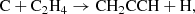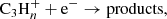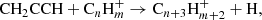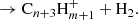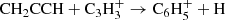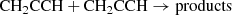| Issue |
A&A
Volume 647, March 2021
|
|
|---|---|---|
| Article Number | L10 | |
| Number of page(s) | 6 | |
| Section | Letters to the Editor | |
| DOI | https://doi.org/10.1051/0004-6361/202140553 | |
| Published online | 18 March 2021 | |
Letter to the Editor
Discovery of the propargyl radical (CH2CCH) in TMC-1: One of the most abundant radicals ever found and a key species for cyclization to benzene in cold dark clouds⋆
1
Instituto de Física Fundamental, CSIC, Calle Serrano 123, 28006 Madrid, Spain
e-mail: marcelino.agundez@csic.es, jose.cernicharo@csic.es
2
Observatorio Astronómico Nacional, IGN, Calle Alfonso XII 3, 28014 Madrid, Spain
3
Observatorio de Yebes, IGN, Cerro de la Palera s/n, 19141 Yebes, Guadalajara, Spain
Received:
12
February
2021
Accepted:
5
March
2021
We present the first identification in interstellar space of the propargyl radical (CH2CCH). This species was observed in the cold dark cloud TMC-1 using the Yebes 40 m telescope. The six strongest hyperfine components of the 20, 2–10, 1 rotational transition, lying at 37.46 GHz, were detected with signal-to-noise ratios from 4.6σ to 12.3σ. We derived a column density of 8.7 × 1013 cm−2 for CH2CCH, which translates to a fractional abundance relative to H2 of 8.7 × 10−9. This radical has a similar abundance as methyl acetylene, with an abundance ratio CH2CCH/CH3CCH close to one. The propargyl radical is thus one of the most abundant radicals detected in TMC-1, and it is probably the most abundant organic radical with a certain chemical complexity ever found in a cold dark cloud. We constructed a gas-phase chemical model and find calculated abundances that agree with, or fall two orders of magnitude below, the observed value depending on the poorly constrained low-temperature reactivity of CH2CCH with neutral atoms. According to the chemical model, the propargyl radical is essentially formed by the C + C2H4 reaction and by the dissociative recombination of C3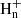 ions with n = 4−6. The propargyl radical is believed to control the synthesis of the first aromatic ring in combustion processes, and it probably plays a key role in the synthesis of large organic molecules and cyclization processes to benzene in cold dark clouds.
ions with n = 4−6. The propargyl radical is believed to control the synthesis of the first aromatic ring in combustion processes, and it probably plays a key role in the synthesis of large organic molecules and cyclization processes to benzene in cold dark clouds.
Key words: astrochemistry / line: identification / molecular processes / ISM: molecules / radio lines: ISM
© ESO 2021
1. Introduction
Cold dark clouds such as TMC-1 have been revealed as extraordinary chemical laboratories that are able to synthesize a great variety of molecules in situ. Most detected species are neutral. Some cations have been detected, mostly protonated forms of closed-shell abundant molecules (e.g., Agúndez et al. 2015a; Marcelino et al. 2020; Cernicharo et al. 2020a, 2021a,b), and a few hydrocarbon and nitrile anions have also been observed (e.g., Cernicharo et al. 2020b), but the vast majority of the species detected are electrically neutral. In general, molecular ions are observed with low abundances, below 10−10 relative to H2, due to their high reactivity.
A large fraction of the neutral species observed in cold dark clouds, and the most abundant ones, are closed-shell molecules. Among them, the long known unsaturated carbon chains stand out as the most prevalent type of molecules (Agúndez & Wakelam 2013). However, in recent times, it has been found that cold dark clouds also contain a variety of organic molecules of increasing complexity, going from the nearly saturated propylene (Marcelino et al. 2007), various isomers of the partially saturated molecules C4H4, C5H4, C4H3N, and C5H3N (Cernicharo et al. 2021c,d; Marcelino et al. 2021; McGuire et al. 2020; Lee et al. 2021), the five-membered ring C5H5CN (McCarthy et al. 2020), and the aromatic ring C6H5CN (McGuire et al. 2018; Burkhardt et al. 2021). These detections reveal that there are chemical processes that are not yet well characterized, which are able to synthesize large complex organic molecules under very cold conditions.
About two-thirds of the neutral species detected in these cold environments are open-shell radicals. With the exception of a few small radicals, such as OH, CH, C2H, C4H, and NO, observed radicals have low abundances because, as ions, they are highly reactive species (see, e.g., Agúndez & Wakelam 2013). In addition, they tend to suffer from spectral dilution due to line splitting resulting from the coupling of the electron spin with the rotation and, also often, with the spin of nuclei. These facts complicate the detection of radicals in cold clouds. Recent examples of detections of radicals are HCCO, HCS, and NCO (Agúndez et al. 2015b, 2018; Marcelino et al. 2018). However, the chemical routes to form molecules of increasing complexity are made of reactions involving reactive species such as radicals and ions. Detecting them is therefore of paramount importance to unveil the synthetic pathways postulated by chemical models. Here we report the detection of the propargyl radical (CH2CCH) in TMC-1. This species is one of the most abundant radicals ever found in cold dark clouds, and it is a potential key intermediate in the formation of complex organic molecules such as aromatic rings.
2. Observations
The propargyl radical (CH2CCH) is an asymmetric rotor, with a planar geometry, C2v symmetry, and a ground electronic state 2B1. Calculations indicate that this radical has a very small electric dipole moment of 0.14 D, according to Botschwina et al. (1995), and 0.115 D according to Woon & Herbst (2009). This is something that was confirmed experimentally by measuring it to be 0.150 D (Küpper et al. 2002). In spite of this small dipole moment, Tanaka et al. (1997) were able to measure, in the laboratory, the fine and hyperfine structure of various rotational transitions lying at 18.7 GHz and in the 37−38 GHz range, with frequency uncertainties of a few kilohertz.
Based on their laboratory measurements, Tanaka et al. (1997) searched for the 20, 2–10, 1 transition of CH2CCH, lying at 37.46 GHz, toward TMC-1 using the Nobeyama 45 m telescope. They did not detect the radical after an integration time of 6 h in which they reached a noise level of 12 mK per 37 kHz channel. Our line survey of TMC-1 carried out with the Yebes 40 m telescope has a much higher sensitivity, with a 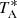 rms noise level of 0.30 mK per 38.15 kHz channel in the region around 37.46 GHz, which has enabled a robust detection of the propargyl radical.
rms noise level of 0.30 mK per 38.15 kHz channel in the region around 37.46 GHz, which has enabled a robust detection of the propargyl radical.
The data in which the detection of CH2CCH is based are part of a line survey of TMC-1 in the Q band carried out with the Yebes 40 m telescope. We used the cryogenic receiver for the Q band, built within the Nanocosmos project1, which covers the 31.0−50.4 GHz frequency range with horizontal and vertical polarizations. Receiver temperatures vary from 17 K at 32 GHz to 25 K at 50 GHz. The spectrometers are FFTS, which cover a bandwidth of 8 × 2.5 GHz in each polarization with a spectral resolution of 38.15 kHz. The system is described in detail by Tercero et al. (2021). The line survey was carried out during various observing sessions and several results have already been published. Results such as the detection of the negative ions C3N− and C5N− (Cernicharo et al. 2020b), the discoveries of HC4NC (Cernicharo et al. 2020c), HC3O+ (Cernicharo et al. 2020a), and HC5NH+ (Marcelino et al. 2020) were based on the data taken during November 2019 and February 2020. An additional observing run was carried out in October 2020, which allowed us to improve the sensitivity and resulted in the detection of HDCCN (Cabezas et al. 2021), HC3S+ (Cernicharo et al. 2021a), and CH3CO+ (Cernicharo et al. 2021b), and the observational study of C4H3N isomers (Marcelino et al. 2021). A final observing run was performed in December 2020 and January 2021, resulting in the discovery of CH2CHCCH (Cernicharo et al. 2021c) and CH2CCHCCH (Cernicharo et al. 2021d). All observations were carried out using the frequency switching technique, with a frequency throw of 10 MHz during the two first observing runs and 8 MHz in the later ones. The intensity scale, the antenna temperature 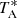 , was calibrated using two absorbers at different temperatures and the atmospheric transmission model (ATM; Cernicharo 1985; Pardo et al. 2001). The uncertainty in
, was calibrated using two absorbers at different temperatures and the atmospheric transmission model (ATM; Cernicharo 1985; Pardo et al. 2001). The uncertainty in 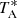 is estimated to be around 10%. To convert to main beam brightness temperature (Tmb), one has to divide
is estimated to be around 10%. To convert to main beam brightness temperature (Tmb), one has to divide 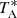 by Beff/Feff. The parameters of the Yebes 40 m antenna2 at the frequency of interest here, 37.46 GHz, are Beff = 0.56 and Feff = 0.97, while the half power beam width (HPBW) is 47.8″. All data have been reduced with the program CLASS from the GILDAS software package3.
by Beff/Feff. The parameters of the Yebes 40 m antenna2 at the frequency of interest here, 37.46 GHz, are Beff = 0.56 and Feff = 0.97, while the half power beam width (HPBW) is 47.8″. All data have been reduced with the program CLASS from the GILDAS software package3.
3. Results
The spectrum of TMC-1 at 37.46 GHz has a 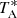 rms noise level of 0.30 mK and shows various emission lines which can be assigned to the six strongest hyperfine components of the 20, 2–10, 1 transition of ortho CH2CCH (see Fig. 1). The energy levels, transition frequencies, and line strengths of CH2CCH were taken from the Cologne Database for Molecular Spectroscopy (CDMS; Müller et al. 2005)4, which is based on a fit to the laboratory frequencies measured by Tanaka et al. (1997), and is implemented in MADEX (Cernicharo 2012). We considered the ortho and para species separately and adopted a dipole moment of 0.150 D, as measured by Küpper et al. (2002).
rms noise level of 0.30 mK and shows various emission lines which can be assigned to the six strongest hyperfine components of the 20, 2–10, 1 transition of ortho CH2CCH (see Fig. 1). The energy levels, transition frequencies, and line strengths of CH2CCH were taken from the Cologne Database for Molecular Spectroscopy (CDMS; Müller et al. 2005)4, which is based on a fit to the laboratory frequencies measured by Tanaka et al. (1997), and is implemented in MADEX (Cernicharo 2012). We considered the ortho and para species separately and adopted a dipole moment of 0.150 D, as measured by Küpper et al. (2002).
 |
Fig. 1. Spectrum of TMC-1 taken with the Yebes 40 m telescope around 37.46 GHz (black histogram). The frequencies of the six most intense hyperfine components of the 20, 2–10, 1 transition of ortho CH2CCH are indicated by red dotted vertical lines. Transition quantum numbers, frequencies, and derived line parameters are given in Table 1. The synthetic spectrum (red line) was computed for a column density of ortho CH2CCH of 6.5 × 1013 cm−2, a rotational temperature of 10 K, an emission size of 40″ of radius, and a linewidth of 0.72 km s−1 (see main text). |
The six observed lines are precisely centered at the calculated frequencies from the CDMS, adopting a systemic velocity of 5.83 km s−1 for TMC-1 (Cernicharo et al. 2020c). The values of VLSR derived are very close to this value (see Table 1), with deviations in frequency of 32 kHz for one line and < 20 kHz for the rest, which is within the uncertainty given by the spectral resolution of 38.15 kHz and the error in the Gaussian fit. The six lines are also detected at a significant level between 4.6σ and 12.3σ (see Table 1). We therefore consider that the detection of CH2CCH in TMC-1 is robust.
Observed line parameters of CH2CCH in TMC-1.
To further support the detection of the propargyl radical in TMC-1, we calculated a synthetic spectrum. The observed lines have similar upper level energies and thus it is not possible to derive a rotational temperature. Nevertheless, given the low dipole moment of CH2CCH (0.150 D), which results in low critical densities (probably as low as a few 102 cm−3), we can safely assume that rotational levels are thermalized and the rotational temperature is equal to the kinetic temperature of ∼10 K (Fehér et al. 2016). To compute the synthetic spectrum, we adopted a full width at half maximum (FWHM) of 0.72 km s−1, which is the arithmetic mean of the values derived from the six observed lines (see Table 1), and assumed that the emission from CH2CCH is distributed in the sky as a circle with a radius of 40″, as observed for various hydrocarbons in TMC-1 (Fossé et al. 2001). The observed intensities are reproduced with a column density of ortho CH2CCH of 6.5 × 1013 cm−2. As shown in Fig. 1, the computed relative and absolute intensities of the six hyperfine components agree with the observed ones well. It is also seen that weaker components are not detected because the calculated intensities are within the noise of the spectrum. That is to say, there are no missing hyperfine components from the 20, 2–10, 1 transition.
There are two rotational transitions of para CH2CCH covered by our Q band line survey of TMC-1, the 21, 2–11, 1 and the 21, 1–11, 0, lying a 37.2 GHz and 37.8 GHz, respectively. The ground state of the para species is located 14.3 K above the ground state of the ortho species. Treating ortho and para as separate species and assuming an ortho-to-para ratio equal to the statistical value of 3, the most intense component of these two transitions was calculated with 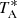 = 0.5 mK, which is within the noise in these spectral regions. A deeper integration that reduces the noise level should allow one to detect these lines. Therefore, the nondetection of these two transitions of para CH2CCH is consistent with the detection of the transition of ortho CH2CCH shown in Fig. 1. Assuming an ortho-to-para ratio of three, the column density derived for CH2CCH (including ortho and para species) in TMC-1 is 8.7 × 1013 cm−2. Adopting a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987), the fractional abundance of CH2CCH relative to H2 is 8.7 × 10−9.
= 0.5 mK, which is within the noise in these spectral regions. A deeper integration that reduces the noise level should allow one to detect these lines. Therefore, the nondetection of these two transitions of para CH2CCH is consistent with the detection of the transition of ortho CH2CCH shown in Fig. 1. Assuming an ortho-to-para ratio of three, the column density derived for CH2CCH (including ortho and para species) in TMC-1 is 8.7 × 1013 cm−2. Adopting a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987), the fractional abundance of CH2CCH relative to H2 is 8.7 × 10−9.
4. Discussion
It is remarkable that the propargyl radical is one of the most abundant radicals ever found in TMC-1. Only a few simple radicals, such as OH, CH, C4H, and NO, have been detected with abundances above that derived for CH2CCH (see, e.g., Agúndez & Wakelam 2013). Moreover, CH2CCH has an abundance similar to its closed-shell counterpart CH3CCH, which has a column density of (1.1−1.3) × 1014 cm−2 (Gratier et al. 2016; Cabezas et al. 2021). Therefore, the abundance ratio CH2CCH/CH3CCH is close to one. This fact is unusual since in TMC-1, in particular, and in cold dark clouds, in general, a radical resulting from removing one hydrogen atom from a closed-shell molecule is usually less abundant than the corresponding closed-shell molecule. The only other example of a radical being more abundant than the corresponding closed-shell molecule is the cyanomethyl radical, in which case the abundance ratio CH2CN/CH3CN in TMC-1 is in the range from 3 to 9 (Gratier et al. 2016; Cabezas et al. 2021). The large abundance of CH2CCH and the fact that it is as abundant as, but more reactive than, CH3CCH makes this radical a very interesting intermediate in the build-up of chemical complexity in cold dark clouds such as TMC-1.
To get insight into the chemical implications of the detection of the propargyl radical in TMC-1, we carried out chemical model calculations. We are interested in understanding the following aspects: whether the high abundance derived for CH2CCH can be accounted for by a gas-phase chemical model, the main formation and destruction pathways of CH2CCH, and which kind of chemical routes are opened by the presence of this abundant radical. We adopted typical parameters of cold dark clouds, that is to say a gas kinetic temperature of 10 K, a volume density of H2 of 2 × 104 cm−3, a visual extinction of 30 mag, a cosmic-ray ionization rate of H2 of 1.3 × 10−17 s−1, and the set of “low-metal” elemental abundances (see, e.g., Agúndez & Wakelam 2013). We used the gas-phase chemical network RATE12 from the UMIST database (McElroy et al. 2013), where we have revised the reactions involved in the formation and destruction of CH2CCH and the related molecules CH3CCH and CH2CCH2 (see Table A.1).
The abundance calculated for the propargyl radical is shown in Fig. 2, together with those of methyl acetylene and allene. It can be seen that the calculated abundances of CH3CCH and CH2CCH2 are very similar at any time, which points to allene being as abundant as methyl acetylene in cold dark clouds. Moreover, the peak abundance of CH3CCH is close to the observed value. In the case of the radical CH2CCH, the calculated abundance lies well below the observed value and the CH2CCH/CH3CCH abundance ratio falls well below one, in contrast with observations. To better understand the behavior of the calculated abundances, it is useful to look at the main formation and destruction processes of each species in the chemical model. The propargyl radical is mostly formed through the neutral-neutral reaction
 |
Fig. 2. Calculated fractional abundances of CH2CCH and related molecules as a function of time. The black dashed line corresponds to the calculated abundance of CH2CCH when the reactions of this radical with O and N atoms were removed. The abundances observed in TMC-1 are indicated by dotted horizontal lines. |
which was measured to be rapid down to 15 K (Chastaing et al. 1999) and yields propargyl radical as a main product, as indicated by extensive experimental and theoretical evidence (Kaiser et al. 1996; Bergeat & Loison 2001; Gepper et al. 2003; Chin et al. 2012; Mandal et al. 2018). A second formation pathway, which is also the main route to the two C3H4 isomers, is provided by the dissociative recombination of C3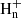 ions with electrons
ions with electrons
with n = 4−7, since ions with n > 7 are not included in the chemical network. Information on the product branching ratios is available to different degrees of detail for the dissociative recombination of the different ions C3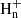 (Angelova et al. 2004; Gepper et al. 2004; Ehlerding et al. 2003; Larsson et al. 2005). For example, it is known that the CH2CCH radical is the main product in the dissociative recombination of C3
(Angelova et al. 2004; Gepper et al. 2004; Ehlerding et al. 2003; Larsson et al. 2005). For example, it is known that the CH2CCH radical is the main product in the dissociative recombination of C3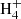 (Gepper et al. 2004), while it does not form at all during the dissociative recombination of C3
(Gepper et al. 2004), while it does not form at all during the dissociative recombination of C3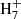 (Ehlerding et al. 2003; Larsson et al. 2005). Thus, the ions C3
(Ehlerding et al. 2003; Larsson et al. 2005). Thus, the ions C3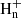 with n = 4−6 contribute to the formation of CH2CCH, while the closed-shell hydrocarbons CH3CCH and CH2CCH2 are essentially formed upon the dissociative recombination of C3
with n = 4−6 contribute to the formation of CH2CCH, while the closed-shell hydrocarbons CH3CCH and CH2CCH2 are essentially formed upon the dissociative recombination of C3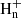 ions with n = 5−7 (Ehlerding et al. 2003; Angelova et al. 2004; Larsson et al. 2005). The synthesis of the ions C3
ions with n = 5−7 (Ehlerding et al. 2003; Angelova et al. 2004; Larsson et al. 2005). The synthesis of the ions C3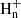 occurs through a series of reactions involving cations (see, e.g., Herbst & Leung 1989).
occurs through a series of reactions involving cations (see, e.g., Herbst & Leung 1989).
Reactions with neutral atoms are the main destruction process for the propargyl radical and the closed-shell molecules methyl acetylene and allene. There is experimental evidence that C atoms react quickly with CH3CCH and CH2CCH2 (Loison & Bergeat 2004), although these hydrocarbons do not react rapidly with either O or N atoms at low temperatures due to the presence of activation barriers (Adusei et al. 1996; Michael & Lee 1977). Information on the reactivity of CH2CCH with neutral atoms is more limited. The propargyl radical reacts quickly with O atoms at room and high temperature (Slagle et al. 1991), although it is not known whether this behavior is maintained at very low temperatures. The radical is also thought to react quickly with C and N atoms (Smith et al. 2004; Loison et al. 2017), although there is no experimental or theoretical evidence. The reaction between CH2CCH and H atoms has an activation barrier to produce any of the C3H2 isomers (Miller & Klippenstein 2003), and we assume that this reaction does not occur at 10 K. Reactions with O and N atoms are one of the main destruction channels of CH2CCH in the chemical model and the main cause of the low calculated abundance of this radical. If we assume that, as the C3H4 isomers, the propargyl radical does not react with O or N atoms, then the calculated abundance of CH2CCH experiences an important enhancement, approaching those of the C3H4 isomers and becoming closer to the observed value (see black dashed line in Fig. 2). A study of the reactions of CH2CCH with O and N atoms at low temperatures would certainly shed light on the chemistry of this important radical. If these reactions are found to be rapid at low temperatures, then a powerful formation mechanism of propargyl, in addition to reactions (1) and (2), would be needed to explain the high abundance of CH2CCH observed in TMC-1. Alternatively, the gas-phase abundance of O atoms in TMC-1 could be lower than given by the chemical model if oxygen is significantly depleted on grains. If the C/O gas-phase elemental ratio is assumed to be above one, then the calculated abundance of propargyl increases up to the observed value. However, the abundances of other C-bearing molecules also increase in many cases well above the observed values (see, e.g., Agúndez & Wakelam 2013).
Reactions involving CH2CCH can be an important source of organic molecules of a certain chemical complexity, including aromatic rings under cold conditions. As a matter of fact, CH2CCH is one of the most abundant radicals found in TMC-1 and, given its radical nature, it is expected to show an enhanced reactivity compared to closed-shell molecules. On the other hand, CH2CCH is a resonance-stabilized radical because it has a π-conjugated system (see discussion by Tanaka et al. 1997), which makes it less reactive than other radicals that do not have electron delocalization.
An illustration of a route to complex molecules under cold conditions is provided by the reactions of C3H4 isomers with CN and C2H radicals (Carty et al. 2001; Balucani et al. 2002; Zhang et al. 2009), which are thought to form C4H3N and C5H4 isomers, respectively, in TMC-1 (Marcelino et al. 2021; Cernicharo et al. 2021d). Similarly, reactions of the C3H3 radical with HCN and C2H2 could do the same job producing C4H3N and C5H4 isomers, respectively, with an even higher efficiency, given that C3H3/C3H4 ∼ 1 and HCN/CN > 1 (and probably C2H2/C2H > 1). However, the propargyl radical does not react with either C2H2 or C4H2 at low temperatures due to the existence of activation barriers (da Silva 2017; Indarto 2011). Therefore, reactions of CH2CCH with polyynes and cyanopolyynes are probably not efficient to form complex molecules in cold dark clouds. Reactions of CH2CCH with abundant radicals, such as CN, C2H, and C4H, could however be efficient in producing complex molecules if they are rapid at low temperatures, given the high abundance of the reactants involved. Information on the kinetics of these reactions is, however, not available. Another route to form large molecules from the propargyl radical consists of condensation reactions with hydrocarbon ions of the type
These reactions were assumed to proceed quickly by Herbst & Leung (1989), although some empirical or theoretical evidence is needed to verify this hypothesis. In particular, it will be of great interest to investigate if these kinds of reactions can lead to aromatic rings.
The CH2CCH radical has a key role in the synthesis of benzene and polycyclic aromatic hydrocarbons (PAHs) in combustion processes, where it is thought that the reaction of two propargyl radicals is the main route to the formation of the first aromatic ring (Miller & Melius 1992). The recent detection of benzonitrile (C6H5CN) in TMC-1 (McGuire et al. 2018) and the experimental evidence that it is most likely formed from benzene reacting with CN (Cooke et al. 2020) highlights that benzene, and perhaps PAHs as well, are formed in situ in cold dark clouds. These environments have conditions very different to those of combustion flames, where the pressures and temperatures allow three-body recombination processes and reactions with activation barriers to proceed efficiently. However, the detection of abundant propargyl in TMC-1 presented here suggests that this radical could play a key role in the cyclization toward the first aromatic ring, as it does in combustion chemistry.
Herbst & Leung (1989) consider that the reaction
proceeds quickly, resulting in the cyclic species C6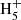 , which can then lead to benzene after radiative association with H2 and the dissociative recombination of the ion C6H
, which can then lead to benzene after radiative association with H2 and the dissociative recombination of the ion C6H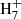 with electrons (McEwan et al. 1999). Reaction (4) is included in our chemical model and it turns out to be one of the main sources of C6
with electrons (McEwan et al. 1999). Reaction (4) is included in our chemical model and it turns out to be one of the main sources of C6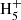 , and thus of benzene, in cold dark clouds. In the protoplanetary nebula CRL 618, the synthesis of benzene, detected by Cernicharo et al. (2001), also relies on the precursor ion C6
, and thus of benzene, in cold dark clouds. In the protoplanetary nebula CRL 618, the synthesis of benzene, detected by Cernicharo et al. (2001), also relies on the precursor ion C6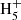 , although in that case it is mainly formed through the radiative association of C2H2 and C4
, although in that case it is mainly formed through the radiative association of C2H2 and C4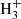 (Woods et al. 2002).
(Woods et al. 2002).
The formation of benzene could also occur through the reaction CH2CCH + C3H4 → C6H6 + H, although kinetic modeling of combustion experiments point to the presence of activation barriers for the two isomers of C3H4 (Faravelli et al. 2000). It is unknown if reactions of CH2CCH with other closed-shell hydrocarbons, such as the recently discovered C4H4 and C5H4 isomers (Cernicharo et al. 2021c,d), could lead to cyclization without an activation barrier. The self-reaction of propargyl radicals
is a very attractive candidate to form benzene in cold dark clouds. Reaction (5) has been widely studied for conditions relevant to combustion experiments, as it is believed to be the key cyclization step to benzene and PAHs in flames. Reaction (5) is relatively fast at room temperature, with a slight negative dependence on temperature, and a variety of products such as benzene, fulvene (c–C5H4 = CH2), and C6H5 + H, with branching ratios depending on the temperature (Miller & Klippenstein 2001; Shafir et al. 2003). It would be of great interest to study the behavior of reaction (5) at low temperatures. In particular, it would be interesting to see if the channels leading directly to benzene through a radiative association and to C6H5 + H are open at the low temperatures of cold dark cloud conditions.
5. Conclusions
We have reported the detection of the propargyl radical (CH2CCH) in the cold dark cloud TMC-1. The high column density derived, 8.7 × 1013 cm−2, is similar to that of the closed-shell counterpart CH3CCH, making it one of the most abundant radicals detected in TMC-1. Due to its high abundance, it probably plays a key role in the synthesis of large organic molecules. In particular, it could be the key species in the cyclization to form aromatic rings similar to benzene in cold dark clouds.
Acknowledgments
We acknowledge the anonymous referee for a constructive report. We acknowledge funding support from Spanish MICIU through grants AYA2016-75066-C2-1-P, PID2019-106110GB-I00, and PID2019-107115GB-C21 and from the European Research Council (ERC Grant 610256: NANOCOSMOS). M.A. also acknowledges funding support from the Ramón y Cajal programme of Spanish MICIU (grant RyC-2014-16277).
References
- Adusei, G. Y., Blue, A. S., & Fontijn, A. 1996, J. Phys. Chem., 100, 16921 [Google Scholar]
- Agúndez, M., & Wakelam, V. 2013, Chem. Rev., 113, 8710 [Google Scholar]
- Agúndez, M., Cernicharo, J., de Vicente, P., et al. 2015a, A&A, 579, L10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Cernicharo, J., & Guélin, M. 2015b, A&A, 577, L5 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Marcelino, N., Cernicharo, J., & Tafalla, M. 2018, A&A, 611, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Angelova, G., Novotny, O., Mitchell, J. B. A., et al. 2004, Int. J. Mass Spectrom., 235, 7 [Google Scholar]
- Balucani, N., Asvany, O., Kaiser, R. I., & Osamura, Y. 2002, J. Phys. Chem. A, 106, 4301 [CrossRef] [Google Scholar]
- Baulch, D. L., Bowman, C. T., Cobos, C. J., et al. 2005, J. Phys. Chem. Ref. Data, 34, 757 [NASA ADS] [CrossRef] [Google Scholar]
- Bergeat, A., & Loison, J.-C. 2001, Phys. Chem. Chem. Phys., 3, 2038 [CrossRef] [Google Scholar]
- Botschwina, P., Oswald, R., Flügge, J., & Horn, M. 1995, Z. Phys. Chem., 188, 29 [Google Scholar]
- Bouwman, J., Goulay, F., Leone, S. R., & Wilson, K. R. 2012, J. Phys. Chem. A, 116, 3907 [CrossRef] [Google Scholar]
- Burkhardt, A. M., Loomis, R. A., Shingledecker, C. N., et al. 2021, Nat. Astron., 5, 181 [Google Scholar]
- Cabezas, C., Endo, Y., Roueff, E., et al. 2021, A&A, 646, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Canosa, A., Sims, I. R., Travers, D., et al. 1997, A&A, 323, 644 [Google Scholar]
- Canosa, A., Páramo, A., Le Picard, S., & Sims, I. R. 2007, Icarus, 187, 558 [NASA ADS] [CrossRef] [Google Scholar]
- Carty, D., Le Page, V., Sims, I. R., & Smith, I. W. M. 2001, Chem. Phys. Lett., 344, 310 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J. 1985, IRAM Internal Report 52 [Google Scholar]
- Cernicharo, J. 2012, in European Conference on Laboratory Astrophysics, eds. C. Stehlé, C. Joblin, & L. d’Hendecourt, EAS Publ. Ser., 58, 251 [Google Scholar]
- Cernicharo, J., & Guélin, M. 1987, A&A, 176, 299 [Google Scholar]
- Cernicharo, J., Heras, A. M., Tielens, A. G. G. M., et al. 2001, ApJ, 546, L123 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Marcelino, N., Agúndez, M., et al. 2020a, A&A, 642, L17 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Marcelino, N., Pardo, J. R., et al. 2020b, A&A, 641, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Marcelino, N., Agúndez, M., et al. 2020c, A&A, 642, L8 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Endo, Y., et al. 2021a, A&A, 646, L3 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Bailleux, S., et al. 2021b, A&A, 646, L7 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Cabezas, C., et al. 2021c, A&A, 647, L2 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2021d, A&A, 647, L3 [EDP Sciences] [Google Scholar]
- Chastaing, D., James, P. L., Sims, I. R., & Smith, I. W. M. 1999, Phys. Chem. Chem. Phys., 1, 2247 [Google Scholar]
- Chin, C.-H., Chen, W.-K., Huang, W.-J., et al. 2012, J. Phys. Chem. A, 116, 7615 [Google Scholar]
- Cooke, I. R., Gupta, D., Messinger, J. P., & Sims, I. R. 2020, ApJ, 891, L41 [Google Scholar]
- da Silva, G. 2017, J. Phys. Chem. A, 121, 2086 [Google Scholar]
- Ehlerding, A., Arnold, S. T., & Viggiano, A. A. 2003, J. Phys. Chem. A, 107, 2179 [CrossRef] [Google Scholar]
- Faravelli, T., Goldaniga, A., Zappella, L., et al. 2000, Proc. Combust. Inst., 28, 2601 [Google Scholar]
- Fehér, O., Tóth, L. V., Ward-Thompson, D., et al. 2016, A&A, 590, A75 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fossé, D., Cernicharo, J., Gerin, M., & Cox, P. 2001, ApJ, 552, 168 [Google Scholar]
- Gepper, W. D., Naulin, C., Costes, M., et al. 2003, J. Chem. Phys., 119, 10607 [Google Scholar]
- Gepper, W. D., Thomas, R., Ehlerding, A., et al. 2004, Int. J. Mass Spectrom., 237, 25 [Google Scholar]
- Goulay, F., Trevitt, A. J., Meloni, G., et al. 2009, J. Am. Chem. Soc., 131, 993 [CrossRef] [Google Scholar]
- Gratier, P., Majumdar, L., Ohishi, M., et al. 2016, ApJS, 225, 25 [Google Scholar]
- Herbst, E., & Leung, C. M. 1989, ApJS, 69, 271 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Indarto, A. 2011, Jurnal Teknik Kimia Indonesia, 10, 81 [Google Scholar]
- Kaiser, R. I., Lee, Y. T., & Suits, A. G. 1996, J. Chem. Phys., 105, 8705 [NASA ADS] [CrossRef] [Google Scholar]
- Küpper, J., Merritt, J. M., & Miller, R. E. 2002, J. Chem. Phys., 117, 647 [Google Scholar]
- Larsson, M., Ehlerding, A., Geppert, W. D., et al. 2005, J. Chem. Phys., 122 [Google Scholar]
- Lee, K. L. K., Loomis, R. A., Burkhardt, A. M., et al. 2021, J. Chem. Phys., 908, L11 [Google Scholar]
- Loison, J.-C., & Bergeat, A. 2004, Phys. Chem. Chem. Phys., 6, 5396 [CrossRef] [Google Scholar]
- Loison, J.-C., Agúndez, M., Wakelam, V., et al. 2017, MNRAS, 470, 4075 [NASA ADS] [CrossRef] [Google Scholar]
- Mandal, M., Ghosh, S., & Maiti, B. 2018, J. Phys. Chem. A, 122, 3556 [Google Scholar]
- Marcelino, N., Cernicharo, J., Agúndez, M., et al. 2007, ApJ, 665, L127 [Google Scholar]
- Marcelino, N., Agúndez, M., Cernicharo, J., et al. 2018, A&A, 612, L10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Marcelino, N., Agúndez, M., Tercero, B., et al. 2020, A&A, 643, L6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Marcelino, N., Tercero, B., Agúndez, M., & Cernicharo, J. 2021, A&A, 646, L7 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- McCarthy, M. C., Lee, K. L. K., Loomis, R. A., et al. 2020, Nat. Astron., 5, 176 [Google Scholar]
- McElroy, D., Walsh, C., Markwick, A. J., et al. 2013, A&A, 550, A36 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- McEwan, M. J., Scott, G. B. I., Adams, N. G., et al. 1999, ApJ, 513, 287 [NASA ADS] [CrossRef] [Google Scholar]
- McGuire, B. A., Burkhardt, A. M., Kalenskii, S., et al. 2018, Science, 359, 202 [Google Scholar]
- McGuire, B. A., Burkhardt, A. M., Loomis, R. A., et al. 2020, ApJ, 900, L10 [Google Scholar]
- Michael, J. V., & Lee, J. H. 1977, Chem. Phys. Lett., 51, 303 [Google Scholar]
- Miller, J. A., & Klippenstein, S. J. 2001, J. Phys. Chem. A, 105, 7254 [Google Scholar]
- Miller, J. A., & Klippenstein, S. J. 2003, J. Phys. Chem. A, 107, 2680 [CrossRef] [Google Scholar]
- Miller, J. A., & Melius, C. F. 1992, Combust. Flame, 91, 21 [Google Scholar]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct., 742, 215 [Google Scholar]
- Pardo, J. R., Cernicharo, J., & Serabyn, E. 2001, IEEE Trans. Antennas Propag., 49, 1683 [Google Scholar]
- Shafir, E. V., Slagle, I. R., & Knyazev, V. D. 2003, J. Phys. Chem. A, 107, 8893 [Google Scholar]
- Slagle, I. R., Gmurczyk, G. W., Batt, L., & Gutman, D. 1991, Symp. (Int.) Combust. Proc., 23, 115 [Google Scholar]
- Smith, I. W. M., Herbst, E., & Chang, Q. 2004, MNRAS, 350, 323 [NASA ADS] [CrossRef] [Google Scholar]
- Tanaka, K., Sumiyoshi, Y., Ohshima, Y., et al. 1997, J. Chem. Phys., 107, 2728 [Google Scholar]
- Tercero, F., López-Pérez, J. A., Gallego, J. D., et al. 2021, A&A, 645, A37 [EDP Sciences] [Google Scholar]
- Woods, P. M., Millar, T. J., Zijlstra, A. A., & Herbst, E. 2002, ApJ, 574, L167 [NASA ADS] [CrossRef] [Google Scholar]
- Woon, D. E., & Herbst, E. 2009, ApJS, 185, 273 [NASA ADS] [CrossRef] [Google Scholar]
- Zhang, F., Kim, S., & Kaiser, R. I. 2009, Phys. Chem. Chem. Phys., 11, 4707 [Google Scholar]
Appendix A: Updates with respect to UMIST RATE12
Table A.1 lists the chemical reactions involving CH2CCH, CH3CCH, and CH2CCH2 used in the chemical model, which either are not included in the gas-phase chemical network RATE12 from the UMIST database (McElroy et al. 2013) or are included with different rate coefficients. We have adopted rate coefficient expressions which are adequate, or at least reasonable, for a kinetic temperature of 10 K. Some of the reactions in Table A.1 are included in the RATE12 network but with rate coefficients expressions that result in unrealistic values at 10 K. This is the case for the reactions CH + C2H4 and C2H + CH3CHCH2. In those cases in which there is information on product branching ratios, this information has been taken into account.
Reactions involving CH2CCH, CH3CCH, and CH2CCH2 modified with respect to the UMIST RATE12 chemical network.
All Tables
Reactions involving CH2CCH, CH3CCH, and CH2CCH2 modified with respect to the UMIST RATE12 chemical network.
All Figures
 |
Fig. 1. Spectrum of TMC-1 taken with the Yebes 40 m telescope around 37.46 GHz (black histogram). The frequencies of the six most intense hyperfine components of the 20, 2–10, 1 transition of ortho CH2CCH are indicated by red dotted vertical lines. Transition quantum numbers, frequencies, and derived line parameters are given in Table 1. The synthetic spectrum (red line) was computed for a column density of ortho CH2CCH of 6.5 × 1013 cm−2, a rotational temperature of 10 K, an emission size of 40″ of radius, and a linewidth of 0.72 km s−1 (see main text). |
| In the text | |
 |
Fig. 2. Calculated fractional abundances of CH2CCH and related molecules as a function of time. The black dashed line corresponds to the calculated abundance of CH2CCH when the reactions of this radical with O and N atoms were removed. The abundances observed in TMC-1 are indicated by dotted horizontal lines. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.