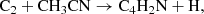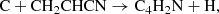| Issue |
A&A
Volume 693, January 2025
|
|
|---|---|---|
| Article Number | L14 | |
| Number of page(s) | 5 | |
| Section | Letters to the Editor | |
| DOI | https://doi.org/10.1051/0004-6361/202453419 | |
| Published online | 13 January 2025 | |
Letter to the Editor
Identification of the interstellar 1-cyano propargyl radical (HCCCHCN) in TMC-1
1
Departamento de Astrofísica Molecular, Instituto de Física Fundamental (IFF-CSIC), C/ Serrano 121, 28006 Madrid, Spain
2
Observatorio de Yebes, IGN, Cerro de la Palera s/n, 19141 Yebes Guadalajara, Spain
3
Observatorio Astronómico Nacional (OAN, IGN), C/ Alfonso XII, 3, 28014, Madrid, Spain
4
Department of Basic Science, Graduate School of Arts & Sciences, The University of Tokyo, Komaba 3-8-1, Meguro-ku, Tokyo 153-8902, Japan
5
Department of Applied Chemistry, Science Building II, National Yang Ming Chiao Tung University, Hsinchu 300098, Taiwan
⋆ Corresponding authors; carlos.cabezas@csic.es; jose.cernicharo@csic.es
Received:
12
December
2024
Accepted:
29
December
2024
We report the first detection in interstellar medium of the 1-cyano propargyl radical, HC3HCN. This species is an isomer of the 3-cyano propargyl radical (CH2C3N), which was recently discovered in TMC-1. The 1-cyano propargyl radical was observed in the cold dark cloud TMC-1 using data from the ongoing QUIJOTE line survey, which is being carried out with the Yebes 40m telescope. A total of seven rotational transitions with multiple hyperfine components were detected in the 31.0–50.4 GHz range. We derived a column density of (2.2 ± 0.2) × 1011 cm−2 and a rotational temperature of 7±1 K. The abundance ratio between HC3HCN and CH2C3N is 1.4. The almost equal abundance of these isomers indicates that the two species may be produced in the same reaction with a similar efficiency, probably in the reaction C + CH2CHCN and perhaps also in the reaction C2 + CH3CN and the dissociative recombination with electrons of CH2C3NH+.
Key words: astrochemistry / line: identification / ISM: molecules / ISM: individual objects: TMC-1
© The Authors 2025
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1. Introduction
The cold dark cloud TMC-1 has a rich and complex chemistry that leads to the formation of a great variety of molecules. Two molecular line surveys of this cloud, QUIJOTE1 (Cernicharo et al. 2021a) and GOTHAM2 (McGuire et al. 2018), have detected more than 70 molecular species in the last four years. Recent discoveries include pure hydrocarbons (Cernicharo et al. 2021b), sulphur-bearing species such as HC3S and NC3S (Cernicharo et al. 2024a), and cyano-substituted polycyclic aromatic hydrocarbons such as cyanonaphthalene (McGuire et al. 2021), cyanoacenapthylene (Cernicharo et al. 2024b), and cyanopyrene (Wenzel et al. 2024a,b). Around 75% of the latest molecular discoveries in TMC-1 are closed-shell species and the remaining 25% are open-shell radicals.
Among the radicals detected with QUIJOTE, we can find hydrocarbons as CH2CCH (Agúndez et al. 2021) and c-C5H (Cabezas et al. 2022a), sulphur-bearing radicals such as the mentioned HC3S and NC3S (Cernicharo et al. 2024a), NCS (Cernicharo et al. 2021c), and HCCS+(Cabezas et al. 2022b), and also nitrile-bearing radicals like HC3N+ (Cabezas et al. 2024), HC5N+, and HC7N+ (Cernicharo et al. 2024c), CH2CCN (Cabezas et al. 2023), and CH2CCCN (Cabezas et al. 2021a). Due to their high reactivity, all the cations and radicals have low abundances. However, a special case to note is CH2CCH, which is extremely abundant due to its well-known low reactivity (see e.g. Agúndez & Wakelam 2013). This added to the spectral dilution resulting from line splitting due to the different interactions between angular momenta makes the detection of radicals fairly challenging. The complexity of the rotational spectrum of a radical is related to the number of nuclei with non-zero nuclear spin. As the number of nuclei with non-zero nuclear spin increases, the prediction and analysis of the fine and hyperfine structure of the rotational spectrum becomes more complicated. Hence, the radio-astronomical discovery of these species is highly dependent on the availability of precise rotational spectroscopic laboratory data. However, thanks to the high sensitivity and resolution of QUIJOTE, some radicals, which display almost trivial spectral patterns, have been identified in TMC-1 without any laboratory data. This is the case for HCCS+ (Cabezas et al. 2022b), which has one non-zero nuclear spin nucleus, and HC3N+ (Cabezas et al. 2024), HC5N+, and HC7N+ (Cernicharo et al. 2024b), which have two non-zero nuclear spin nuclei.
In the case of open-shell radicals with three non-zero nuclear spin nuclei, even very precise ab initio calculations are not enough to unravel their spectral features in the line surveys. Three nuclear spins cause much more complex rotational spectra, with rotational transitions split into dozens of hyperfine components due to the interaction between the rotational, electron spin, and nuclear spin angular momenta. In such cases, the laboratory experiments are invaluable tools, as shown in the identification of the radicals CH2CCN (Cabezas et al. 2023) and CH2C3N (Cabezas et al. 2021a). Only aminomethylidyne, H2NC (Cabezas et al. 2021b), a radical with three non-zero nuclear spin nuclei, has been discovered in space without laboratory experimental support.
This work is another example of the astronomical discovery of a radical with a complex rotational spectrum using high-resolution rotational spectroscopy experiment data. The 3-cyano propargyl radical, CH2C3N, was detected in TMC-1 in 2021 by Cabezas et al. (2021a) using the molecular constants derived from laboratory experiments (Chen et al. 1998; Tang et al. 2001). One of the routes to form CH2C3N in space is the reaction C + CH2CHCN → CH2C3N + H. This reaction has been studied using crossed molecular beam experiments and theoretical calculations (Su et al. 2005; Guo et al. 2006a). These studies indicate that the reaction is barrier-less and occurs through H atom elimination, yielding as main products the radicals 3-cyano propargyl (CH2C3N) and also its isomer 1-cyano propargyl (HC3HCN; see Fig. 1). The latter is inferred to be produced at least twice more efficiently than the former (Guo et al. 2006a). On this basis, in 2022 we investigated the rotational spectrum of HC3HCN and carried out an astronomical search of TMC-1 using the QUIJOTE survey (Cabezas et al. 2022c). We did not detect this species at that time but did derive a 3σ upper limit to its column density of 6.0×1011 cm−2, which implies an abundance ratio HC3HCN/CH2C3N < 3.8. The non-observation of HC3HCN was rationalized by the large partition function for this radical compared to that for CH2C3N. For example, the partition function at 10 K for HC3HCN is 9462, while that for CH2C3N is 1718. Hence, the expected intensity of the lines of HC3HCN, assuming the same column density as for CH2C3N, will be much lower, which makes its detection more difficult. However, as stated in our previous work, a deeper integration in TMC-1 would lead to the detection of the HC3HCN radical in this source. The present QUIJOTE dataset provides a more sensitive spectrum than what was used in Cabezas et al. (2022c), and thus now we revisit the astronomical search for 1-cyano propargyl, reporting the first detection of this radical in space.
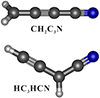 |
Fig. 1. Molecular structures of the CH2C3N and HC3HCN radicals. |
2. Observations
The astronomical observations presented in this work are from the ongoing Yebes 40m Q-band line survey of TMC-1, the QUIJOTE line survey. A detailed description of the line survey and the data-analysis procedure is provided in Cernicharo et al. (2021a, 2022). Briefly, QUIJOTE consists of a line survey in the Q band (31.0–50.3 GHz) at the position of the cyanopolyyne peak of TMC-1 (αJ2000 = 4h41m41.9s and δJ2000 = +25° 41′27.0″). This survey is being carried out using a receiver built within the Nanocosmos project3 consisting of two cooled high-electron-mobility-transistor amplifiers covering the Q band with horizontal and vertical polarization. Fast Fourier transform spectrometers with 8 × 2.5 GHz and a spectral resolution of 38.15 kHz provide the whole coverage of the Q band in both polarizations. Receiver temperatures are 16 K at 32 GHz and 30 K at 50 GHz. The experimental setup is described in detail by Tercero et al. (2021).
All observations were performed using frequency-switching observing mode with a frequency throw of 10 and 8 MHz. The total observing time on the source for data taken with frequency throws of 10 MHz and 8 MHz is 772.6 and 736.6 hours, respectively. Hence, the total observing time on source is 1509.2 hours. The QUIJOTE sensitivity varies between 0.08 mK at 32 GHz and 0.2 mK at 49.5 GHz. The main beam efficiency can be given across the Q band as Beff = 0.797 exp[−(ν(GHz)/71.1)2]. The forward telescope efficiency is 0.97. The telescope beam size at half power intensity is 54.4″ at 32.4 GHz and 36.4″ at 48.4 GHz. The absolute calibration uncertainty is 10%. The data were analysed with the GILDAS package4.
3. Results
As mentioned before, the rotational spectrum of HC3HCN (2A″) was measured in the laboratory by Cabezas et al. (2022c). This transient species was produced by electric discharges, and its rotational spectrum was characterized using a Balle-Flygare narrowband-type Fourier-transform microwave spectrometer operating in the frequency region of 4–40 GHz (Cabezas et al. 2022c). The spectral analysis was supported by high-level ab initio calculations. A total of 193 fine and hyperfine components that originated from 12 rotational transitions, a and b type, were measured for the HC3HCN. The analysis allowed us to accurately determine 22 molecular constants, including rotational and centrifugal distortion constants as well as the fine and hyperfine constants. Electric dipole moment components along the a and b inertial axes have been calculated to be 3.7 and 1.7 D (Cabezas et al. 2022c).
We used the SPCAT program (Pickett 1991) to predict the line frequencies from the spectroscopic parameters determined in Cabezas et al. (2022c). The predictions, which are also available in the CDMS catalogue (entry number 064527; Müller et al. 2005), have an accuracy better than 10 kHz in the Q band (30–50 GHz) for transitions with Ka = 0 and 1. Hence, they should be sufficiently accurate and extensive for searches in cold sources. The frequency predictions were implemented in the MADEX code (Cernicharo 2012) to compute synthetic spectra assuming local thermodynamic equilibrium. We used the values of the dipole moment components mentioned above and assumed a systemic velocity for TMC-1 of VLSR = 5.83 km s−1 (Cernicharo et al. 2020).
Initially, our search focused on a-type transitions since their predicted intensity is 4.7 times stronger than that of b-type transitions. A total of 12 a-type transitions with Ka = 0 and 1 are covered by our QUIJOTE survey. The four N-triplets, with N = 6, 7, 8, and 9, are centred around 32, 37, 43, and 48 GHz. Of these twelve transitions, we detected seven of them well (shown in Fig. 2): 60, 6 − 50, 5, 61, 5 − 51, 4, 71, 7 − 61, 6, 70, 7 − 60, 6, 71, 6 − 61, 5, 80, 8 − 70, 7, and 81, 7 − 71, 6. For all these transitions, the most intense hyperfine features, resulting from the collapse of several components, are clearly detected, while not all the weak components are well detected. As can be seen, the contamination with other species and with negative features produced in the folding of the frequency-switching data precludes the detection of the weak components. In the case of the 61, 6 − 51, 5 transition (top panel of Fig. 2), the most intense component overlaps with a strong line of CH2DC4H, but the overall spectral pattern predicted is fully consistent with the observed spectrum. The remaining transitions covered in the Q band, 81, 8 − 71, 7, 91, 9 − 81, 8, 90, 9 − 80, 8, and 91, 8 − 81, 7 around 43 and 48 GHz, are predicted with similar intensities to those of the analogue lower-N lines, but they are not detected because the noise is higher in that region of the spectrum due to the increase in the atmospheric attenuation and emissivity by the O2 molecular band around 60 GHz. The b-type transitions are expected to be 4.7 times weaker than the a-type ones. Hence, their expected emission is below our detection limit. An analysis of the observed intensities using a line profile fitting method (Cernicharo et al. 2021d) provides a rotational temperature of 7 ± 1 K, while the observed intensities are reproduced with a column density of (2.2 ± 0.2)× 1011 cm−2. The derived column density for HC3HCN is about three times lower than the upper limit estimated in Cabezas et al. (2022c). Given the column density of CH2C3N in TMC-1, 1.6 × 1011 cm−2 (Cabezas et al. 2021a), the column density ratio between the two isomers, HC3HCN/CH2C3N, is 1.4.
 |
Fig. 2. Observed lines of HC3HCN in TMC-1 from the QUIJOTE line survey (black histogram) and synthetic spectra (red curve) calculated adopting a column density of 2.2 × 1011 cm−2. Blanked channels correspond to negative features produced in the folding of the frequency-switching data. For each rotational transition, the most intense hyperfine components are shown. The abscissa corresponds to the rest frequency assuming a local standard of rest velocity of 5.83 km s−1. The ordinate is the antenna temperature in millikelvins. |
4. Chemical modelling
The chemistry of the radical 3-cyano propargyl (CH2C3N) in cold dense clouds has been discussed in Cabezas et al. (2021a). Here we revisit the chemistry of cyano propargyl radicals in the light of the discovery of the isomer 1-cyano propargyl (HCCCHCN) in TMC-1. To that purpose, we ran chemical modelling calculations, adopting typical parameters of a cold dense cloud: a gas temperature of 10 K, a volume density of H nuclei of 2 × 104 cm−3, a cosmic-ray ionization rate of 1.3 × 10−17 s−1, a visual extinction of 30 mag, and low metal elemental abundances (Agúndez & Wakelam 2013). We used a chemical network from a more recent version of the UMIST database (Millar et al. 2024) than that used in Cabezas et al. (2021a). Unlike in the previous version, the radical CH2C3N is now included in the UMIST network with several of the reactions of formation and destruction discussed in Cabezas et al. (2021a). However, other isomers such as HCCCHCN are not included, and, since in many of the reactions involving CH2C3N it is difficult to distinguish between different isomers, we considered that CH2C3N comprises all possible C4H2N isomers.
The calculated abundance of C4H2N is shown as a function of time in Fig. 3 (solid blue line). It is seen that the peak abundance, reached at a time of around 105 yr, is a few 10−10 relative to H2, while the observed abundances of the two isomers CH2C3N and HCCCHCN lie around one order of magnitude below. That is, the chemical model is too efficient at producing cyano propargyl radicals. This is in contrast with the results obtained in Cabezas et al. (2021a), where the calculated abundance of CH2C3N remained below the observed value (in the case in which the reaction N + C4H3 was neglected). The reason is that the UMIST 2022 network does not include reactions of destruction of CH2C3N with neutral atoms, such as C, N, and O, which were included in Cabezas et al. (2021a). If these reactions are included, then the calculated peak abundance of C4H2N decreases by two orders of magnitude (see the dashed blue line in Fig. 3), in agreement with the calculations shown in Cabezas et al. (2021a). This illustrates how critical the reactions with neutral atoms are for establishing how abundant certain molecules can be in cold dense clouds. Unfortunately, in the case of cyano propargyl radicals, the kinetics of the reactions with neutral atoms is not known.
 |
Fig. 3. Calculated fractional abundance of cyano propargyl radicals (C4H2N, where all possible isomers are included) as a function of time. The solid blue line corresponds to the standard UMIST 2022 case, in which reactions of C4H2N with neutral atoms are not included, while the dashed blue line corresponds to the case in which C4H2N radicals react fast with neutral C, N, and O atoms. The horizontal dotted lines correspond to the observed abundances of the two cyano propargyl isomers detected in TMC-1, CH2C3N, and HCCCHCN, adopting a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). |
According to the chemical model, the main reactions of formation of cyano propargyl radicals (C4H2N) are the neutral-neutral reactions
and the dissociative recombination of the cation CH2C3NH+ with electrons, which is assumed to yield C4H2N isomers and H atoms as fragments. As far as we know, reaction (1) has not been studied theoretically or experimentally, although related reactions have been studied and some information can be gained from such studies. The reaction C + CH3CN has been experimentally found to be rapid at low temperatures, with a rate coefficient in the range (3–4) × 10−10 cm3 s−1 and with H atom elimination being the main channel, with a measured branching ratio of 0.63 (Hickson et al. 2021). Similarly, the reaction C2 + CH3CCH has been measured to be rapid at low temperatures, with a rate coefficient in the range (4–5) × 10−10 cm3 s−1 (Daugey et al. 2008), while theoretical calculations and crossed-beam experiments indicate that the H atom elimination channel is also the preferred one, with an estimated branching ratio of 0.65 (Guo et al. 2006b; Mebel et al. 2006). The behaviour of the C + CH3CN and C2 + CH3CCH reactions suggests that reaction (1) could behave similarly (i.e. fast at low temperatures and with H atom elimination as the main channel). It would be interesting to study this reaction to have evidence on whether or not this is the case, and if so, to determine the branching ratios of the different C4H2N isomers formed. Reaction (2) has been studied through theoretical calculations and using crossed-beam experiments (Su et al. 2005; Guo et al. 2006a). According to these studies, the reaction is barrier-less and the main channel is again H atom elimination. Moreover, the isomer 1-cyano propargyl (HCCCHCN) is inferred to be twice more abundant than the 3-cyano propargyl radical (CH2C3N). This later point is of great interest given that in TMC-1 1-cyano propargyl is observed to be 1.4 times more abundant than 3-cyano propargyl. The agreement between TMC-1 observations and the study by Guo et al. (2006a) on the C + CH2CHCN reaction and the preference of HCCCHCN over CH2C3N supports a scenario in which this reaction would be a major source of cyano propargyl radicals in cold dense clouds.
In summary, the observed abundance of cyano propargyl radicals in TMC-1 and their observed abundance ratio suggest that the reaction C + CH2CHCN is an important source of these radicals. Still, there are various open questions regarding the chemistry of these species, namely: (1) whether or not cyano propargyl radicals react fast with neutral atoms; (2) whether or not the reaction C2 + CH3CN is rapid at low temperatures and occurs through H atom elimination, and if so, what the product branching ratios are; (3) what branching ratios of the channels lead to cyano propargyl radicals in the dissociation recombination of the ions CH2C3NH+ and CH3C3NH+ with electrons; and (4) whether or not the reaction N + C4H3 could be a source of cyano propargyl radicals (see Cabezas et al. 2021a for a discussion on this point).
5. Conclusions
We have reported the detection of the 1-cyano propargyl radical (HC3HCN) in the cold dark cloud TMC-1. This discovery was achieved using a combination of high-resolution rotational spectroscopy experiments and ultra-sensitive astronomical observations. A total of seven rotational transitions with several hyperfine components were observed in our Q-band survey of TMC-1. We find that this radical is slightly more abundant than its structural isomer 3-cyano propargyl radical (CH2C3N). The investigation of the chemical routes of these radicals indicates that both can be formed in the neutral-neutral reaction C + CH2CHCN, with uncertain contributions from the reaction C2 + CH3CN and the dissociative recombination with electrons of CH2C3NH+.
Acknowledgments
This work was based on observations carried out with the Yebes 40m telescope (projects 19A003, 20A014, 20D023, 21A011, 21D005, and 23A024). The 40m radiotelescope at Yebes Observatory is operated by the Spanish Geographic Institute (IGN, Ministerio de Transportes y Movilidad Sostenible). We acknowledge funding support from Spanish Ministerio de Ciencia e Innovación through grants PID2019-106110GB-I00, PID2019-107115GB-C21, PID2019-106235GB-I00, and PID2022-136525NA-I00. We thank the Consejo Superior de Investigaciones Científicas (CSIC) for funding through project PIE 202250I097. The present study was also supported by Ministry of Science and Technology of Taiwan and CSIC under the MoST-CSIC Mobility Action 2021 (Grants 11-2927-I-A49-502 and OSTW200006). Y. Endo thanks Ministry of Science and Technology of Taiwan through grant MOST108-2113-M-009-25.
References
- Agúndez, M., & Wakelam, V. 2013, Chem. Rev., 113, 8710 [Google Scholar]
- Agúndez, M., Cabezas, C., Tercero, B., et al. 2021, A&A, 647, L10 [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2021a, A&A, 654, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2021b, A&A, 654, A45 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Fuentetaja, R., et al. 2022a, A&A, 663, L2 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2022b, A&A, 657, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Nakajima, M., Chang, C. H., et al. 2022c, A&A, 657, A24 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Tang, J., Agúndez, M., et al. 2023, A&A, 676, L5 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Endo, Y., et al. 2024, A&A, 687, L22 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J. 2012, in European Conference on Laboratory Astrophysics, eds. C. Stehlé, C. Joblin, & L. d’Hendecourt, EAS Pub. Ser., 58, 251 [Google Scholar]
- Cernicharo, J., & Guélin, M. 1987, A&A, 176, 299 [Google Scholar]
- Cernicharo, J., Marcelino, N., Pardo, J. R., et al. 2020, A&A, 641, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Kaiser, R., et al. 2021a, A&A, 652, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Cabezas, C., et al. 2021b, A&A, 649, L15 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2021c, A&A, 648, L3 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Endo, Y., et al. 2021d, A&A, 646, L3 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Fuentetaja, R., et al. 2022, A&A, 663, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2024a, A&A, 688, L13 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Fuentetaja, R., et al. 2024b, A&A, 690, L13 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2024c, A&A, 686, L15 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Chen, W., McCarthy, M. C., Travers, M. J., et al. 1998, ApJ, 492, 849 [NASA ADS] [CrossRef] [Google Scholar]
- Daugey, N., Caubet, P., Bergeat, A., et al. 2008, PCCP, 10, 729 [Google Scholar]
- Guo, Y., Gu, X., Zhang, F., et al. 2006a, PCCP, 8, 5454 [CrossRef] [Google Scholar]
- Guo, Y., Gu, X., Balucani, N., & Kaiser, R. I. 2006b, J. Phys. Chem. A, 110, 6245 [NASA ADS] [CrossRef] [Google Scholar]
- Hickson, K. M., Loison, J.-C., & Wakelam, V. 2021, ACS Earth Space Chem., 5, 824 [NASA ADS] [CrossRef] [Google Scholar]
- McGuire, B. A., Burkhardt, A. M., Kalenskii, S., et al. 2018, Science, 359, 202 [Google Scholar]
- McGuire, B. A., Loomis, R. A., Burkhardt, A. M., et al. 2021, Science, 371, 1265 [Google Scholar]
- Mebel, A. M., Kislov, V. V., & Kaiser, R. I. 2006, J. Phys. Chem. A, 110, 2421 [NASA ADS] [CrossRef] [Google Scholar]
- Millar, T. J., Walsh, C., Van de Sande, M., & Markwick, A. J. 2024, A&A, 682, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct., 742, 215 [Google Scholar]
- Pickett, H. M. 1991, J. Mol. Spectr., 148, 371 [Google Scholar]
- Su, H.-F., Kaiser, R. I., & Chang, A. H. H. 2005, J. Chem. Phys., 122, 074320 [NASA ADS] [CrossRef] [Google Scholar]
- Tang, J., Sumiyoshi, Y., & Endo, Y. 2001, ApJ, 552, 409 [NASA ADS] [CrossRef] [Google Scholar]
- Tercero, F., López-Pérez, J. A., Gallego, J. D., et al. 2021, A&A, 645, A37 [EDP Sciences] [Google Scholar]
- Wenzel, G., Speak, T. H., Changala, P. B., et al. 2024a, Nat. Astron., https://doi.org/10.1038/s41550-024-02410-9 [Google Scholar]
- Wenzel, G., Cooke, I. R., Changala, P. B., et al. 2024b, Science, 386, 810 [NASA ADS] [CrossRef] [Google Scholar]
All Figures
 |
Fig. 1. Molecular structures of the CH2C3N and HC3HCN radicals. |
| In the text | |
 |
Fig. 2. Observed lines of HC3HCN in TMC-1 from the QUIJOTE line survey (black histogram) and synthetic spectra (red curve) calculated adopting a column density of 2.2 × 1011 cm−2. Blanked channels correspond to negative features produced in the folding of the frequency-switching data. For each rotational transition, the most intense hyperfine components are shown. The abscissa corresponds to the rest frequency assuming a local standard of rest velocity of 5.83 km s−1. The ordinate is the antenna temperature in millikelvins. |
| In the text | |
 |
Fig. 3. Calculated fractional abundance of cyano propargyl radicals (C4H2N, where all possible isomers are included) as a function of time. The solid blue line corresponds to the standard UMIST 2022 case, in which reactions of C4H2N with neutral atoms are not included, while the dashed blue line corresponds to the case in which C4H2N radicals react fast with neutral C, N, and O atoms. The horizontal dotted lines correspond to the observed abundances of the two cyano propargyl isomers detected in TMC-1, CH2C3N, and HCCCHCN, adopting a column density of H2 of 1022 cm−2 (Cernicharo & Guélin 1987). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.