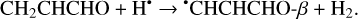 (4)
(4)
The enthalpies 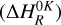 of the corresponding reactions for trans-CH2CHCHO (cis-CH2CHCHO) molecules computed at the (U)B3LYP/cc-pVTZ//(U)B3LYP/cc- pVTZ+ZPVE((U)B3LYP/cc-pVTZ) level are −71.8 (−92.9), 16.5 (−18.3), 18.7 (18.8), 18.1 (23.4) kJ mol−1, while those of the barriers
of the corresponding reactions for trans-CH2CHCHO (cis-CH2CHCHO) molecules computed at the (U)B3LYP/cc-pVTZ//(U)B3LYP/cc- pVTZ+ZPVE((U)B3LYP/cc-pVTZ) level are −71.8 (−92.9), 16.5 (−18.3), 18.7 (18.8), 18.1 (23.4) kJ mol−1, while those of the barriers 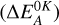 are −2.3 (−3.0), 42.6 (39.0), 46.3 (46.1), 46.9 (50.7) kJ mol−1 respectively (Fig. 1). The “negative” barrier for the trans-CH2CH•CO (•CH2CHCO) formation appears after taking into account the ZPVE energy in the harmonic approximation and it implies that the barrier for Reaction (1), if it exists, may be expected to be very low (see, for example, Zasimov et al. 2022). It is interesting to notice that the CH2CH•CO radical with the CCO-bend fragment (trans-CH2CH•CO) appears as the result of the reaction of H atoms with trans-CH2CHCHO while that of with the CCO-linear fragment (•CH2CHCO) is formed in the case of cis-one (Fig. A1). At the same time, we failed to find the CCO-bend radical resulting from the cis-CH2CHCHO (it is predicted to be the thermodynamically least stable radical of this type; Das & Lee 2013). The CCO-linear radical is thermodynamically more stable than trans-CH2CH•CO one by 12.3 kJ mol−1 which agrees well with the literature data (Das & Lee 2013). Most probably the stabilization is achieved due to the more efficient CH2=CH–•C=O ↔ •CH2–CH=C=O resonance. A similar situation was found for the CH2•CCHO radical: a CCC-bend structure for the trans-CH2CHCHO and a CCC-linear structure for the cis-CH2CHCHO (Fig. A1) with the latter one being ca. 26.1 kJ mol−1 more stable (most probably owing to the more efficient CH2=•C–CH=O ↔ CH2=C=CH–O• resonance). The abstraction of an H atom from the CH2 group (Reactions (3) and (4)) is both kinetically unfavorable and endothermic. Therefore, these reactions most likely do not proceed in astrophysical conditions at low temperatures.
are −2.3 (−3.0), 42.6 (39.0), 46.3 (46.1), 46.9 (50.7) kJ mol−1 respectively (Fig. 1). The “negative” barrier for the trans-CH2CH•CO (•CH2CHCO) formation appears after taking into account the ZPVE energy in the harmonic approximation and it implies that the barrier for Reaction (1), if it exists, may be expected to be very low (see, for example, Zasimov et al. 2022). It is interesting to notice that the CH2CH•CO radical with the CCO-bend fragment (trans-CH2CH•CO) appears as the result of the reaction of H atoms with trans-CH2CHCHO while that of with the CCO-linear fragment (•CH2CHCO) is formed in the case of cis-one (Fig. A1). At the same time, we failed to find the CCO-bend radical resulting from the cis-CH2CHCHO (it is predicted to be the thermodynamically least stable radical of this type; Das & Lee 2013). The CCO-linear radical is thermodynamically more stable than trans-CH2CH•CO one by 12.3 kJ mol−1 which agrees well with the literature data (Das & Lee 2013). Most probably the stabilization is achieved due to the more efficient CH2=CH–•C=O ↔ •CH2–CH=C=O resonance. A similar situation was found for the CH2•CCHO radical: a CCC-bend structure for the trans-CH2CHCHO and a CCC-linear structure for the cis-CH2CHCHO (Fig. A1) with the latter one being ca. 26.1 kJ mol−1 more stable (most probably owing to the more efficient CH2=•C–CH=O ↔ CH2=C=CH–O• resonance). The abstraction of an H atom from the CH2 group (Reactions (3) and (4)) is both kinetically unfavorable and endothermic. Therefore, these reactions most likely do not proceed in astrophysical conditions at low temperatures.
Furthermore, the abstraction of an H atom from these radicals may result in the formation of propadienone (H2CCCO), propynal (HCCCHO), and cyclopropenone (c-C3H2O)molecules (Reactions (5)–(10); XH• +H• → X+H2 process in Fig. 1), which are found to be thermodynamically favorable with the enthalpy diminishment lying within the range of 139.8–302.0 kJ mol−1 (Fig. 1).
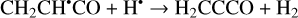 (5)
(5)
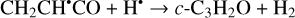 (6)
(6)
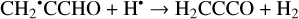 (7)
(7)
 (8)
(8)
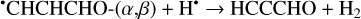 (9)
(9)
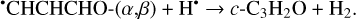 (10)
(10)
Besides abstraction, an H atom can also form a bond with a CH2CHCHO molecule, resulting in the formation of several types of hydrogen-addition radicals (Reactions (11)–(14); XH2 + H• → XH3• process in Fig. 1)1:
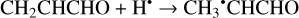 (11)
(11)
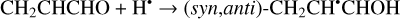 (12)
(12)
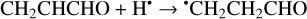 (13)
(13)
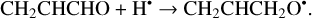 (14)
(14)
The enthalpies for the formation of the CH3•CHCHO, •CH2CH2CHO, and CH2CHCH2O• radicals for trans- CH2CHCHO (cis-CH2CHCHO) molecules are −186.0 (−197.2), −129.4 (−141.2), −60.9 (−72.2) kJ mol−1, while those of the barriers are 3.3 (2.2), 12.7 (8.2), 21.1 (15.8) kJ mol−1 respectively. As related to the syn-CH2CH•CHOH and anti-CH2CH•CHOH species, the enthalpies of their formation in the case of trans- CH2CHCHO (cis-CH2CHCHO) are −161.5 (−173.8) and −159.7 (−169.9) kJ mol−1, accordingly (Fig. 1). At the same time, it seems that only the anti-trans-CH2 CH• CHOH and syn-cis- CH2 CH• CHOH can be formed directly as a result of a hydrogen atom reaction with trans- and cis-CH2CHCHO molecules, respectively (the barriers of the corresponding reactions are 18.3 and 14.6 kJ mol−1) while the two other radical conformers are most likely produced via the intermediate formation of anti-trans-CH2CH•CHOH and syn-cis-CH2CH•CHOH species (we believe that the energy of the CH2CHCHO + H• systems is generally higher than the anti- ↔ syn- interconversion barrier). It is interesting to note that the barriers for the formation of cis-species are generally slightly smaller than those for transspecies, while the enthalpies are slightly larger. This observation may be explained by the smaller steric repulsion in the case of the cis-species. Concluding this paragraph, one may point out that the thermodynamically and kinetically most favorable H-atom addition channel is the formation of the CH3•CHCHO radical.
The second step of an H addition to these radicals may result in the formation of the propanal (CH3CH2CHO), propenol enol (CH3CHCHOH), and allyl alcohol (CH2CHCH2OH) molecules (Reactions (15)–(21); XH3 + H• → XH4 process in Fig. 1). All of these reactions are thermodynamically favorable with the enthalpy diminishment lying within the range of 279.5– 480.1 kJ mol−1 (Fig. 1).
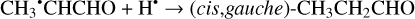 (15)
(15)
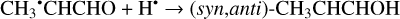 (16)
(16)
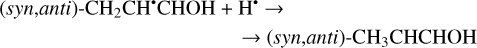 (17)
(17)
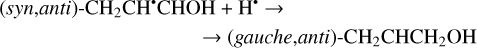 (18)
(18)
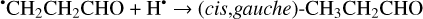 (19)
(19)
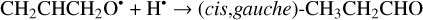 (20)
(20)
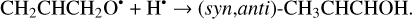 (21)
(21)
Another possibility of the further chemical evolution of the radicals formally may be considered as an H-atom mediated isomerization of CH2CHCHO molecules (Reactions (22)–(27); XH• + H• → XH2 and XH3• + H• → XH2 + H2 processes in Fig. 1). The products of such isomerization are methylketene (CH3CHCO), cyclopropanone (c-CH2CH2CO), and hydroxypropadiene (syn-CH2CCHOH, and anti-CH2CCHOH). All of these reactions are thermodynamically favorable with the enthalpy diminishment lying within the range of 163.6–369.5 kJ mol−1 (Fig. 1). It is worth noting that according to our computations, only methylketene is more thermodynamically stable than trans-CH2CHCHO (−6.5 kJ mol−1) while the energies of c-CH2CH2CO, syn-CH2CCHOH, and anti-CH2CCHOH are 86.6, 98.1 and 106.2 kJ mol−1 higher than that of trans- CH2CHCHO (one should notice that the computations at the CCSD(T)/cc-pVTZ level show (Field-Theodore & Taylor 2022) similar energy differences for the c-CH2CH2CO, syn- CH2CCHOH, and anti-CH2CCHOH species but predict methylketene being 1.9 kJ mol−1 higher in energy than trans- CH2CHCHO). Thus, it should be stressed that in the case of the H-atom mediated isomerization of CH2CHCHO the energy release is caused by a (formal) recombination of H atoms to H2 occurring as a result of the H-atom addition and H-atom abstraction (and vice versa) cycle (Fig. 1; the (U)B3LYP/cc- pVTZ//(U)B3LYP/cc-pVTZ+ZPVE((U)B3LYP/cc-pVTZ) enthalpy of the 2H• → H2 reaction is −434.8 kJ mol−1; Tsai et al. 2022).
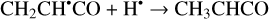 (22)
(22)
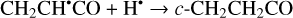 (23)
(23)
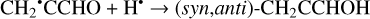 (24)
(24)
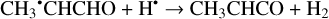 (25)
(25)
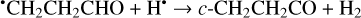 (26)
(26)
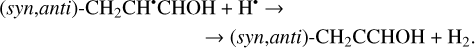 (27)
(27)
 |
Fig. 1 Potential energy surface of the reactions of H-atom abstraction (e) and addition (a) with trans- and cis-CH2CHCHO molecules. The energies are provided in kJ mol−1 relative to the trans-CH2CHCHO (+2H•) species. The radical–radical reaction pathways hypothesized theoretically and generally expected, without computational verification, are indicated with dashed lines. |
3.1.2 Reactions of H atoms with CH3CH2CHO
As related to the CH3CH2CHO molecule, the conformational analysis revealed two conformers, cis-CH3CH2CHO and gauche- CH3CH2CHO, respectively, with the former one being more thermodynamically stable by 4.5 kJ mol−1 which is in a very good agreement with previously published theoretical data (Yan et al. 2023). The reaction of hydrogen atoms with CH3CH2CHO molecules may lead to the H-atom abstraction from one of three possible positions (Reactions (28)–(30); XH2 + H• → XH• + H2 process in Fig. 2):
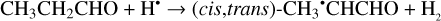 (28)
(28)
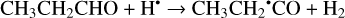 (29)
(29)
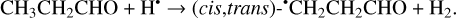 (30)
(30)
The enthalpies of the corresponding reactions for cis- CH3CH2CHO (gauche-CH3CH2CHO) molecules are −82.3 (−84.4), −76.2 (−79.4), −26.2 (−27.4) kJ mol−1, while those of the barriers are 8.7 (7.7), −3.4 (−2.9), and 25.1 (25.1) kJ mol−1, respectively (Fig. 2). The H-atom abstraction from the CHO fragment represents the most kinetically favorable pathway, computed with a “negative” barrier attributed to approximations and numerical uncertainties in the computations, similar to that observed with CH2CHCHO molecules. In contrast, not the CH3CH2•CO radical is the thermodynamically most stable H-atom abstraction product but rather the CH3• CHCHO radical (Fig. A2). The resonance CH3–•CH–CH=O ↔ CH3– CH=CH–O• may contribute significantly to the stabilization of this radical. At the same time, the abstraction of an H atom from the terminal methyl group (Reaction (30)) is kinetically and thermodynamically the least favored as in the case of CH2CHCHO molecules.
Furthermore, the abstraction of an H atom from the radicals mentioned above may result in the formation of CH3CHCO, CH2CHCHO, and c-CH2CH2CO molecules (Reactions (31)–(36); XH• + H• → X + H2 process in Fig. 2). All of these reactions were computed to be thermodynamically favorable with the enthalpy diminishment lying within the range of 165.5– 305.1 kJ mol−1 (Fig. 2).
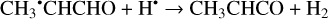 (31)
(31)
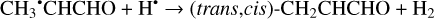 (32)
(32)
 (33)
(33)
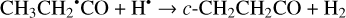 (34)
(34)
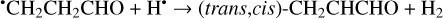 (35)
(35)
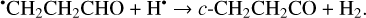 (36)
(36)
The addition of an H atom to a CH3CH2CHO molecule may occur either to the aldehyde carbon or oxygen atom (Reactions (37)–(38); XH2 + H• → XH3• process in Fig. 2).
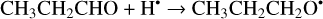 (37)
(37)
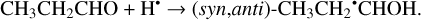 (38)
(38)
The enthalpies for the formation of the CH3CH2CH2O• radicals from cis-CH3CH2CHO (gauche-CH3CH2CHO) molecules are −73.0 (−73.6) kJ mol−1 while those of the barriers are 20.2 (18.4) kJ mol−1, respectively. As related to the syn-CH3CH2•CHOH and anti-CH3CH2•CHOH species, the enthalpies of their formation in the case of cis-CH3CH2CHO (gauche-CH3CH2CHO) are −101.8 (−108.0) and –103.6 (−108.9) kJ mol−1, respectively (Fig. 2). It is worth noting that it seems that only the syn-cis-CH3CH2•CHOH and syn-gauche- CH3CH2•CHOH can be formed directly as a result of an H-atom reaction with cis- and gauche-CH3CH2CHO molecules, respectively (the barriers of the corresponding reactions we found to be are 27.3 and 24.8 kJ mol−1) while the two other radical conformers are probably formed via the intermediate production of syn-cis-CH3CH2•CHOH and syn-gauche-CH3CH2•CHOH radicals. Therefore the CH3CH2•CHOH radicals, despite being the most thermodynamically stable product of the reaction with one H atom, are virtually the least kinetically favored products (Fig. A2).
The second step of the H-atom addition to these radicals may result in the formation of the different (five: anti-gauche, gauche- gauche-1, gauche-anti, anti-anti, and gauche-gauche-2; ag, gg-1, ga, aa, and gg-2; Troya 2019) conformers of 1-propanol (Reactions (39)–(40); XH3 + H• → XH4 process in Fig. 2). Both of the reactions are thermodynamically favorable with the enthalpy diminishment lying within the range of 375.2–407.2 kJ mol−1 (Fig. 2).
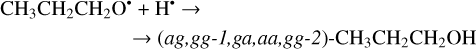 (39)
(39)
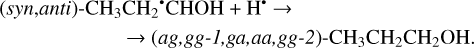 (40)
(40)
And, finally, the H-atom mediated isomerization of the CH3CH2CHO molecules may occur and lead to the formation of the enol (CH3CHCHOH) species which has four possible conformers (syn-trans, syn-cis, anti-trans, anti-cis). It is represented by Reactions (41)–(42) (XH• + H• → XH2 and XH3• + H• → XH2 + H2 processes in Fig. 2). Both of the reactions are thermodynamically favorable with the enthalpy diminishment lying within the range of 287.4–316.2 kJ mol−1 (Fig. 2).
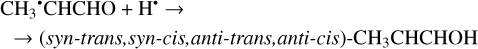 (41)
(41)
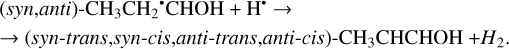 (42)
(42)
The Cartesian coordinates, energies, and vibrational frequencies of CH2CHCHO and CH3CH2CHO as well as their reaction products computed at the (U)B3LYP/cc- pVTZ//(U)B3LYP/cc-pVTZ level are provided in Figs. A1 and A2 and Tables A1–A4. These theoretical data are discussed further in Sects. 3.2.1, 3.2.2, and 4.
 |
Fig. 2 Potential energy surface of the reactions of H-atom abstraction (e) and addition (a) with cis- and gauche-CH3CH2CHO molecules. The energies are provided in kJ mol−1 relative to the cis-CH3CH2CHO (+2H•) species. The radical–radical reaction pathways hypothesized theoretically and generally expected, without computational verification, are indicated with dashed lines. |
3.2 Experimental results
3.2.1 Reactions of H atoms with CH2CHCHO
A number of rather strong absorption bands were observed in the FTIR spectra (Fig. B1) of the non-irradiated CH2CHCHO/para- H2 and CH2CHCHO/Cl2/para-H2 matrices (Table C1). These features were attributed to the trans-CH2CHCHO and cis- CH2 CHCHO molecules isolated in solid para-H2 based on the results of the quantum-chemical computations (Bokareva et al. 2009 and Table A2) matrix isolation data (Blom et al. 1980), and gas phase values (Osborne & Ramsay 1973; Hamada et al. 1985). Rough integrated intensity analysis of the CH2, scis mode of CH2CHCHO taking into account the anharmonic IR absorption coefficients computed at the B3LYP/cc-pVTZ level (Table A2) indicates that the samples contain predominantly the trans-conformer (Fig. B1): proportion of the cis-conformer is at ca. 3–5% level. The minor signals of H2O and CO2 (Tam & Fajardo 2000; Fajardo & Lindsay 2008) were also found in the FTIR spectra of the non-irradiated matrices. Additional absorption bands in the CH2CHCHO/Cl2/para-H2 as compared to CH2CHCHO/para-H2 likely may be tentatively attributed to the CH2CHCHO ⋯ Cl2 associates (Table C1).
Photolysis of the CH2CHCHO/para-H2 and CH2 CHCHO/Cl2 /para-H2 samples with the 365 nm light results in the interconversion of the trans-CH2CHCHO molecules to the cis-ones (Fig. B1). The observed photoinduced interconversion process (Blom et al. 1980; Johnstone & Sodeau 1992; and a reverse interconversion under the 225 nm light irradiation, Fig. B1) helped us distinguish between the absorption bands of the trans- and cis-conformers Table (C1) in addition to the literature data (Osborne & Ramsay 1973; Blom et al. 1980; Hamada et al. 1985; Bokareva et al. 2009). The proportion of the cis-CH2CHCHO increased approximately up to 5–9% level after an hour of the UV photolysis. In addition to the conformational conversion, the UV photolysis also leads to the dissociation of chlorine molecules into chlorine atoms in the samples doped with Cl2, which was evidenced by the appearance of the absorption bands of Cl atoms (5094.9 cm−1; Raston et al. 2015), chlorine superoxide (ClOO•, 1442.2 cm−1; Jacox 1998, which is the reaction product of Cl atoms with O2 impurity), and hydrogen chloride (HCl, 2894.5 cm−1 and a number of satellites nearby; Raston & Anderson 2006; Raston et al. 2015). Weak features of •CH2CHCO, trans-CH2CH•CO, and carbon monoxide (CO) were also observed after the UV photolysis which may be explained by the formation of a small amount of H atoms already during the 365 nm photolysis because these bands were not found in the chlorine-free sample after photolysis.
Subsequent 2217 nm irradiation of the CH2 CHCHO/Cl2 /para-H2 samples results in the generation of H atoms via the photoinduced reaction of H2 molecules with Cl atoms, which is followed by the tunneling diffusion and reactions of H atoms with the molecules trapped in solid para- H2. One of the main products of the reaction of an H atom with a CH2CHCHO molecule was found to be the •CH2CHCO radical (Das & Lee 2013). Its six vibrational modes were observed in the FTIR spectra of the photolyzed samples (Table 1 and Fig. 3). The features with the maxima at 1829.9 and 1828.8 cm−1 were assigned to the trans-CH2CH•CO radical based on the similarity of these values with that of observed in FTIR spectra of these species isolated in an Ar matrix (Baskir & Nefedov 1996). In addition, we detected the absorption bands with the maxima at 2129.3 and 2126.3 cm−1, which were assigned to the COstr mode of the methylketene based on the literature data (Johnstone & Sodeau 1992; Winter et al. 1998; Das & Lee 2013). The absorption feature with the maximum at 1749.1 cm−1 observed in the FTIR spectra was assigned to CH3 CH2 CHO (one with the maximum at 1756.1 cm−1 was assigned to CH3CH2CHO only tentatively; Fig. 3 and Fig. B2; Guirgis et al. 1998; Köroğlu et al. 2015; Yan et al. 2023). Weak signals of CO (2153.7, 2146.0 with the shoulder at 2147.7, 2145.2, 2142.8 with the shoulder at 2143.8, 2141.2 with the shoulder at 2140.5, 2139.4, 2137.6 with the shoulder at 2136.9 cm−1; Tam & Fajardo 2001; Das & Lee 2013) and hydroperoxy radical (HO2•, 3416.8, 1392.4, and 1100.2 cm−1; Thompson & Jacox 1989) molecules were also found.
In addition to these features, a number of absorption bands were assigned only tentatively based on the results of our computational studies and literature data (Table 1). The signal with the maxima at 1543.2, 1457.3, 1401.0, and 1369.6 cm−1 (Fig. 3) may belong to the modes of trans-CH3•CHCHO radical based on our computational data. As related to the COstr mode (1543.2 cm−1) it is worth noting that the literature gas-phase value for the cis- CH3•CHCHO radical (1578 cm−1; Yacovitch et al. 2011) is rather close to that of in para-H2 taking into the account the possible errors in the gas-phase value determinations (see the note under Table 2 and the Sect. 4). Furthermore, we also observed the absorption features similar to that of tentatively assigned to the COstr mode in the case of CH3CH2CHO (Fig. B3). The signal with the maximum at 1769.1 cm−1 was tentatively ascribed to the trans-•CH2CH2CHO species also based on the detection of a similar absorption band in the CH3CH2CHO/Cl2/para-H2 samples after 365 nm + 2217 nm photolysis (Fig. B3). At the same time, we tentatively attribute the absorption feature with the maximum at 3619.6 cm−1 to the OHstr mode of the syn- trans-CH2CH•CHOH radicals because it is quite similar to the theoretical value of 3601.9 cm−1 (Table 1). Keeping the photolyzed CH2CHCHO/Cl2/para-H2 samples overnight generally results in a minor growth of the above-mentioned absorption features caused by the continuing reactions of hydrogen atoms (Fig. 3).
Besides the photoinduced trans- to cis-CH2CHCHO interconversion, the most prominent changes observed in the FTIR spectra of the CH2CHCHO/Cl2/para-H2 matrices recorded after the UV photolysis (λ = 365 nm) performed on the next day, as a part of the second H-atom generation cycle, are related to the photodecomposition of the •CH2CHCO radical (Das & Lee 2013; Fig. 3). It was found that more than 95% of the radical decomposes as a result of the 365 nm photolysis for 70 min. The photodecomposition implies the C–C bond cleavage with the formation of CO and C2H3• fragments. The latter one readily reacts with the surrounding para-H2 with the formation of the ethyl radical (C2H5•) species (Wu et al. 2004). Indeed, the prominent growth of the CO absorption features (Tam & Fajardo 2001; Das & Lee 2013; Fig. 3) and the formation of the ethyl radicals (3124.9, 3034.5, 3032.5, and 2984.9 cm−1; Wu et al. 2004) were detected.
During the further photolysis of the samples with the 2217 nm irradiation (Fig. 3), we registered the growth of the signals of the CH2CHCHO H-atom abstraction and H-atom addition products mentioned above. In addition, we observed the appearance of the absorption features of the formyl radical (H•CO) (2449.1 and 1864.5 cm−1; Ruzi & Anderson 2012) and ethane (C2H6, 2981.5 and 2980.5 cm−1; Sogoshi et al. 1997) species, which are formed as a result of the H-atom addition to the CO and C2H5•, respectively.
A set of secondary photolyses (λ = 400, 365, 330, 295, 260, and 225 nm) was performed after the second H-atom generation cycle in order to verify the assignments and study the photochemical behavior of the system under consideration (Fig. 3). First, we did not observe the efficient trans- to cis-CH2CHCHO interconversion under the λ = 400, 365, 330, 295, and 260 nm laser irradiation (most likely because the photon fluxes at the sample point and irradiation times were generally smaller as that of the λ = 365 nm UV LED). More interestingly, the reverse (cis- to trans-CH2CHCHO) interconversion taking place under the 225 nm irradiation was found (Fig. B1). Second, the photoinduced decomposition of the •CH2CHCO radical as a result of the λ = 295, 260, and 225 nm photolyses was observed (the growth of the absorption features at the laser photolysis with larger wavelength most probably may be explained by the ongoing reactions of H atoms which restore this radical faster than it photodecomposes; Fig. 3). It is worth noting that its efficiency noticeably increases when the photolysis is performed with the λ = 260 or 225 nm irradiation. The decomposition of the trans-CH2CH•CO radical in this wavelength region was also observed. In addition to the noticeable growth of the CO and C2H5• absorption features under the 225 nm photolysis, the formation of the traces of the C2H4 (1440.0 and 948.5 cm−1; Das & Lee 2013) and C2H2 (3273.4 and 733.7 cm−1; Das & Lee 2013) species were also registered; we suppose that their formation is most likely related to the •CH2CHCO (trans-CH2CH•CO) radicals photodecomposition. Among the other important spectra changes, we would like to emphasize that the diminishment of the absorption features attributed to the trans-CH3•CHCHO radicals and increase of the CH3CHCO signals under the λ = 260 or 225 nm photolysis were detected as well (Fig. 3). The efficiency of the CH3CHCO photoinduced formation was found to be sufficiently greater in the Cl2-doped samples which most probably implies that the reaction trans-CH3•CHCHO ⇝ CH3CHCO + H• may be the main source of the methylketene production under the λ = 260 or 225 nm irradiation of the CH2CHCHO/Cl2/para-H2 matrices. We also suppose that the photodecomposition of the trans-CH3•CHCHO radicals may be a source of CO in the investigated systems, which may explain the greater CO absorption band growth after the 225 nm photolysis than after the 260 nm one (Fig. 3).
 |
Fig. 3 Difference FTIR spectra of the CH2CHCHO/Cl2/para-H2 sample illustrating the effect of the sequential 365 nm (60 min) + 2217 nm (100 min) irradiation (a), free sample standing after photolysis for 480 min (b), 365 nm (70 min) photolysis (c), 2217 nm (100 min) photolysis (d), 400 nm (20 min) photolysis (e), 330 nm (20 min) photolysis (f), 260 nm (20 min) photolysis (g), and 225 nm (20 min) photolysis (h). We note that the center-left panel is multiplied by 0.3 for a better representation. The absorption features attributed to the trans-CH2CHCHO and cis-CH3CH2CHO are marked with “a” and “p”, respectively. The unassigned absorption features are labeled with question signs. |
3.2.2 Reactions of H atoms with CH3CH2CHO
Several relatively strong absorption features were detected in the FTIR spectra of the non-irradiated CH3CH2CHO/para-H2 and CH3CH2CHO/Cl2/para-H2 matrices (Table C1). These bands were assigned to the cis-CH3CH2CHO and gauche-CH3CH2CHO species isolated in solid para-H2 on the basis of the values obtained by means of quantum-chemical computations (Yan et al. 2023 and Table A4), matrix isolation data (Yan et al. 2023), and gas-phase data (Guirgis et al. 1998; Köroğlu et al. 2015). Rough integrated intensity analysis of the COstr mode of CH3CH2CHO taking into account the anharmonic IR absorption coefficients computed at the B3LYP/cc-pVTZ level (Table A4) indicates that the samples contain in large part cis-conformer (Fig. B2): proportion of the gauche-conformer is at ca. 30–40% level which is quite close to the theoretical value of 32.1% (at T = 298.15 K). The traces of H2O and CO2 (Tam & Fajardo 2000; Fajardo & Lindsay 2008) were also registered in the FTIR spectra of the non-irradiated samples. Additional minor features in the CH3CH2CHO/Cl2/para-H2 as compared to CH3CH2CHO/para-H2 likely may be tentatively attributed to the CH3CH2CHO ⋯ Cl2 associates (Table C1).
In the FTIR spectra of the CH3CH2CHO/Cl2/para-H2 matrices after the UV photolysis (λ = 365 nm) one may find the absorption features of Cl atoms (Raston et al. 2015), ClOO• (Jacox 1998), and HCl (Raston & Anderson 2006; Raston et al. 2015), which evidences the dissociation of Cl2 molecules under the irradiation. Weak features of CH3CHCO and CO were also observed after the UV photolysis. The non-observation of these bands in the chlorine-free samples indicates that the formation of a small amount of H atoms already during the 365 nm photolysis and their subsequent reactions are most likely responsible for the production of the considered species. At the same time, no prominent spectral changes except a kind of CH3CH2CHO absorption band “relaxation” were found in the FTIR spectra of the CH3CH2CHO/para-H2 matrices recorded after photolysis (Fig. B2).
A set of novel spectral features was observed in the FTIR spectra recorded after the 2217 nm photolysis of the CH3CH2CHO/Cl2/para-H2 samples (Fig. 4 and Table 2). In addition to the features of CH3CHCO and CO, we observed the absorption bands with the maxima at 1849.2 and 1864.1 cm−1 which, on the basis of our theoretical results, were assigned to the cis- and gauche-CH3CH2•CO radicals, respectively. We also tentatively ascribed the 1543.4 and 1548.1 cm−1 features to the trans- and cis-CH3•CHCHO radicals and 1784.7, 1781.3 cm−1 ones to the cis-•CH2CH2CHO species based on the similarity of these absorption bands with those one observed in the case of CH2CHCHO (Fig. B3). At the same time, the absorption band with the maximum at 3612.5 cm−1 was tentatively attributed to the syn-cis-CH3CH2•CHOH species. The overnight standing of the photolyzed CH3CH2CHO/Cl2/para-H2 samples, when H atoms can diffuse and react, generally leads to an incense of the absorption bands mentioned above related to the ongoing reactions of H atoms.
The UV photolysis (λ = 365 nm) of the CH3CH2CHO/Cl2/para-H2 matrices performed on the next day, as a part of the second H-atom generation cycle, results in the growth of the absorption features attributed to the CO, CH3CHCO, cis-CH3CH2•CO, gauche-CH3CH2•CO, cis-CH3•CHCHO, and trans-CH3•CHCHO species. The subsequent 2217 nm photolysis results in the further growth of the CO, CH3CHCO, cis-CH3CH2•CO, gauche-CH3CH2•CO, cis-•CH2CH2CHO, and cis-CH3•CHCHO molecule absorption bands as well as a growth of the syn-cis-CH3CH2•CHOH ones (Fig. 4).
The subsequent photolyses with the wavelengths 400, 365, 330, and 295 nm were found to have virtually no effect on the 365 nm + 2217 nm photolyzed CH3CH2CHO/Cl2/para-H2 samples. (A relatively small growth of the cis-CH3CH2•CO, gauche-CH3CH2•CO, and cis-CH3•CHCHO radical absorption features observed in Fig. 4 is most likely related to the ongoing reactions of H atoms). At the same time, photolysis at 225 nm resulted in the growth of the CH3CHCO and CO features and the decay of the signals attributed to the cis-CH3CH2•CO, gauche-CH3CH2•CO, cis-CH3•CHCHO, cis-CH3•CHCHO, trans-CH3•CHCHO, and syn-cis-CH3CH2•CHOH species and appearance of the C2H5• (Wu et al. 2004) absorption bands. (A similar effect but to a lesser extent was observed during the 260 nm photolysis as well; Fig. 4). Most likely these changes are related to the occurrence of the (cis,gauche)-CH3CH2·•CO ⇝ C2H5·• + CO and cis-CH3·•CHCHO ⇝ CH3CHCO + H• photoinduced reactions. The photodecomposition of the syn-cis-CH3CH2•CHOH radicals may result in the formation of CH3CH2CHO. However, it is difficult to ascertain if the absorption bands of CH3CH2CHO increase during photolysis due to this reaction, given the overlapping effect of the “relaxation” of the CH3CH2CHO absorption features. This relaxation may be related to the media’s relaxation, where isolated molecules occupy thermodynamically more favorable positions in the matrix (see Fig. B2). It is worth noting that virtually no changes except a kind of “relaxation” of CH3CH2CHO absorption features occur as a result of the λ = 260 and 225 nm photolysis of the chlorine-free samples (Fig. B2).
4 Discussion
Our theoretical results predict that the kinetically most favored pathway of the H-atom reaction both with CH2 CHCHO and CH3CH2CHO is the H-atom abstraction from the aldehyde (CHO) fragment (Figs. 1 and 2) which should either have no barrier or with a very small barrier (Reactions (1) and (29)). In both cases, this abstraction reaction is also highly favored thermodynamically. In agreement with the theoretical results, the products of this type of H-atom abstraction reaction were found to be formed in solid para-H2 matrices (Figs. 3 and 4): both for CH2CHCHO and CH3CH2CHO several absorption features were found in the region of 1820–1870 cm−1 (Tables 1 and 2).
It is worth noting that such vibrational frequencies are quite typical for the (usually rather) intensive COstr mode of the R•CO radicals (see, for instance, Baskir & Nefedov 1996; Ruzi & Anderson 2012; Das & Lee 2014) and therefore were assigned to trans-CH2CH•CO and (cis,gauche)-CH3CH2•CO radicals, respectively. Based on our theoretical data (Table 2) the high- and low-frequency (1864.1 and 1849.2 cm−1) absorption bands appeared in the FTIR spectra after H-atom generation (that is, in the spectra of the photolyzed CH3CH2CHO/Cl2/para-H2 samples) were assigned to the gauche- and cis-conformers of the CH3CH2•CO radicals, respectively. The gauche to (gauche+cis) ratio of the absorption intensities of these IR features normalized by the corresponding absorption coefficients (Table 2) also falls in the region of 30–45% and is close to the corresponding ratio for the CH3 CH2 CHO conformers in the non-photolyzed samples (see Sect. 2.2), which supports the assignment. In addition to a rather good agreement with the theoretical data (Tables 1 and 2) the observed photochemical transformations also support the assignment of the CH2CH•CO and CH3CH2•CO species (Tables 1 and 2 and Figs. 3 and 4). It was found that the UV photolysis results in the formation of CO and C2H3• (C2H5•) fragments, along with the decrease in the features attributed to the CH2CH•CO (CH3CH2•CO) radicals. This decrease most probably indicates photoinduced C–C bond cleavage in these radicals.
In addition to the CCO-bend trans-CH2CH•CO species the quantum-chemical computations also predict a minimum for the •CH2 CHCO radical which is, first, thermodynamically more stable (by 12.3 kJ mol−1), and, second, has sufficiently higher frequency for the COstr vibrational mode (Fig. 1 and Table 1). Both of these effects are likely explained by a more efficient CH2=CH–•C=O ↔ •CH2–CH=C=O resonance in the •CH2CHCO species. The •CH2CHCO radical absorption bands were also observed in the FTIR spectra of the photolyzed CH2CHCHO/Cl2/para-H2 matrices and were assigned on the basis of our theoretical predictions (Table 1), literature data (Das & Lee 2013), and observed UV-photoinduced C–C bond cleavage (see Sect. 2.2). It is interesting to notice that, taking into account thermodynamics, one should expect solely the formation of the •CH2CHCO radical. In contrast, kinetic considerations suggest the observation of solely trans-CH2CH•CO species (the latter is the only direct product of the trans-CH2CHCHO aldehyde H abstraction reaction with the trans-CH2CHCHO molecules being the dominant CH2CHCHO conformer (>90%) in the pre-2217 nm photolyzed para-H2 matrices). However, taking into account that both radicals were observed in the recorded FTIR spectra, we expect that the energy excess of the trans- CH2CHCHO + H• system (more than 70 kJ mol−1) may result in the over-the-barrier formation of the •CH2CHCO from the trans-CH2CHCHO molecules.
Absorption bands with the maxima at 1543.2 cm−1 and 1543.4, 1548.1 cm−1 were also observed in the FTIR spectra of the photolyzed CH2CHCHO/Cl2/para-H2 and CH3CH2CHO/Cl2/para-H2 matrices, respectively (Figs. 3 and 4). Taking into account the gas-phase value of 1578 cm−1 for the cis-CH3•CHCHO radical (Yacovitch et al. 2011) and our theoretical predictions at 1535.2 and 1541.9 cm−1 for the COstr mode of the trans-CH3•CHCHO and cis-CH3•CHCHO species, we tentatively assigned these signals to trans-CH3•CHCHO and cis-CH3•CHCHO, respectively. Given the noticeable difference of approximately 30 cm−1 between the para-H2 and gas-phase value, we may suggest that the gas-phase number could potentially be an overestimation. This could be due to the broadness of the peak corresponding to that absorption band. Unfortunately, Yacovitch et al. (2011) did not provide the error bars for this vibrational frequency measurement. It is worth noting that, in the case of CH2CHCHO, we also detected additional absorption features tentatively assigned in a good agreement with the computations to the CH2, scis, CCHbend, and CH3, s-str modes of the trans-CH3•CHCHO radicals (Table 1). Furthermore, we also noticed the correlation between the growths of the methylketene absorption bands and the decrease of the signals attributed to the CH3•CHCHO species during the 225 nm photolysis (Figs. 3 and 4). It may indicate the occurrence of the CH3·•CHCHO ⇝ CH3CHCO + H• photoinduced reaction, therefore supporting the assignment. It is worth noting that in the case of CH3CH2CHO we did not find any additional features belonging to the CH2, scis, CCHbend, and CH3, s-str modes of the cis-CH3•CHCHO radicals, which is most likely explained by their relatively low intensities (the unassigned features with the maxima at 1451.5, 1450.4, and 1374.4 cm−1 have different photochemistry than that attributed to the COstr mode of the radical and therefore cannot be assigned to these species): the main absorption feature (COstr mode) of the CH3•CHCHO radical is roughly six times more intensive in the case of CH2CHCHO than that in the case of CH3CH2CHO. The possible routes for the formation of the CH3• CHCHO radicals in the systems under study are the H-atom addition to CH2CHCHO and H-atom abstraction from CH3CH2CHO molecules (Reactions (11) and (28), respectively). Theoretical results (Figs. 1 and 2) also demonstrate that the formation of these species is highly favored thermodynamically and predict a quite small barrier for their formation (less than 3.4 kJ mol−1 for CH2CHCHO and 8.8 kJ mol−1 for CH3CH2CHO). Thus, the formation of the CH3•CHCHO species is very probable in the investigated matrices. It is very interesting to notice that both cis- and trans-conformers of this radical are formed in the case of CH3CH2CHO while only the trans-one is formed in the case of CH2CHCHO molecules (Tables 1 and 2). We believe that trans-CH3•CHCHO radicals are formed from the trans-CH2CHCHO and gauche-CH3CH2CHO molecules while cis-ones are produced from the cis-CH2 CHCHO and cis- CH3 CH2 CHO molecules, respectively. Therefore, taking into account that the percentage of the trans-CH2CHCHO in the non-photolyzed matrices is larger than 90% while that of cis- CH3 CH2 CHO is only 60–70%, one may expect the observed results.
We also observed the appearance of absorption features with maxima at 1769.1, 3619.6 cm−1 and 1784.7, 1781.3, 3612.5 cm−1 in the FTIR spectra in the CH2CHCHO/Cl2/para-H2 and CH3 CH2 CHO/Cl2 /para-H2 matrices, respectively (Figs. 3 and 4), after H-atom generation. It is reasonable to assign them to aldehyde COstr and alcohol OHstr modes, accordingly. Owing to statistical reasons, we believe that the formation of the products of the single hydrogen addition or abstraction is much more probable than the double hydrogen addition or abstraction; therefore, these absorption modes most probably belong to the open-shell species. In the case of the trans-CH2CHCHO which should be the dominant (>90%) conformer in the nonphotolyzed samples, our computations predict (Fig. 1) only one thermodynamically possible open shell product with the COstr mode close to the original aldehyde – trans-•CH2CH2CHO radical. In the case of CH3CH2CHO, the only product of this type is also •CH2CH2CHO radical. Therefore, based on the results of the computations we tentatively assigned the absorption features with the maxima at 1769.1 and 1784.7, 1781.3 cm−1 to the trans- and cis-•CH2CH2CHO species, respectively (Tables 1 and 2). The •CH2CH2CHO radicals may be produced from CH2CHCHO and CH3CH2CHO by the H-atom addition and abstraction (Reactions (13) and (30), respectively). As related to the alcohol radicals, there is only one type of process which may produce this type of radical – the addition of a hydrogen atom to the aldehyde oxygen atom of CH2CHCHO or CH3CH2CHO (Reactions (12) and (38), accordingly). Therefore, we tentatively assigned the absorption features with the maxima at 3619.6 and 3612.5 cm−1 to syn-trans-CH2CH•CHOH (CH2CHCHO) and syn-cis-CH3CH2•CHOH (CH3CH2CHO) radicals because the agreement of the experimental results with syn-conformers was found to be better: the corresponding anti-conformers generally have 35–65 cm−1 higher theoretical anharmonic frequencies (Tables A2 and A4). The detection of 1-propanol in the mass spectra of CH3CH2CHO ice after a hydrogen atom bombardment (Qasim et al. 2019) also suggests that the formation of syn-cis-CH3CH2•CHOH radical may be possible under the conditions of our experiments. On the other hand, we did not observe the absorption features in the region 1300–1100 cm−1 which should have rather prominent intensity in the case of the R•CHOH-radicals (Tables A2 and A4). At the same time, it is hard to determine the reason why only cis-•CH2CH2CHO and syn-cis-CH3CH2•CHOH radicals were observed in the case of CH3 CH2 CHO (Fig. B3); therefore, we assigned these species only tentatively. Despite the formation of these species being theoretically predicted to be thermodynamically possible, another reason why we assigned the corresponding absorption bands only tentatively is the generally rather high barriers of the formation: ca. 8.1–12.7 and 25.0–25.1 kJ mol−1 for •CH2CH2CHO radicals from CH2CHCHO and CH3CH2CHO and 14.6–18.4 and 24.7–27.3 kJ mol−1 for CH2CH•CHOH (CH2CHCHO) and CH3CH2•CHOH (CH3CH2CHO) radicals, respectively (Figs. 1 and 2). However, taking into account that the reactions of H atoms in solid para-H2 are driven by the H-atom tunneling (Haupa et al. 2019b), tunneling control (Schreiner 2017; Qiu & Schreiner 2023) may take place in this case. It makes the width of a reaction barrier much more important than its height: the reactions with relatively high but narrow barriers may occur even more readily than those with low but broad reaction barriers (Schreiner 2017; Qiu & Schreiner 2023). Thus, an interesting issue for further studies is the estimation of the barrier widths for the investigated chemical reactions. As related to the possible hydrogen-assisted isomerization of the investigated aldehydes to enols or the double hydrogen addition to the C=O fragment leading to the formation of alcohol, we did not find any clear evidence of the O–H bond formation except the above-mentioned signals with the maxima at 3619.6 (CH2CHCHO) and 3612.5 (CH3 CH2 CHO) cm−1, which were tentatively attributed to the OHstr mode. However, because two subsequent reactions with H atoms are generally less probable than the reaction with one H atom, we believe that these absorption features most likely belong to the open-shell species containing the O–H group.
The main second-step hydrogenation product according to our experimental results (Figs. 3 and 4) is methylketene. It is interesting to notice that its IR signatures, which are well-known in para-H2 (Das & Lee 2013) and Ar matrices (Johnstone & Sodeau 1992; Winter et al. 1998), were observed in the FTIR spectra after H-atom generation both in CH2CHCHO/Cl2/para-H2 and CH3CH2CHO/Cl2/para-H2 samples (Fig. B3). This molecule may have formed either via hydrogen addition to the •CH2CHCO (trans-CH2CH•CO) radicals in the CH2 CHCHO/Cl2 /para-H2 samples or via H-atom abstraction from the CH3• CHCHO radicals (Reactions (22) and (25)) and via abstraction either from the CH3CH2•CO or CH3•CHCHO radicals in the CH3CH2CHO/Cl2/para-H2 matrices (Reactions (31) and (33)). These reactions are thermodynamically highly favored (Figs. 1 and 2) and are expected to have no barrier (at least on the singlet PES).
The absorption band with the maximum at 1749.1 cm−1, observed in the FTIR spectra of the CH2CHCHO/Cl2/para- H2 samples after H-atom generation (Fig. 3), was assigned to CH3CH2CHO (the 1756.1 cm−1 one was attributed only tentatively). Thus, CH3CH2CHO is another second-step hydrogenation product of CH2CHCHO which is most likely produced via the hydrogenation of the CH3•CHCHO radicals (Reaction (15)). It is worth noting that these results are in agreement with the previous data (Qasim et al. 2019) on the CH3 CH2 CHO formation as a result of the H-atom bombardment of CH2CHCHO ices. On the other hand, CH2CHCHO does not appear to be produced as a result of a two-step H-atom reaction of CH3 CH2 CHO, most likely because the formation of methylketene (Reaction (31)) or the reformation of CH2CHCHO (Reaction (15)) are more favorable, potentially due to steric factors. The general scheme of the proposed chemical reactions is provided in Fig. 5 (the full scheme is provided in Fig. B4).
 |
Fig. 4 Difference FTIR spectra of the CH3CH2CHO/Cl2/para-H2 sample illustrating the effect of the sequential 365 nm (60 min) + 2217 nm (90 min) irradiation (a), free sample standing after photolysis for 420 min (b), 365 nm (30 min) photolysis (c), 2217 nm (100 min) photolysis (d), 400 nm (20 min) photolysis (e), 330 nm (20 min) photolysis (f ), 260 nm (20 min) photolysis (g), and 225 nm (20 min) photolysis (h). The absorption features attributed to the cis-CH3CH2CHO are marked with “p”. The unassigned absorption features are labeled with question signs. |
Maxima of the absorption bands observed in the FTIR spectra of the 365 nm + 2217 nm photolyzed CH2CHCHO/Cl2/para-H2 matrices and the corresponding computed values.
Maxima of the absorption bands observed in the FTIR spectra of the 365 nm + 2217 nm photolyzed CH3CH2CHO/Cl2/para-H2 matrices and the corresponding computed values.
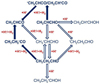 |
Fig. 5 General scheme of the hydrogen atom reactions with CH2CHCHO and CH3CH2CHO in solid para-H2. The tentatively assigned products are in non-bold and tentatively proposed reactions are outlined with the empty arrows |
5 Summary and conclusions
In the present work, we investigated the H-atom reactions of CH2CHCHO and CH3CH2CHO insolid para-H2 at 3.1 K. It was found that for both CH2CHCHO and CH3CH2CHO molecules, H atoms can abstract another H atom from the aldehyde group, resulting in the formation of the R•CO radicals: CH2CH•CO (•CH2CHCO) and CH3CH2•CO, respectively. Furthermore, we have tentatively identified the CH3•CHCHO species which formed both in the H-atom addition reaction of CH2CHCHO and in the H-atom abstraction reaction of CH3CH2CHO while no solid evidence of the formation of the closed-shell alcohols was found in the experimental data. Further hydrogenation of the R•CO and CH3• CHCHO species results in the formation of methylketene which was found to have an energy close to trans-CH2CHCHO. This H-atom assisted isomerization product of CH2 CHCHO was clearly identified in our experiments. Another second-step hydrogenation product which was detected in the FTIR spectra of the photolyzed CH2CHCHO/Cl2/para-H2 matrices is CH3CH2CHO. The formation of the •CH2CH2CHO, CH2CH•CHOH, and CH3CH2•CHOH radicals was also tentatively proposed in the considered systems.
6 Astrophysical implications
Saturated and unsaturated aldehydes are abundant species in the ISM (Bennett & Kaiser 2007; Abplanalp et al. 2015; Singh et al. 2022). The present study reveals that the identified interstellar aldehydes, CH3CH2CHO and trans-CH2CHCHO, are most likely chemically linked in the ISM through H-atom reaction via the CH3•CHCHO radicals.
An isomer of CH2CHCHO, CH3CHCO, was also recently detected in the ISM (Fuentetaja et al. 2023). Although many possible interstellar formation pathways were considered for CH3 CHCO (Fuentetaja et al. 2023), the H-atom assisted isomerization of trans-CH2 CHCHO was not considered as a possibility. The present study clearly shows that H-atom assisted isomerization may serve as a chemical link between these species in dark molecular clouds.
We can assume that the formation of the different types of carbon-centerd radicals from the hydrogenation of CH2CHCHO and CH3CH2CHO in principle may also take place in the ISM: both in the gas phase and solid environment. The radicals may be formed in interstellar ices if the efficiency of the bimolecular radical–radical reactions is not high due to the low radical concentrations and in the absence of (or well-protected by the ice mantle from) destructive high-energy radiation (photons and charged particles) which promotes the monomolecular decomposition. Furthermore, they potentially can go through the desorption process without the decomposition eventually finding themselves in the gas phase where these species can be detected (see, for instance, the example of hydroxyl radical (• OH); Tsuge & Watanabe 2021). Thus, we believe that even if H-atom reactions are less effective in the gas phase implying the predominant formation of these species in the solid phase with they further desorption into the gas phase, H-atom reactions could still contribute to the gas-phase abundances of these radicals. Since some of the radicals resulting from aldehydes have already been characterized by laboratory microwave spectroscopy (for example, vinyloxy radical (•CH2CHO); Chahbazian et al. 2024), therefore opening a possibility to obtain the corresponding data for the radicals derived from propenal and propanal, it would be straightforward and worthwhile to investigate the presence in the ISM using radio astronomy. Both in the gas phase and solid environment the radicals may further react with each other resulting in the formation of a variety of polyfunctional compounds (for example, dialdehydes). Therefore, these processes can lead to the complexification of the chemical composition of the ISM and potentially contribute to the prebiotic astrochemistry.
Moreover, together with the previous reports (see Haupa et al. 2019b; Schneiker et al. 2022; Tsai et al. 2022; Schneiker et al. 2024), this research proposes that the i-COMs can participate both in H-atom abstraction and H-atom addition reaction. It creates a mechanism in which an H2 molecule is produced from two H atoms and implies that i-COMs might be the important species in interstellar H2 formation, therefore stimulating further studies in this field.
Data availability
The data that support the findings of this study are available within this article and in its online Supplementary Material (https://doi.org/10.5281/zenodo.14178828).
See Appendices A, B, and C of the Supplementary Material for the FTIR spectra of the non-photolyzed CH2CHCHO/para-H2 and CH3CH2CHO/para-H2 samples and difference FTIR spectra illustrating the effect of the 365 and 225 nm photolyses on these samples, the difference FTIR spectra of the CH2CHCHO/Cl2/para-H2 and CH3CH2CHO/Cl2/para-H2 matrices illustrating the effect of the sequential 365 nm + 2217 nm photolyses on these matrices; energies, optimized molecular geometries, harmonic and anharmonic vibrational frequencies, and IR intensities for the CH2CHCHO, CH3 CH2 CHO, and the products of their reactions with hydrogen atoms as well as the corresponding transition states, and absorption maxima of the main features of the CH2CHCHO and CH3 CH2 CHO molecules isolated in a solid para-H2 matrix.
Acknowledgements
This work was financially supported by the Lendület program of the Hungarian Academy of Sciences and the Hungarian Scientific Research Fund (Grant No. OTKA K143196) and by the ELTE Institutional Excellence Program (Grant No. TKP2021-NKTA-64), both funded by the National Research, Development and Innovation Fund. Pavel V. Zasimov thanks the Alexander von Humboldt Foundation (AvH) for a research scholarship. Sándor Góbi was supported by the “Bolyai” Scholarship of the Hungarian Academy of Sciences as well as by the “ÚNKP-23-5 New National Excellence Program” of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. Barbara Keresztes acknowledges the “ÚNKP-23-3 New National Excellence Program” and “DKOP-23 Doctoral Excellence Program” of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund. Author Contributions Statement: P. V. Zasimov – Investigation, Validation, Formal Analysis, Visualization, Writing – original draft; B. Keresztes – Investigation, Validation, Writing – original draft; S. Góbi – Investigation, Validation, Writing – original draft; A. D. Volosatova – Investigation, Writing – original draft; G. Tarczay – Conceptualization, Validation, Supervision, Project administration, Funding acquisition, Writing – review & editing.
References
- Abplanalp, M. J., Borsuk, A., Jones, B. M., & Kaiser R. I. 2015, ApJ, 814, 45 [NASA ADS] [CrossRef] [Google Scholar]
- Agúndez, M., Marcelino, N., Tercero, B., et al. 2021, A&A, 649, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Loison, J. C., et al. 2023, A&A, 673, A34 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Baskir, E. G., & Nefedov, O. M. 1996, Russ. Chem. Bull., 45, 99 [Google Scholar]
- Bahou, M., Das, P., Lee, Y. F., Wu, Y. J., & Lee, Y. P. 2014, Phys. Chem. Chem. Phys., 16, 2200 [Google Scholar]
- Balabanoff, M. E., Ruzi, M., & Anderson, D. T. 2018, Phys. Chem. Chem. Phys., 20, 422 [Google Scholar]
- Barone, V. J. 2005, Chem. Phys., 122, 014108 [NASA ADS] [Google Scholar]
- Bazsó, G., Csonka, I. P., Góbi, S., & Tarczay, G. 2021, Rev. Sci. Inst., 92, 124104 [Google Scholar]
- Bennett, C. J., & Kaiser, R. I. 2007, ApJ, 661, 899 [Google Scholar]
- Blom, C. E., Müller, R. P., & Günthard, H. H. 1980, Chem. Phys. Lett., 73, 483 [NASA ADS] [CrossRef] [Google Scholar]
- Bokareva, O. S., Bataev, V. A., & Godunov, I. A. 2009, Russ. J. Phys. Chem. A, 83, 81 [Google Scholar]
- Burluka, A. A., Harker, M., Osman, H., Sheppard, C. G. W., & Konnov, A. A. 2010, Fuel, 89, 2864 [Google Scholar]
- Cavaliere, A., Ciajolo, A., D’Anna, A., Mercogliano, R., & Ragucci, R. 1993, Combust. Flame, 93, 279 [NASA ADS] [CrossRef] [Google Scholar]
- Chahbazian, R., Martin-Drumel, M. A., & Pirali, O. 2024, J. Phys. Chem. A, 128, 370 [NASA ADS] [CrossRef] [Google Scholar]
- Clayden, J., Greeves, N., & Warren, S. 2012, Organic Chemistry, 2nd edn. (Oxford: OUP Oxford), 1234 [Google Scholar]
- Cleaves II, H. J. 2003, Monatsh. Chem., 134, 585 [Google Scholar]
- Das, P., & Lee, Y. P. 2013, J. Chem. Phys., 139, 084320 [CrossRef] [Google Scholar]
- Das, P., & Lee, Y. P. 2014, J. Chem. Phys., 140, 244303 [NASA ADS] [CrossRef] [Google Scholar]
- da Silva, G., & Bozzelli, J. W. 2006, J. Phys. Chem. A, 110, 13058 [Google Scholar]
- de Marcellus, P., Meinert, C., Myrgorodska, L., et al. 2015, Proc. Natl. Acad. Sci., 112, 965 [NASA ADS] [CrossRef] [Google Scholar]
- Dickens, J. E., Irvine, W. M., Nummelin, A., et al. 2001, Spectrochim. Acta A, 57, 643 [Google Scholar]
- Fajardo, M. E., & Lindsay, C. M. 2008, J. Chem. Phys., 128, 014505 [NASA ADS] [CrossRef] [Google Scholar]
- Field-Theodore, T. E., & Taylor, P. R. 2022, Phys. Chem. Chem. Phys., 24, 19184 [Google Scholar]
- Folliard, V., Tommaso, J. D., & Dubois, J. L. 2021, Catalysts, 11, 229 [CrossRef] [Google Scholar]
- Frisch, M. J., et al. 2016, Gaussian 16, Rev. A.03 (Wallingford, CT: Gaussian Inc.) [Google Scholar]
- Fuentetaja, R., Bermúdez, C., Cabezas, C., et al. 2023, A&A, 671, L6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fushitani, M., & Momose, T. 2003, Low Temp. Phys., 29, 740 [NASA ADS] [CrossRef] [Google Scholar]
- Grosjean, D., & Williams II, E. L. 1994, Sci. Total Environ., 153, 195 [Google Scholar]
- Guirgis, G. A., Drew, B. R., Gounev, T. K., & Durig, J. R. 1998, Spectrochim. Acta A, 54, 123 [Google Scholar]
- Hamada, Y., Nishimura, Y., & Tsuboi, M. 1985, Chem. Phys., 100, 3, 365 [CrossRef] [Google Scholar]
- Haupa, K. A., Johnson, B. A., Sibert, E. L., & Lee, Y. P. 2017, J. Chem. Phys., 147, 154305 [NASA ADS] [CrossRef] [Google Scholar]
- Haupa, K. A., Strom, A. I., Anderson, D. T., & Lee, Y. P. 2019a, J. Chem. Phys., 151, 234302 [NASA ADS] [CrossRef] [Google Scholar]
- Haupa, K. A., Tarczay, G., & Lee, Y. P. 2019b, J. Am. Chem. Soc., 141, 11614 [CrossRef] [Google Scholar]
- Haupa, K. A., Ong, W. S., & Lee, Y. P. 2020, Phys. Chem. Chem. Phys., 22, 6192 [Google Scholar]
- Haupa, K. A., Joshi, P. R., & Lee, Y. P. 2022, J. Chin. Chem. Soc., 69, 1159 [NASA ADS] [CrossRef] [Google Scholar]
- Hänni, N., Altwegg, K., Baklouti, D., et al. 2023, A&A, 678, A22 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hollis J. M., Lovas F. J., & Jewell P. R. 2000, ApJ, 540, L107 [NASA ADS] [CrossRef] [Google Scholar]
- Hollis, J. M., Jewell, P. R. Lovas, F. J., Remijan, A. & Møllendal, H. 2004, ApJ, 610, L21 [NASA ADS] [CrossRef] [Google Scholar]
- Jacox, M. E. 1998, J. Phys. Chem. Ref. Data, 27, 115 [Google Scholar]
- Johnstone, D. E., & Sodeau, J. R. 1992, J. Chem. Soc. Faraday Trans., 88, 409 [CrossRef] [Google Scholar]
- Jonusas, M., Guillemin, J. C., & Krim, L. 2017, MNRAS, 468, 4592 [Google Scholar]
- Joshi, P. R., & Lee, Y. P. 2022, Commun. Chem., 5, 62 [CrossRef] [Google Scholar]
- Joshi, P. R., How, K. C. Y., & Lee, Y. P. 2021, ACS Earth Space Chem., 5, 106 [CrossRef] [Google Scholar]
- Krim, L., Jonusas, M., Guillemin, J. C., Yanez, M., & Lamsabhi, A. M. 2018, Phys. Chem. Chem. Phys., 20, 19971 [NASA ADS] [CrossRef] [Google Scholar]
- Keresztes, B., Góbi, S., Csonka, I. P., et al. 2023, MNRAS, 521, 2649 [CrossRef] [Google Scholar]
- Keresztes, B., Aazaad, B., Schneiker, A., et al. 2024a, A&A, 689, A21 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Keresztes, B., Góbi, S., & Tarczay, G. 2024b, MNRAS, 527, 12027 [Google Scholar]
- Köroğlu, B., Loparo, Z., Nath, J., Peale, R. E., & Vasu, S. S. 2015, JQSRT, 152, 107 [Google Scholar]
- Le Crâne, J. P., Villenave, E., Hurley, M. D., Wallington, T. J., & Ball, J. C. 2005, J. Phys. Chem., A 109, 11837 [Google Scholar]
- Lewars, E., & Liebman, J. F. 2013, Struct. Chem., 24, 741 [Google Scholar]
- Lykke, J. M., Coutens, A., Jørgensen, J. K., et al. 2017, A&A, 597, A53 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Manigand, S., Coutens, A., Loison, J.-C., et al. 2021, A&A, 645, A53 [EDP Sciences] [Google Scholar]
- Momose, T., & Shida, T. 1998, Bull. Chem. Soc. Jpn., 71, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Osborne, G. A., & Ramsay, D. A. 1973, Can. J. Phys., 51, 1170 [CrossRef] [Google Scholar]
- Raston, P. L., & Anderson, D. T. 2006, Phys. Chem. Chem. Phys., 8, 3124 [NASA ADS] [CrossRef] [Google Scholar]
- Raston, P. L., Kettwich, S. C., & Anderson, D. T. 2015, J. Mol. Spectrosc., 310, 72 [NASA ADS] [CrossRef] [Google Scholar]
- Requena-Torres, M. A., Martín-Pintado, J., Martin, S., & Morris, M. R. 2008, ApJ, 672, 352 [NASA ADS] [CrossRef] [Google Scholar]
- Ruzi, M., & Anderson, D. T. 2012, J. Chem. Phys., 137, 194313 [NASA ADS] [CrossRef] [Google Scholar]
- Ruzi, M., & Anderson, D. T. 2015, J. Phys. Chem. A, 119, 12270 [Google Scholar]
- Qasim, D., Fedoseev, G., Chuang, K. J., et al. 2019, A&A, 627, A1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Qiu, G., & Schreiner, P. R. 2023, ACS Cent. Sci., 9, 2129 [CrossRef] [Google Scholar]
- Schneiker, A., Góbi, S., Joshi, P. R., et al. 2021, J. Phys. Chem. Lett., 12, 6744 [CrossRef] [Google Scholar]
- Schneiker, A., Ragupathy, G., Bazsó, G., & Tarczay, G. 2022, J. Phys. Chem. A, 126, 2832 [NASA ADS] [CrossRef] [Google Scholar]
- Schneiker, A., Góbi, S., Ragupathy, G., et al. 2024, J. Chem. Phys., 160, 214303 [NASA ADS] [CrossRef] [Google Scholar]
- Schreiner, P. R. 2017, J. Am. Chem. Soc., 139, 15276 [NASA ADS] [CrossRef] [Google Scholar]
- Singh, S. K., Kleimeier, N. F., Eckhardt, A. K. & R. I. Kaiser, 2022, ApJ, 941, 2, 103 [NASA ADS] [CrossRef] [Google Scholar]
- Sogoshi, N., Wakabayashi, T., Momose, T., & Shida, T. 1997, J. Phys. Chem. A, 101, 522 [Google Scholar]
- Tam, S., & Fajardo, M. E. 2000, Low Temp. Phys., 26, 653 [NASA ADS] [CrossRef] [Google Scholar]
- Tam, S., & Fajardo, M. E. 2001, J. Low Temp. Phys., 122, 345 [CrossRef] [Google Scholar]
- Tarczay, G., Haupa, K., & Lee, Y. P. 2019, Proc. Int. Astron. Union, 15, 394 [Google Scholar]
- Thompson, W. E., & Jacox, M. E. 1989, J. Chem. Phys., 91, 3826 [NASA ADS] [CrossRef] [Google Scholar]
- Troya, D. 2019, J. Phys. Chem. A, 123, 1044 [Google Scholar]
- Tsai, S. Y., Haupa, K. A., & Lee, Y. P. 2022, J. Am. Chem. Soc., 144, 12339 [NASA ADS] [CrossRef] [Google Scholar]
- Tsuge M., & Lee, Y. P. 2020, in Molecular and Laser Spectroscopy: Advances and Applications, Vol. 2, eds. V. P. Gupta, & Y. Ozaki (Amsterdam, Netherlands: Elsevier), 167 [Google Scholar]
- Tsuge, M., & Watanabe, N. 2021, Acc. Chem. Res., 54, 471 [CrossRef] [Google Scholar]
- Tsuge, M., Tseng, C. Y., & Lee, Y. P. 2018, Phys. Chem. Chem. Phys., 20, 5344 [Google Scholar]
- Winter, P. R., Rowland, B., Hess, W. P., et al. 1998, J. Phys. Chem. A, 102, 3238 [Google Scholar]
- Wu, Y. J., Yang, X., & Lee, Y. P. 2004, J. Chem. Phys., 120, 1168 [NASA ADS] [CrossRef] [Google Scholar]
- Yacovitch, T. I., Kim, J. B., Garand, E., van der Poll, D. G., & Neumark, D. M. 2011, J. Chem. Phys., 134, 134307 [NASA ADS] [CrossRef] [Google Scholar]
- Yan, C., McKinnon, A., Moore, B., et al. 2023, Low Temp. Phys., 49, 1072 [NASA ADS] [CrossRef] [Google Scholar]
- Yoshioka, K., Raston, P. L., & Anderson, D. T., 2006, Int. Rev. Phys. Chem., 25, 469 [NASA ADS] [CrossRef] [Google Scholar]
- Zasimov, P. V., Tyurin, D. A., Ryazantsev, S. V., & Feldman, V. I. 2022, J. Am. Chem. Soc., 144, 8115 [NASA ADS] [CrossRef] [Google Scholar]
- Zasimov, P. V., Volosatova, A. D., Góbi, S., et al. 2024, J. Chem. Phys., 160, 024308 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Maxima of the absorption bands observed in the FTIR spectra of the 365 nm + 2217 nm photolyzed CH2CHCHO/Cl2/para-H2 matrices and the corresponding computed values.
Maxima of the absorption bands observed in the FTIR spectra of the 365 nm + 2217 nm photolyzed CH3CH2CHO/Cl2/para-H2 matrices and the corresponding computed values.
All Figures
 |
Fig. 1 Potential energy surface of the reactions of H-atom abstraction (e) and addition (a) with trans- and cis-CH2CHCHO molecules. The energies are provided in kJ mol−1 relative to the trans-CH2CHCHO (+2H•) species. The radical–radical reaction pathways hypothesized theoretically and generally expected, without computational verification, are indicated with dashed lines. |
| In the text | |
 |
Fig. 2 Potential energy surface of the reactions of H-atom abstraction (e) and addition (a) with cis- and gauche-CH3CH2CHO molecules. The energies are provided in kJ mol−1 relative to the cis-CH3CH2CHO (+2H•) species. The radical–radical reaction pathways hypothesized theoretically and generally expected, without computational verification, are indicated with dashed lines. |
| In the text | |
 |
Fig. 3 Difference FTIR spectra of the CH2CHCHO/Cl2/para-H2 sample illustrating the effect of the sequential 365 nm (60 min) + 2217 nm (100 min) irradiation (a), free sample standing after photolysis for 480 min (b), 365 nm (70 min) photolysis (c), 2217 nm (100 min) photolysis (d), 400 nm (20 min) photolysis (e), 330 nm (20 min) photolysis (f), 260 nm (20 min) photolysis (g), and 225 nm (20 min) photolysis (h). We note that the center-left panel is multiplied by 0.3 for a better representation. The absorption features attributed to the trans-CH2CHCHO and cis-CH3CH2CHO are marked with “a” and “p”, respectively. The unassigned absorption features are labeled with question signs. |
| In the text | |
 |
Fig. 4 Difference FTIR spectra of the CH3CH2CHO/Cl2/para-H2 sample illustrating the effect of the sequential 365 nm (60 min) + 2217 nm (90 min) irradiation (a), free sample standing after photolysis for 420 min (b), 365 nm (30 min) photolysis (c), 2217 nm (100 min) photolysis (d), 400 nm (20 min) photolysis (e), 330 nm (20 min) photolysis (f ), 260 nm (20 min) photolysis (g), and 225 nm (20 min) photolysis (h). The absorption features attributed to the cis-CH3CH2CHO are marked with “p”. The unassigned absorption features are labeled with question signs. |
| In the text | |
 |
Fig. 5 General scheme of the hydrogen atom reactions with CH2CHCHO and CH3CH2CHO in solid para-H2. The tentatively assigned products are in non-bold and tentatively proposed reactions are outlined with the empty arrows |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


