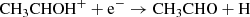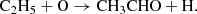| Issue |
A&A
Volume 693, January 2025
|
|
|---|---|---|
| Article Number | L20 | |
| Number of page(s) | 7 | |
| Section | Letters to the Editor | |
| DOI | https://doi.org/10.1051/0004-6361/202453459 | |
| Published online | 28 January 2025 | |
Letter to the Editor
Detection of thioacetaldehyde (CH3CHS) in TMC-1: Sulfur-oxygen differentiation along the hydrogenation sequence⋆
1
Instituto de Física Fundamental, CSIC, Calle Serrano 123, E-28006 Madrid, Spain
2
Observatorio Astronómico Nacional, IGN, Calle Alfonso XII 3, E-28014 Madrid, Spain
3
Observatorio de Yebes, IGN, Cerro de la Palera s/n, E-19141 Yebes, Guadalajara, Spain
⋆⋆ Corresponding authors; marcelino.agundez@csic.es; jose.cernicharo@csic.es
Received:
16
December
2024
Accepted:
7
January
2025
In recent years, the chemistry of sulfur in the interstellar medium has experienced renewed interest due to the detection of a large variety of molecules containing sulfur. We report the first identification in space of a new S-bearing molecule, thioacetaldehyde (CH3CHS), which is the sulfur counterpart of acetaldehyde (CH3CHO). The astronomical observations are part of QUIJOTE, a Yebes 40 m Q-band line survey of the cold dense cloud TMC-1. We detected seven individual lines corresponding to A and E components of the four most favorable rotational transitions of CH3CHS covered in the Q band (31.0–50.3 GHz). Assuming a rotational temperature of 9 K, we derive a column density of 9.8 × 1010 cm−2 for CH3CHS, which implies that it is 36 times less abundant than its oxygen counterpart CH3CHO. By comparing the column densities of the O- and S-bearing molecules detected in TMC-1, we find that as molecules increase their degree of hydrogenation, sulfur-bearing molecules become less abundant than their oxygen analog. That is, hydrogenation seems to be less favored for S-bearing molecules than for O-bearing ones in cold sources such as TMC-1. We explored potential formation pathways to CH3CHS and implemented them into a chemical model, which underestimates the observed abundance of thioacetaldehyde by several orders of magnitude, however. Quantum chemical calculations carried out for one of the potential formation pathways, the S + C2H5 reaction, indicate that formation of CH3CHS is only a minor channel in this reaction.
Key words: astrochemistry / line: identification / ISM: molecules / radio lines: ISM / ISM: individual objects: TMC-1
© The Authors 2025
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1. Introduction
There are still many gaps in our knowledge of the chemistry of sulfur in interstellar space. This is well illustrated by the so-called missing-sulfur problem, which states that most of the sulfur budget in cold dense clouds is still pending characterization (Ruffle et al. 1999; Martín-Domenech et al. 2016; Vidal et al. 2017; Navarro-Almaida et al. 2020). In the past years, many new sulfur-bearing molecules have been reported in dense clouds, namely, S2H (Fuente et al. 2017), HCS, HSC (Agúndez et al. 2018), NS+, HC3S+, NCS, HCCS, H2CCS, H2CCCS, C4S, C5S, HCSCN, HCSCCH (Cernicharo et al. 2018, 2021a,b,c), HCOSH (Rodríguez-Almeida et al. 2021), HCCS+ (Cabezas et al. 2022), HC4S (Fuentetaja et al. 2022), HSO (Marcelino et al. 2023), HNSO (Sanz-Novo et al. 2024), NCCHCS (Cabezas et al. 2024), HCNS, NC3S, HC3S (Cernicharo et al. 2024a,b), and surprisingly, the metal-bearing molecules MgS and NaS (Rey-Montejo et al. 2024). However, none of the above molecules are a major reservoir of sulfur. For example, in TMC-1 the inventory of S-bearing molecules detected according to our records (see below in Sect. 4) accounts for only 0.15% of the cosmic abundance of sulfur (Asplund et al. 2009), if we adopt a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). This means that 99.85% of sulfur is still missing and awaiting identification.
The large number of detected S-bearing molecules did not help to solve the missing-sulfur problem, but it has allowed us to place exquisite observational constraints on the chemistry of this element, in particular, in cold dense clouds. Although the oxygenated counterparts of many of the detected sulfurated molecules have been detected as well, there are significant differences between the chemistries of sulfur and oxygen. For example, the carbon chains C2S, C3S, and C5S are far more abundant than their oxygen counterparts (Cernicharo et al. 2021b,d). The sulfur counterparts of the hydrogenated O-bearing molecules HCO, H2CO, HCCO, CH2CO, HC3O, and HCCCHO have been detected (Agúndez & Wakelam 2013; Loison et al. 2016; Cernicharo et al. 2020a, 2021b,c,d, 2024b), although the oxygen molecule is in some cases more abundant than the sulfur molecule, while in other cases, the opposite is found. For many O-bearing complex organic molecules, the sulfur counterpart also remains elusive. These are C2H3OH, C2H5OH, CH3OCH3, HCOOH, HCOOCH3, HC5O, HC7O, c-H2C3O, C2H3CHO, CH3COCH3, and C2H5CHO (Loison et al. 2016; McGuire et al. 2017; Cordiner et al. 2017; Cernicharo et al. 2020a; Agúndez et al. 2021, 2023a).
In this Letter we report the detection in the cold dense cloud TMC-1 of thioacetaldehyde (CH3CHS), the sulfur analog of acetaldehyde (CH3CHO). Acetaldehyde is a well-known interstellar molecule that was detected in space in the early years of radioastronomy (Gottlieb et al. 1973). It is known to be present in TMC-1 (Matthews et al. 1985), and its chemistry is relatively well understood (Lamberts et al. 2019; Vazart et al. 2020; Enrique-Romero et al. 2021; Fedoseev et al. 2022; Ferrero et al. 2022; Molpeceres et al. 2022a). However, its sulfur cousin CH3CHS has so far eluded detection in interstellar space (Margulès et al. 2020), and very little is known about its chemistry.
2. Astronomical observations
The astronomical observations on which this work is based belong to the ongoing Yebes 40 m Q-band line survey of TMC-1, QUIJOTE (Q-band Ultrasensitive Inspection Journey to the Obscure TMC-1 Environment; Cernicharo et al. 2021e). Briefly, QUIJOTE is a Q-band line survey (31.0–50.3 GHz) of the cold dense cloud TMC-1 at the position of the cyanopolyyne peak (αJ2000 = 4h41m41.9s and δJ2000 = +25 ° 41′27.0″). The observations were carried out in frequency-switching observing mode, with a frequency throw of either 8 or 10 MHz. We used a 7 mm dual linear polarization receiver connected to a set of eight fast Fourier transform spectrometer, which allowed us to cover the full Q band in one shot with a spectral resolution of 38.15 kHz in both polarizations (Tercero et al. 2021). The intensity scale is the antenna temperature, TA*, which has an estimated uncertainty due to calibration of 10%. The main-beam brightness temperature, Tmb, can be obtained by dividing TA* by Beff/Feff (see note in Table 1). The half-power beam width (HPBW) can be approximated as HPBW(″) = 1763/ν (GHz). We used the latest QUIJOTE dataset, which includes observations carried out between November 2019 and July 2024. The total on-source telescope time is 1509.2 h, of which 736.6 h correspond to a frequency throw of 8 MHz and 772.6 h to a throw of 10 MHz. The TA* rms noise level varies between 0.06 mK at 32 GHz and 0.18 mK at 49.5 GHz. The procedure used to reduce and analyze the data was described in Cernicharo et al. (2022). All data were analyzed using the GILDAS software1.
Observed line parameters of CH3CHS in TMC-1.
3. Results
Thioacetaldehyde (CH3CHS) is an asymmetric rotor in which the internal rotation of the CH3 group, which has a C3v symmetry, couples to the overall rotation of the molecule. As a result, the rotational levels split into A and E substates and the molecule can exist in different torsional states. All lines observed here belong to the ground torsional state vt = 0. The next torsional state vt = 1 lies 229 K above the ground torsional state and is therefore not populated at the cold temperatures of TMC-1. The rotational spectrum of thioacetaldehyde was measured in the laboratory in the microwave range (7–41 GHz) by Kroto et al. (1974) and Kroto & Landsberg (1976), and more recently, at millimeter and submillimeter waves (150–660 GHz) by Margulès et al. (2020). The total dipole moment was measured to be 2.33 ± 0.02 D, with components along the a- and b-axes of 2.26 ± 0.02 D and 0.56 ± 0.01 D, respectively (Kroto & Landsberg 1976). All transitions observed here are a-type. We adopted the rotational spectroscopy of CH3CHS from the Lille Spectroscopic Database2, which is based on the aforementioned references.
For a rotational temperature of 9 K, which is the gas kinetic temperature in TMC-1 (Agúndez et al. 2023b), the most intense predicted lines of CH3CHS in the Q band correspond to the transitions 30, 3-20, 2 at 33.16 GHz, 41, 4-31, 3 at 43.34 GHz, 40, 4-30, 3 at 44.20 GHz, and 41, 3-31, 2 at 45.11 GHz, each of them consisting of a doublet with two components A and E that are separated by 0.6–2.4 MHz and have nearly identical intensities. We detected these four line doublets in the Q-band scan of TMC-1. The line parameters obtained from a Gaussian fit to the line profiles are given in Table 1, and the lines are shown in Fig. 1. The lines are weak, with intensities well below 1 mK in antenna temperature, although they are detected with signal-to-noise ratios above 3σ, in the range 3.5–8.1σ (see Table 1). The E component of the 30, 3-20, 2 transition at 33160.973 MHz lies very close in frequency to a brighter line, the 20-10 transition of CH3C13CH at 33160.940 MHz (Cernicharo et al., in prep.), and thus, it cannot be adequately fit. The seven fit components are centered at the systemic velocity of TMC-1, 5.83 km s−1 (Cernicharo et al. 2020b). The A/E doublets of the transitions 31, 3-21, 2 at 32.51 GHz and 31, 2-21, 1 at 33.83 GHz are predicted with about half of the intensity of the four line doublets shown in Fig. 1. They are not clearly detected above the noise level, which is consistent with their low predicted intensities. Although the signal-to-noise ratios of the detected lines are moderately low, the availability of the four brightest doublets in the Q band and the fact that no bright line is missing makes us sure about the detection of thioacetaldehyde in TMC-1.
 |
Fig. 1. Lines of CH3CHS observed in TMC-1 (black histogram). The negative features correspond to artifacts caused by the frequency-switching technique. The line parameters are given in Table 1. In magenta we show the LTE calculated line profiles adopting a column density of 9.8 × 1010 cm−2, a rotational temperature of 9.0 K, a full width at half maximum of 0.76 km s−1 (the average of the values observed for the seven detected components; see Table 1), and a circular emission distribution with a diameter of 80″. |
In order to determine the column density of thioacetaldehyde in TMC-1, we assumed local thermodynamic equilibrium (LTE). The narrow range of upper-level energies of the seven detected components (3.2–7.5 K) and the sizable errors in the velocity-integrated intensities do not allow us to constrain the rotational temperature precisely. We thus adopted a rotational temperature of 9 K, the gas kinetic temperature in TMC-1 (Agúndez et al. 2023b), which is consistent with the observed relative intensities, and an emission distribution consisting of a circle with a diameter of 80″, which is consistent with the emission size of most of the molecules mapped in TMC-1 (Cernicharo et al. 2023). We derive a column density of 9.8 × 1010 cm−2 for CH3CHS in TMC-1. The line profiles calculated under LTE for this column density are compared with the observed ones in Fig. 1. A variation of 1 K in the adopted rotational temperature has only a moderate impact of just 5% on the value of the determined column density.
4. Discussion
The cold dense cloud TMC-1 contains a remarkably rich variety of sulfur-containing molecules. Even though sulfur is ∼40 times less abundant than oxygen (Asplund et al. 2009), the number of S-bearing molecules identified to date in TMC-1 is comparable to that of O-bearing molecules, 33 and 39, respectively, according to our records (see Table 2). The oxygen counterparts of most of the S-bearing molecules detected in TMC-1 are detected as well, as illustrated in Table 2. The cases in which no oxygen counterpart is detected, such as HSC, H2C3S, C4S, HC4S, NS+, NC3S, and NCCHCS, reflect the existing chemical differences between sulfur and oxygen. For example, the metastable isomer HOC has not been observed in space, probably because its formation from H and CO is endothermic, unlike in the case of sulfur (Marenich & Boggs 2003; Puzzarini 2005). As discussed by Loison et al. (2016) and Shingledecker et al. (2019), chemical kinetics explain the nondetection of the most stable C3H2O isomer, propadienone (H2C3O), and the detection of the two isomers that are higher in energy, propynal (HCCCHO) and cyclopropenone (c-H2C3O), while for sulfur, the two most stable isomers (H2C3S and HCCCHS) are detected (Cernicharo et al. 2021b,c). Further differences are illustrated by the fact that no oxygen counterparts are detected for the sulfur carbon chains C4S, HC4S, and NC3S and the cation NS+ (Cernicharo et al. 2018, 2021b,d, 2024b; Fuentetaja et al. 2022), while NCCHCS is more abundant than its oxygen analog NCCHCO (Cabezas et al. 2024).
Observed column densities of O- and S-bearing molecules in TMC-1.
Many of the sulfur-bearing molecules with a detected oxygen counterpart still show remarkable differences in how their abundances behave. For example, the unsaturated carbon chains C2S, C3S, and C5S are significantly more abundant than their oxygen analogs (Cernicharo et al. 2021b,d). Nonetheless, as these carbon chains become hydrogenated, the behavior changes. We can make a meaningful comparison between sulfur and oxygen by focusing on the series of partially hydrogenated species HxCnS and HxCnO (with n = 1, 2, 3, and x = 0 − 4), for which there are extensive observational constraints. In TMC-1, the oxygen counterparts of molecules with n = 1 CS, HCS, H2CS, CH3SH3, with n = 2 CCS, HCCS, CH2CS, CH3CHS, and with n = 3 C3S, HC3S, and HCCCHS are detected. As listed in Table 2, the oxygenated molecule is in some cases more abundant than the sulfurated molecule, while in other cases, it is the opposite. However, a clear trend emerges for the column density ratio between the S- and O-bearing species as a function of the degree of hydrogenation4. Figure 2 shows that S-bearing molecules HxCnS with a low degree of hydrogenation (x < 1) are more abundant than their corresponding oxygen counterpart, but as the degree of hydrogenation increases, the abundance of S-bearing molecules with respect to their oxygen counterparts drops. In general, for moderate to high degrees of hydrogenation (x > 1), O-bearing molecules are more abundant than their corresponding sulfur analogs. A possible interpretation is that hydrogenation, whether it occurs in the gas phase or on grain surfaces, is less efficient for S-bearing than for O-bearing molecules, although this is probably a too simplistic view of the rather complex network of reactions involving these sulfur hydrogenated molecules (Oba et al. 2018; Molpeceres et al. 2022b; Shingledecker et al. 2022). Given the differentiation between the chemistry of sulfur and oxygen, it is not surprising that no sulfur counterpart is deteced for the hydrogenated O-bearing complex organic molecules detected in TMC-1, such as C2H3OH, C2H5OH, CH3OCH3, HCOOCH3, C2H3CHO, CH3COCH3, and C2H5CHO (Agúndez et al. 2021, 2023a). If the trend shown in Fig. 2 holds at larger hydrogenation degrees, we would expect the C2H5SH/C2H5OH ratio to be lower than the CH3CHS/CH3CHO ratio of 0.028, which means that the column density of C2H5SH would be lower than 3.1 × 1010 cm−2. In warmer environments, the observed C2H5SH/C2H5OH ratios are also rather low, < 0.008 in Sgr B2 (Müller et al. 2016), 0.05 in Orion KL (Kolesniková et al. 2014), and 0.07 in G+0.693−0.027 (Rodríguez-Almeida et al. 2021).
To investigate the formation of thioacetaldehyde in TMC-1, we carried out chemical modeling calculations. We adopted a standard model of a cold dense cloud (Agúndez & Wakelam 2013) and the chemical network associated with the UMIST Database for Astrochemistry 2022 (Millar et al. 2024). Thioacetaldehyde is not included in the UMIST 2022 network, but its oxygen counterpart acetaldehyde is included. According to our cold dense cloud model, CH3CHO is mainly formed through the dissociative recombination with electrons of protonated acetaldehyde, CH3CHOH+, together with the neutral-neutral reaction O + C2H5, which was theoretically studied recently by Vazart et al. (2020). In our chemical model, however, the calculated peak abundance of CH3CHO lies around 20 times below the value observed in TMC-1 (see Fig. 3). We explored whether formation pathways similar to those leading to CH3CHO could explain the observed abundance of CH3CHS in TMC-1. To do this, we included CH3CHS as a new species in the chemical network, where we assumed that it is formed through the dissociative recombination of CH3CHSH+ and the neutral-neutral reaction S + C2H5. We adopted the same rate coefficients as for the analog oxygen reactions, with the exception of S + C2H5, for which we carried out specific quantum chemical calculations that are presented in Appendix A. We calculated a total rate coefficient of 2.0 × 10−10 cm3 s−1 at 40 K and branching ratios of 0.73 for the channel leading to H2CS + CH3, 0.24 for C2H4 + SH, 0.025 for CH3CHS + H, 0.012 for CH2CHSH + H, and 3.1 × 10−5 for cyclic CH2SCH2 + H. The channel leading to thioformaldehyde is therefore a minor channel, according to our calculations. The calculated abundance of CH3CHS is well below the observed value (see Fig. 3) by more than four orders of magnitude. The reason for the strong disagreement between the chemical model and the observations is difficult to identify, but it is certainly related to our poor knowledge of the sulfur chemistry. As shown in Fig. 3, the calculated abundance of the ethyl radical (C2H5), which is a potential precursor of both CH3CHO and CH3CHS, is quite low, and this might be at the origin of the low abundance predicted for CH3CHO, although it would hardly explain the four order-of-magnitude disagreement between model and observation for CH3CHS. The formation of thioacetaldehyde in TMC-1 therefore remains unknown. We note that the molecule has been observed as a product in solid-state experiments at 10 K in which SH, C2H2, and H atoms react on ices (Santos et al. 2024). The formation on ices is therefore a possibility, although it is uncertain how it would desorb to the gas phase after formation.
 |
Fig. 3. Calculated fractional abundances of CH3CHO and CH3CHS, together with the abundance of C2H5, shown as a function of time. The horizontal dotted lines correspond to the values observed in TMC-1 adopting the column densities in Table 2 and a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). |
5. Conclusions
We reported the first detection in space of thioacetaldehyde (CH3CHS), the sulfur analog of acetaldehyde (CH3CHO). Thioacetaldehyde was detected toward the cyanopolyyne peak of the starless core TMC-1 using data from the QUIJOTE program of the Yebes 40 m telescope. We derived a column density of 9.8 × 1010 cm−2 for CH3CHS, which means that it is 36 times less abundant than CH3CHO. A comparison of the inventory of O- and S-bearing molecules in TMC-1 revealed a clear trend in which the abundance ratio of a sulfur-bearing molecule and its oxygen counterpart decreases with increasing hydrogenation degree. This probably means that hydrogenation is less favored for S-bearing than for O-bearing molecules.
Methyl mercaptan (CH3SH) is observed in TMC-1 through the A component of the 21, 1–11, 0 line at 50058.809 MHz. The corresponding E component at 50599.288 MHz lies outside the frequency coverage of QUIJOTE. We assumed a rotational temperature of 9 K and a source size diameter of 80″ to derive the column density value given in Table 2.
A notable exception to the trend shown in Fig. 2 is the CS/CO column density ratio, which takes a value of 6.5 × 10−5 in TMC-1.
 |
Fig. 2. Column density ratio between the S-bearing molecule and the O-bearing counterpart in TMC-1 for the series of species HxC1S, HxC2S, and HxC3S as a function of the number of H atoms x. The column density ratio CS/CO, 6.5 × 10−5 (see Table 2), would lie outside the represented region and is not shown. |
Acknowledgments
We acknowledge funding support from Spanish Ministerio de Ciencia, Innovación, y Universidades through grants PID2023-147545NB-I00 and RyC-2022-035442-I and from Consejo Superior de Investigaciones Científicas (CSIC) through projects i-LINK 23017 SENTINEL and Proyecto Intramural 20245AT016. We acknowledge the computational resources provided by the DRAGO computer cluster managed by SGAI-CSIC, and the Galician Supercomputing Center (CESGA). The supercomputer FinisTerrae III and its permanent data storage system have been funded by the Spanish Ministry of Science and Innovation, the Galician Government and the European Regional Development Fund (ERDF).
References
- Agúndez, M., & Wakelam, V. 2013, Chem. Rev., 113, 8710 [Google Scholar]
- Agúndez, M., Marcelino, N., Cernicharo, J., & Tafalla, M. 2018, A&A, 611, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Marcelino, N., Tercero, B., et al. 2021, A&A, 649, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Loison, J.-C., Hickson, K. M., et al. 2023a, A&A, 673, A34 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Marcelino, N., Tercero, B., et al. 2023b, A&A, 677, A106 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Asplund, M., Grevesse, N., Sauval, A. J., & Scott, P. 2009, ARA&A, 47, 481 [NASA ADS] [CrossRef] [Google Scholar]
- Becke, A. D. 1993, J. Chem. Phys., 98, 1372 [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2022, A&A, 657, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Endo, Y., et al. 2024, A&A, 686, L3 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., & Guélin, M. 1987, A&A, 176, 299 [Google Scholar]
- Cernicharo, J., Lefloch, B., Agúndez, M., et al. 2018, ApJ, 853, L22 [CrossRef] [Google Scholar]
- Cernicharo, J., Marcelino, N., Agúndez, M., et al. 2020a, A&A, 642, L17 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Marcelino, N., Agúndez, M., et al. 2020b, A&A, 642, L8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Endo, Y., et al. 2021a, A&A, 646, L3 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2021b, A&A, 648, L3 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Endo, Y., et al. 2021c, A&A, 650, L14 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Cabezas, C., et al. 2021d, A&A, 656, L21 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Kaiser, R. I., et al. 2021e, A&A, 652, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Bailleux, S., et al. 2021f, A&A, 646, L7 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Fuentetaja, R., Agúndez, M., et al. 2022, A&A, 663, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Tercero, B., Marcelino, N., et al. 2023, A&A, 674, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Cabezas, C., et al. 2024a, A&A, 682, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2024b, A&A, 688, L13 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cordiner, M. A., Charnley, S. B., Kisiel, Z., et al. 2017, ApJ, 850, 187 [NASA ADS] [CrossRef] [Google Scholar]
- Enrique-Romero, J., Ceccarelli, C., Rimola, A., et al. 2021, A&A, 655, A9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fedoseev, G., Qasim, D., Chuang, K.-J., et al. 2022, ApJ, 924, 110 [NASA ADS] [CrossRef] [Google Scholar]
- Ferrero, S., Grieco, F., Ibrahim Mohamed, A.-S., et al. 2022, MNRAS, 516, 2586 [CrossRef] [Google Scholar]
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. 2016, Gaussian 16 Revision C.01 (Wallingford CT: Gaussian, Inc.) [Google Scholar]
- Fuente, A., Goicoechea, J. R., Pety, J., et al. 2017, ApJ, 851, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Fuentetaja, R., Agúndez, M., Cabezas, C., et al. 2022, A&A, 667, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Georgievskii, Y., & Klippenstein, S. J. 2013, J. Chem. Phys., 118, 5442 [Google Scholar]
- Gottlieb, C. A. 1973, in Molecules in the Galactic Environment, eds. M. A. Gordon, & L. E. Snyder (New York: John Wiley& Sons), 181 [Google Scholar]
- Grimme, S. 2006, J. Chem. Phys., 124, 034108 [Google Scholar]
- Grimme, S., Antony, J., Ehrlich, S., et al. 2010, J. Chem. Phys., 132, 154104 [CrossRef] [PubMed] [Google Scholar]
- Grimme, S., Ehrlich, S., & Goerigk, L. 2011, J. Comp. Chem., 32, 1456 [CrossRef] [Google Scholar]
- Kendall, R. A., Dunning, T. H., Jr., & Harrison, R. J. 1992, J. Chem. Phys., 96, 6796 [NASA ADS] [CrossRef] [Google Scholar]
- Kolesniková, L., Tercero, B., Cernicharo, J., et al. 2014, ApJ, 784, L7 [Google Scholar]
- Kroto, H. W., & Landsberg, B. M. 1976, J. Mol. Spectr., 62, 346 [NASA ADS] [CrossRef] [Google Scholar]
- Kroto, H. W., Landsberg, B. M., Suffolk, R. J., & Vodden, A. 1974, Chem. Phys. Lett., 29, 265 [NASA ADS] [CrossRef] [Google Scholar]
- Lamberts, T., Markmeyer, M. N., Kolb, F. J., & Kästner, J. 2019, ACS Earth Space Chem., 3, 958 [Google Scholar]
- Loison, J.-C., Agúndez, M., Marcelino, N., et al. 2016, MNRAS, 456, 4101 [Google Scholar]
- Loison, J.-C., Wakelam, V., Gratier, P., et al. 2019, MNRAS, 485, 5777 [Google Scholar]
- Maeda, S., Ohno, K., & Morokuma, K. 2013, Phys. Chem. Chem. Phys., 15, 3683 [NASA ADS] [CrossRef] [Google Scholar]
- Maeda, S., Harabuchi, Y., Saita, K., et al. 2018, J. Comp. Chem., 39, 233 [CrossRef] [Google Scholar]
- Maeda, S., Harabuchi, Y., Sumiya, Y., et al. 2023, GRRM23, https://global.hpc.co.jp/products/grrm23/ [Google Scholar]
- Marcelino, N., Puzzarini, C., Agúndez, M., et al. 2023, A&A, 674, L13 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Marenich, A. V., & Boggs, J. E. 2003, J. Phys. Chem. A, 107, 2343 [CrossRef] [Google Scholar]
- Margulès, L., Ilyushin, V. V., McGuire, B. A., et al. 2020, J. Mol. Spectr., 371, 111304 [CrossRef] [Google Scholar]
- Martín-Domenech, R., Jiménez-Serra, I., Muñoz-Caro, G. M., et al. 2016, A&A, 585, A112 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Matthews, H. E., Friberg, P., & Irvine, W. M. 1985, ApJ, 290, 609 [NASA ADS] [CrossRef] [Google Scholar]
- McGuire, B. A., Burkhardt, A. M., Shingledecker, C. N., et al. 2017, ApJ, 843, L28 [NASA ADS] [CrossRef] [Google Scholar]
- Millar, T. J., Walsh, C., Van de Sande, M., & Markwick, A. J. 2024, A&A, 682, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Molpeceres, G., Kästner, J., Herrero, V. J., et al. 2022a, A&A, 664, A169 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Molpeceres, G., Jiménez-Serra, I., Oba, Y., et al. 2022b, A&A, 663, A41 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Belloche, A., Xu, L.-H., et al. 2016, A&A, 587, A92 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Navarro-Almaida, D., Le Gal, R., Fuente, A., et al. 2020, A&A, 637, A39 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Neese, F., Wennmohs, F., Ute, B., & Riplinger, C. 2020, J. Chem. Phys., 152, 224108 [NASA ADS] [CrossRef] [Google Scholar]
- Oba, Y., Tomaru, T., Lamberts, T., et al. 2018, Nat. Astron., 2, 228 [Google Scholar]
- Puzzarini, C. 2005, J. Chem. Phys., 123, 024313 [NASA ADS] [CrossRef] [Google Scholar]
- Raghavachari, K. G., Trucks, G. W., Pople, J., et al. 1989, Chem. Phys. Lett., 6, 479 [NASA ADS] [CrossRef] [Google Scholar]
- Rey-Montejo, M., Jiménez-Serra, I., Martín-Pintado, J., et al. 2024, ApJ, 975, 174 [NASA ADS] [CrossRef] [Google Scholar]
- Rodríguez-Almeida, L. F., Jiménez-Serra, I., Rivilla, V. M., et al. 2021, ApJ, 912, L11 [Google Scholar]
- Ruffle, D. P., Hartquist, T. W., Caselli, P., & Williams, D. A. 1999, MNRAS, 306, 691 [Google Scholar]
- Santos, J. C., Enrique-Romero, J., Lamberts, T., et al. 2024, ACS Earth Space Chem., 8, 1646 [NASA ADS] [CrossRef] [Google Scholar]
- Sanz-Novo, M., Rivilla, V. M., Müller, H. S. P., et al. 2024, ApJ, 965, L26 [Google Scholar]
- Shingledecker, C. N., Álvarez-Barcia, S., Korn, V. H., & Kästner, J. 2019, ApJ, 878, 80 [Google Scholar]
- Shingledecker, C. N., Banu, T., Kang, Y., et al. 2022, J. Phys. Chem. A, 126, 5343 [NASA ADS] [CrossRef] [Google Scholar]
- Tercero, F., López-Pérez, J. A., Gallego, J. D., et al. 2021, A&A, 645, A37 [EDP Sciences] [Google Scholar]
- Vazart, F., Ceccarelli, C., Balucani, N., et al. 2020, MNRAS, 499, 5547 [Google Scholar]
- Vidal, T. H. G., Loison, J.-C., Jaziri, A. Y., et al. 2017, MNRAS, 469, 435 [Google Scholar]
Appendix A: Quantum chemical calculations of the gas-phase C2H5 + S reaction
We tried to explore the unknown chemistry of CH3CHS by establishing a parallelism with the chemistry of CH3CHO. In our gas-phase model, and as mentioned in the main text, two main reactions contribute to the abundance of CH3CHO, namely
Moving to the S-bearing counterparts, the first reaction is difficult to model, as it requires not only to know the (currently unknown) formation routes of CH3CHSH+, but also the complete electronic structure of the cation to formulate a rate for the decay into the CH3CHS + H channel. An easier task involves the study of the C2H5 + S → CH3CHS + H gas phase reaction, that we studied using quantum chemical calculations. The stationary points of the C2H5 + O reaction were reported in Vazart et al. (2020). We note that in their work, Vazart et al. (2020) also consider the reaction CH3OH + CH, but we discarded this route for CH3CHS because the chemical model indicates that even if it is rapid, its contribution to the formation of CH3CHS would be around four orders of magnitude lower than that of the reaction C2H5 + S.
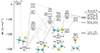 |
Fig. A.1. Energy diagram for the C2H5 + S reaction. Values in square brackets indicate energies for the same well in the C2H5 + O reaction studied in Vazart et al. (2020). Insets correspond to the structures at the potential wells. We note that the products cy-CH2SCH2 + H and C2H3SH + H are almost isoenergetic. |
To obtain the global rate coefficients for the C2H5 + S reaction we reproduced Vazart et al. (2020) calculations in the sulfur atom case. The level of theory for our calculations is selected to be exactly the same as in Vazart et al. (2020), which facilitates any further comparison. This level of theory is CCSD(T)//aug-cc-pVTZ/B2PLYP(D3BJ)/aug-cc-pVTZ (Raghavachari et al. 1989; Kendall et al. 1992; Grimme 2006; Grimme et al. 2010, 2011). Zero point vibrational energies used to correct the electronic energies are also harmonic as in Vazart et al. (2020). Finally, the choice of the code to compute the reaction energetics is made to also be consistent with Vazart et al. (2020), i.e., Gaussian16 (Frisch et al. 2016).
The stationary points that we obtained are gathered in Fig. A.1. Many points are different compared to the potential energy surface (PES) of the oxygen analog reaction studied by Vazart et al. (2020), being the most important ones the several permutations found for the most stable products of the reaction. For example, Vazart et al. (2020) report CH3 elimination, i.e., H2CO + CH3, as the most stable reaction product, whereas in the case of sulfur, C2H4 + SH is the preferred product. Other main changes include the permutation of the stabilities between RI1–RI3 or RI4–RI2, which in turn has an effect on the global rate coefficients, or the different products for TS14, for example. Moreover, one of the characteristic exit channels for the reaction in Vazart et al. (2020), C2H3 + H2O, was not found in the sulfur counterpart (C2H3 + H2S). To confirm this last finding, in addition to test calculations using the equivalent structure with -O we performed additional tests performing a global minima, transition state and dissociation channel search with the single component artificial force induced reaction (SC-AFIR) method (Maeda et al. 2013, 2018) for RI2 and RI3 (the wells conducive to such channel). We used the GRRM code (Maeda et al. 2023) interfaced with ORCA v.5.0.3 (Neese et al. 2020). The search was performed with a model collision parameter γ of 400 kJ mol−1 and the computationally cheaper BHLYP(D3BJ)/6-31+G* model chemistry (Becke 1993; Grimme et al. 2010, 2011). Despite the intensive search, neither the dissociation channel C2H3 + H2S nor their associated TS were found in our exploration.
Values of the fitting parameters for the V(r) attractive potential used in the barrierless association steps of the C2H5 + S reaction. The fit is performed in the range 12-4 Å.
 |
Fig. A.2. Reaction rate coefficients for the reaction C2H5 + S. |
The differences in the energetic profiles reflect on the kinetic behavior of the system. Therefore we computed global reaction rate coefficients for each channel using master equation computations employing RRKM theory to model unimolecular processes with a barrier and classical capture theory in the case of barrierless associations. The latter is obtained by fitting the long range interacion potential to a curve of the type V(r) = − C6/rn functional form, with C6 and n as fitting parameters gathered in Table A.1. Tunneling corrections via an Eckart barrier and symmetry numbers were included, where applicable. The kinetic simulations were performed using the MESS code (Georgievskii & Klippenstein 2013). The temperature dependent rate coefficients are shown in Fig. A.2. As it can be seen from the graph, even though the C2H4 + HS exit channel is the most stable one, it is not the dominant one owing to the competition in RI1 between isomerization to RI2 or RI4 where the latter dominates. Therefore the most dominant channel is H2CS + CH3 as in the case of the O- bearing molecule, followed by C2H4 + HS in a variable proportion within the order of magnitude. The rest of the products, including the molecule subject of this study, CH3CHS, are minor products of this reaction. This indicates that other pathways need to be explored to explain the presence of this molecule in TMC-1. Nevertheless, fitting parameters for easy inclusion of this reaction in astrochemical models, along with branching ratios at 40 K are given for every reaction channel in Table A.2.
Modified Arrhenius parameters a for the fit of the global rate coefficients considered in this section over the temperature range 40-400 K. Branching ratios (BR) are calculated from the ratio between an individual rate coefficient and the total loss rate at 40 K (obtained from the exact values and not from the fit). The A(B) notation indicates AB.
All Tables
Values of the fitting parameters for the V(r) attractive potential used in the barrierless association steps of the C2H5 + S reaction. The fit is performed in the range 12-4 Å.
Modified Arrhenius parameters a for the fit of the global rate coefficients considered in this section over the temperature range 40-400 K. Branching ratios (BR) are calculated from the ratio between an individual rate coefficient and the total loss rate at 40 K (obtained from the exact values and not from the fit). The A(B) notation indicates AB.
All Figures
 |
Fig. 1. Lines of CH3CHS observed in TMC-1 (black histogram). The negative features correspond to artifacts caused by the frequency-switching technique. The line parameters are given in Table 1. In magenta we show the LTE calculated line profiles adopting a column density of 9.8 × 1010 cm−2, a rotational temperature of 9.0 K, a full width at half maximum of 0.76 km s−1 (the average of the values observed for the seven detected components; see Table 1), and a circular emission distribution with a diameter of 80″. |
| In the text | |
 |
Fig. 3. Calculated fractional abundances of CH3CHO and CH3CHS, together with the abundance of C2H5, shown as a function of time. The horizontal dotted lines correspond to the values observed in TMC-1 adopting the column densities in Table 2 and a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). |
| In the text | |
 |
Fig. 2. Column density ratio between the S-bearing molecule and the O-bearing counterpart in TMC-1 for the series of species HxC1S, HxC2S, and HxC3S as a function of the number of H atoms x. The column density ratio CS/CO, 6.5 × 10−5 (see Table 2), would lie outside the represented region and is not shown. |
| In the text | |
 |
Fig. A.1. Energy diagram for the C2H5 + S reaction. Values in square brackets indicate energies for the same well in the C2H5 + O reaction studied in Vazart et al. (2020). Insets correspond to the structures at the potential wells. We note that the products cy-CH2SCH2 + H and C2H3SH + H are almost isoenergetic. |
| In the text | |
 |
Fig. A.2. Reaction rate coefficients for the reaction C2H5 + S. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.