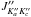| Issue |
A&A
Volume 591, July 2016
|
|
|---|---|---|
| Article Number | A75 | |
| Number of page(s) | 5 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201628140 | |
| Published online | 17 June 2016 | |
The millimeter wave spectrum of methyl cyanate: a laboratory study and astronomical search in space⋆,⋆⋆
1 Grupo de Espectroscopia Molecular (GEM), Edificio Quifima, Área de Química-Física, Laboratorios de Espectroscopia y Bioespectroscopia, Parque Científico UVa, Unidad Asociada CSICUniversidad de Valladolid, 47011 Valladolid, Spain
e-mail: lucie.kolesnikova@uva.es
2 Instituto de Ciencia de Materiales de Madrid, CSIC, C/ Sor Juana Inés de la Cruz 3, 28049 Cantoblanco, Spain
3 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, 11 Allée de Beaulieu, CS 50837, 35708 Rennes Cedex 7, France
Received: 15 January 2016
Accepted: 15 March 2016
Aims. The recent discovery of methyl isocyanate (CH3NCO) in Sgr B2(N) and Orion KL makes methyl cyanate (CH3OCN) a potential molecule in the interstellar medium. The aim of this work is to fulfill the first requirement for its unequivocal identification in space, i.e. the availability of transition frequencies with high accuracy.
Methods. The room-temperature rotational spectrum of methyl cyanate was recorded in the millimeter wave domain from 130 to 350 GHz. All rotational transitions revealed A-E splitting owing to methyl internal rotation and were globally analyzed using the ERHAM program.
Results. The data set for the ground torsional state of methyl cyanate exceeds 700 transitions within J′′ = 10−35 and K"a= 0−13 and newly derived spectroscopic constants reproduce the spectrum close to the experimental uncertainty. Spectral features of methyl cyanate were then searched for in Orion KL, Sgr B2(N), B1-b, and TMC-1 molecular clouds. Upper limits to the column density of methyl cyanate are provided.
Key words: astrochemistry / line: identification / techniques: spectroscopic / astronomical databases: miscellaneous / molecular data
Tables 3 and 4 are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/591/A75
© ESO, 2016
1. Introduction
Organic molecules containing abundant interstellar elements H, C, N, and O are the prime target for both astrophysicists and spectroscopists to intensively search for in the interstellar medium (ISM). One of the simplest, the isocyanic acid (HNCO), was detected with high abundances in a variety of Galactic sources, as well as in the external galaxies (see Quan et al. 2010; Brünken et al. 2010, and references therein). Cyanic acid (HOCN) and fulminic acid (HCNO), two energetically less stable isomers of isocyanic acid with predicted relative energies of 24.7 and 70.7 kcal mol-1, respectively (see Schuurman et al. 2004), were also successfully observed in space (Brünken et al. 2010, 2009; Marcelino et al. 2010, 2009).
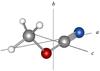 |
Fig. 1 Methyl cyanate depicted in the principal axis system. |
 |
Fig. 2 Section of the methyl cyanate rotational spectrum with the assignment of the A-E doublets of the a-type R-branch transitions (ΔJ = + 1, ΔKa = 0, ΔKc = + 1) within the cluster for J′′ = 13. The quantum numbers of the lower energy levels, |
The choice of new molecules to be searched for is usually justified by analogy with the detected molecules and plausible chemical reactions that occur in the ISM. Methyl substituted derivatives of isocyanic acid and its isomers could thus be suggested as logical candidates. Among the members of the open-chain methyl derivatives, methyl isocyanate (CH3NCO) is the lowest-energy isomer, followed by methyl cyanate (CH3OCN), acetonitrile N-oxide (CH3CNO), and methyl fulminate (CH3ONC). Very recently, methyl isocyanate was detected on the surface of the comet 67P/Churyumov-Gerasimenko (Goesmann et al. 2015) as well as in the molecular clouds Sgr B2 and Orion KL (Halfen et al. 2015; Cernicharo et al. 2016). Owing to relatively high abundances of methyl isocyanate found by Cernicharo et al. (2016), the search for methyl cyanate in space, which uses millimeter wave astronomy, looks promising. Methyl cyanate (Fig. 1) is predicted to be higher in energy with respect to methyl isocyanate by 25.1 kcal mol-1 (Pasinszki & Westwood 2001), i.e. close to that of cyanic acid with respect to isocyanic acid. Moreover, sizable dipole moment components of | μa | = 4.07 (6) D and | μb | = 1.24 (40) D (Sakaizumi et al. 1990) make the possible detection of this metastable isomer relatively favourable.
An attempt to observe methyl cyanate unambiguously in the ISM requires a previous good knowledge of its rotational spectrum in the laboratory. Until this work, only Stark-modulated spectra were recorded up to 50 GHz (Sakaizumi et al. 1990, 1993, 1995). Seven A and seven E transitions, which resulted from the methyl group internal rotation, were reported and analyzed for the ground torsional state and a barrier to internal rotation of 399 cm-1 was derived (Sakaizumi et al. 1995). Lack of accurate laboratory millimeter wave data thus initiated new spectroscopic measurements over the frequency range from 130 to 350 GHz. A new set of spectroscopic constants could reproduce the millimeter wave spectrum within the experimental uncertainty, which could then be used to guide the search for millimeter wave signatures of methyl cyanate towards various interstellar sources. In this work, a search was conducted towards the molecular clouds Orion KL, Sgr B2(N), B1-b and TMC-1, and the results are discussed in Sect. 4.
 |
Fig. 3 Comparison of the predicted positions of the ground torsional state a-type R-branch transitions of methyl cyanate with the experimentally observed spectrum. The blue model shows predictions based on the previous work of Sakaizumi et al. (1995) and Δν quantity provides the difference between the observed and predicted frequencies. Predictions based on the results of this work are represented by the red model. |
2. Experimental details
Methyl cyanate was generated according to Pasinszki & Westwood (1995) by gas-solid reaction of the evaporated O-methyl thiocarbamate (CH3OC(S)NH2) with yellow mercury oxide (HgO, Sigma Aldrich) packed into a tube in half-section in a vacuum line (0.3 mbar pressure). A sample of O-methyl thiocarbamate was prepared according to the method given in Davies & Maclaren (1951). To remove the water, which is a by-product, the reaction products were passed through a short tube (15 cm) filled in half-section with phosphorus pentoxide (P2O5, Sigma Aldrich). Dried products were collected in the liquid nitrogen U-tube trap, which was then connected to the sample cell of the spectrometer. Vapour pressure of the trapped sample near − 60 °C was enough to fill the cell up to a total pressure of about 25 μbar. Undiluted methyl cyanate has been described as an extremely unstable compound (Jensen et al. 1965). Initial experiments were performed in the flow mode. However, later it was found that the measurements could be performed in the closed cell for several hours. Isocyanic acid and methyl isocyanate were found to be major impurities.
The rotational spectra were recorded at room temperature using the Valladolid millimeter wave spectrometer described in detail elsewhere (Daly et al. 2014). The measured frequency region from 130 to 350 GHz was reached by sequential multiplication of the basic synthesizer frequency (≤ 20 GHz) by amplifier-multiplier chains (VDI, Inc.). The synthesizer output was frequency modulated at f = 10.2 kHz with a modulation depth of 30 kHz. The signal was detected by solid-state zero-bias detectors (VDI, Inc.) and further processed by a lock-in amplifier using 2f detection. The resulting second derivative shape of the lines was fitted to the Gaussian profile function and the uncertainty of the isolated well-developed lines was estimated to be better than 50 kHz.
3. Analysis of the spectra
Methyl cyanate is a near-prolate asymmetric top with Cs symmetry and its molecular geometry is shown in Fig. 1. Two characteristic features can be found in the methyl cyanate rotational spectra. Firstly, the dipole moment component along the a-axis is much larger than the component along the b-axis, therefore, the rotational spectrum is dominated by strong a-type R-branch transitions. These transitions usually occur in groups with the central part situated approximately at (B + C)(J′′ + 1). Each group corresponds to specific J′′ + 1 ← J′′ and is formed by transitions with different Ka. Figure 2 illustrates an example of this type of group for J′′ = 13. Two transitions always appear for each Ka> 0 and lower Ka pairs are more widely split than the higher Ka ones (see Fig. 2). Secondly, methyl cyanate contains one C3v internal rotor (methyl group), which can undergo a torsion motion with respect to the rest of the molecule leading to the splitting of the rotational levels into A and E sublevels. As a result, all transitions appear as the A-E doublets with separation from units to tens of MHz (see Fig. 2), which leads to higher line density of the spectrum and complicates its analysis. For completeness, methyl cyanate moreover possess one nucleus with a nonzero electric quadrupole moment (14N), however, no quadrupole hyperfine structure has been observed in the rotational spectra.
 |
Fig. 4 ALMA SV data of three different positions towards Orion KL at selected frequencies, together with the synthetic spectra obtained using the column densities given in Sect. 4 as upper limits. A vLSR of +9.0 km s-1 is assumed. |
Spectroscopic parameters of methyl cyanate in its ground torsional state.
After eliminating the lines that correspond to the isocyanic acid and methyl isocyanate, the assignment was started with the search for the strongest low-Ka transitions using predictions based on the results of Sakaizumi et al. (1995). An example of the predictions of the rotational transitions in the millimeter wave domain is shown in the upper part of Fig. 3. If compared with the experimental spectrum in the middle part of Fig. 3, large discrepancies occur between the observed and predicted frequencies. This is a typical problem of the extrapolation outside the experimentally known data set that makes the confident search in the millimeter wave line surveys impossible. Loomis-Wood-type plots from AABS package (Kisiel et al. 2005) were very useful for reaching conclusive assignments.
The first set of rotational and centrifugal distortion constants was obtained from the separate fit of the A transitions with standard Watson’s A-reduced semirigid rotor Hamiltonian (Watson 1977). Next, the E transitions were searched for and added step-by-step, starting at low J and Ka and moving up progressively. Both A and E transitions were finally assigned within J′′ = 10−35 and 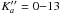 and analyzed simultaneously using the ERHAM program (Groner 1997, 2012). The structural model from Sakaizumi et al. (1993) was adopted to derive initial values of the internal motion parameters ρ and β, which represent the magnitude of the ρ-vector and the angle between this vector and the principal a-axis, respectively. The rotational line sequences of a-type R-branch transitions for J′′ ≤ 25 and
and analyzed simultaneously using the ERHAM program (Groner 1997, 2012). The structural model from Sakaizumi et al. (1993) was adopted to derive initial values of the internal motion parameters ρ and β, which represent the magnitude of the ρ-vector and the angle between this vector and the principal a-axis, respectively. The rotational line sequences of a-type R-branch transitions for J′′ ≤ 25 and 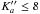 were analyzed using the set of the rotational constants, centrifugal distortion constants up to the sixth order, ρ and β parameters, first two energy tunneling parameters εq (q = 1,2), and two tunneling parameters with q = 1, which were associated with the rotational constants (see Groner 1997, 2012, for definitions and nomenclature of these parameters). Larger discrepancies were found between observed and calculated frequencies for J′′> 25 and for even lower
were analyzed using the set of the rotational constants, centrifugal distortion constants up to the sixth order, ρ and β parameters, first two energy tunneling parameters εq (q = 1,2), and two tunneling parameters with q = 1, which were associated with the rotational constants (see Groner 1997, 2012, for definitions and nomenclature of these parameters). Larger discrepancies were found between observed and calculated frequencies for J′′> 25 and for even lower 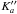 , which could not be rectified with the addition of the tunneling components of the quartic centrifugal distortion constants. These deviations could be partly removed using the tunneling components of the rotational constants with q = 2. The tunneling parameters associated with the Coriolis operators Ja, JaJ2, or
, which could not be rectified with the addition of the tunneling components of the quartic centrifugal distortion constants. These deviations could be partly removed using the tunneling components of the rotational constants with q = 2. The tunneling parameters associated with the Coriolis operators Ja, JaJ2, or 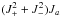 were also applied to decrease those deviations but the ρ and β values were significantly changed leading to the unusual value of the moment of inertia of the methyl group. Therefore, these parameters were finally excluded from the fit. The left and middle part of Table 1 present the 25 spectroscopic parameters determined from the nonlinear least-square fit of 390 A transitions (290 distinct frequency lines owing to the line blending) and 341 E transitions, which lead to the root mean square deviations of 77 kHz and 83 kHz for the sets of A and E transitions, respectively. The moment of inertia of the methyl top, together with other derived internal rotation parameters, are presented on the right side of Table 1. The experimental frequencies can be found in Table 3, along with the assignments to the rotational quantum numbers J, Ka, Kc and νobs−νcalc quantities, where νobs and νcalc are the observed and calculated frequencies, respectively. The spectroscopic parameters from Table 1 were used to predict the transition frequencies and line strengths multiplied by the square of the corresponding dipole moment component, which are indispensable for a correct molecular identification. These predictions are gathered in Table 4 along with the estimated uncertainties of the predicted frequencies and energies of the lower and upper state energy levels.
were also applied to decrease those deviations but the ρ and β values were significantly changed leading to the unusual value of the moment of inertia of the methyl group. Therefore, these parameters were finally excluded from the fit. The left and middle part of Table 1 present the 25 spectroscopic parameters determined from the nonlinear least-square fit of 390 A transitions (290 distinct frequency lines owing to the line blending) and 341 E transitions, which lead to the root mean square deviations of 77 kHz and 83 kHz for the sets of A and E transitions, respectively. The moment of inertia of the methyl top, together with other derived internal rotation parameters, are presented on the right side of Table 1. The experimental frequencies can be found in Table 3, along with the assignments to the rotational quantum numbers J, Ka, Kc and νobs−νcalc quantities, where νobs and νcalc are the observed and calculated frequencies, respectively. The spectroscopic parameters from Table 1 were used to predict the transition frequencies and line strengths multiplied by the square of the corresponding dipole moment component, which are indispensable for a correct molecular identification. These predictions are gathered in Table 4 along with the estimated uncertainties of the predicted frequencies and energies of the lower and upper state energy levels.
Physical parameters of the considered cloud cores.
4. Radioastronomical observations
The new set of the spectroscopic constants of methyl cyanate allow us to attempt a rigorous search for this species in space. The high abundance of molecules containing methoxy (CH3O−) and cyano (−CN) groups in Orion KL (Tercero et al. 2015; López et al. 2014) and the recent detection of CH3NCO as one of the most abundant CH3 −X species towards the source (Cernicharo et al. 2016) could suggest the presence of methyl cyanate (CH3OCN) in Orion KL. Cernicharo et al. (2016) report an upper limit to its column density of 1 × 1014 cm-2 in Orion KL, according to the IRAM 30 m data (Tercero et al. 2010). We obtained similar results searching for CH3OCN in different positions of the ALMA Science Verification (SV) data of this source. Using the MADEX code (Cernicharo 2012), we derived an upper limit of 2 × 1014 cm-2 in the two emission peaks of CH3NCO (see Cernicharo et al. 2016). These positions correspond approximately to the middle of the hot core clumpy structure (see e.g. Favre et al. 2011) and the MM4 source shown in Wu et al. (2014). According to the CH3NCO column densities, we derived the ratio N(CH3NCO)/N(CH3OCN) ≥ 35 and 20 in the hot core and MM4, respectively. Owing to the richness of the compact ridge in molecules containing the methoxy group (Tercero et al. 2015), we also looked for methyl cyanate in this component, but we did not find this species above the detection limit of the data. Adopting the physical parameters of the compact ridge for trans-ethyl methyl ether derived by Tercero et al. (2015), we found an upper limit to the CH3OCN column density of 1 × 1014 cm-2. Figure 4 shows the ALMA SV data of these three positions at selected frequencies, together with the synthetic spectra obtained using the column densities given above as upper limits. Above the limit established by these models, it becames clear that lines were missing or mismatched.
As CH3NCO has also been detected in Sgr B2(N) (Halfen et al. 2015; Cernicharo et al. 2016), we modelled the emission of CH3OCN according to the physical parameters for CH3NCO in Sgr B2(N) (Cernicharo et al. 2016) to search for this species in the public IRAM 30 m spectrum at 3 mm of this source (Belloche et al. 2013). Nevertheless, similar to the Orion case, only upper limits to its column density were derived: 1 × 1016 cm-2 and 5 × 1015 cm-2 for the 63 km s-1 component and for the second component at 73 km s-1, respectively, i.e. a factor ~50 below that of the column density values of CH3NCO (Cernicharo et al. 2016). However, HOCN, an isomer of HNCO, which contains the −OCN group, has been detected in this source (Brünken et al. 2010) so we also expected the presence of methyl cyanate in the cold gas. To explore this possibility, we searched for CH3OCN in the previous data, considering the two extended components at 14 K that were derived by Brünken et al. (2010) for HOCN in Sgr B2(N). Once again, only an upper limit to the column density of 2 × 1013 cm-2 was derived for each component. We also did not find evidence of the presence of this species in the public PRIMOS survey of this source (Neill et al. 2012).
Finally, we concluded this attempt to detect CH3OCN in space by searching for this species in dark clouds. We found an upper limit to its column density of 2 × 1011 cm-2 in B1-b and TMC-1 using the data of Cernicharo et al. (2012). Table 2 shows the physical parameters for each cloud adopted for these calculations, as well as the rotational partition function used to derive the upper limits to the CH3OCN column density.
Acknowledgments
This paper makes use of the following ALMA data: ADS/JAO.ALMA#2011.0.00009.SV. ALMA is a partnership of ESO (representing its member states), NSF (USA), and NINS (Japan) with NRC (Canada), NSC, and ASIAA (Taiwan), and KASI (Republic of Korea), in cooperation with the Republic of Chile. The Joint ALMA Observatory is operated by ESO, AUI/NRAO, and NAOJ. This work was also based on observations carried out with the IRAM 30-m telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain). The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007−2013)/ERC-2013-SyG, Grant Agreement n. 610256 NANOCOSMOS, Ministerio de Ciencia e Innovación (Grants CTQ2013-40717-P, AYA2012-32032, and Consolider-Ingenio 2010 CSD2009-00038 program ASTROMOL) and Junta de Castilla y León (Grant VA175U13). J.-C. G. thanks the Centre National d’Études Spatiales (CNES) and the CNRS program Physique et Chimie du Milieu Interstellaire (PCMI) for financial support. C.B. and E.R.A. also wish to thank the Ministerio de Ciencia e Innovacion for an FPI grant (BES-2011-047695 y BES-2014-067776).
References
- Belloche, A., Müller, H. S. P., Menten, K. M., Schilke, P., & Comito, C. 2013, A&A, 559, A47 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Brünken, S., Gottlieb, C. A., McCarthy, M. C., & Thaddeus, P. 2009, ApJ, 697, 880 [NASA ADS] [CrossRef] [Google Scholar]
- Brünken, S., Belloche, A., Martín, S., Verheyen, L., & Menten, K. M. 2010, A&A, 516, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J. 2012, in ECLA-2011: Proceedings of the European Conference on Laboratory Astrophysics, Laboratory Astrophysics and Astrochemistry in the Herschel/ALMA Era, 58, 251 [Google Scholar]
- Cernicharo, J., Marcelino, N., Roueff, E., et al. 2012, ApJ, 759, L43 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Kisiel, Z., Tercero, B., et al. 2016, A&A, 587, L4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Daly, A., Kolesniková, L., Mata, S., & Alonso, J. 2014, J. Mol. Spectr., 306, 11 [NASA ADS] [CrossRef] [Google Scholar]
- Davies, W., & Maclaren, J. A. 1951, J. Chem. Soc., 1434 [Google Scholar]
- Favre, C., Wootten, H. A., Remijan, A. J., et al. 2011, ApJ, 739, L12 [NASA ADS] [CrossRef] [Google Scholar]
- Goesmann, F., Rosenbauer, H., Bredehöft, J. H., et al. 2015, Science, 349, 689 [CrossRef] [Google Scholar]
- Groner, P. 1997, J. Chem. Phys., 107, 4483 [NASA ADS] [CrossRef] [Google Scholar]
- Groner, P. 2012, J. Mol. Spectr., 278, 52 [Google Scholar]
- Halfen, D. T., Ilyushin, V. V., & Ziurys, L. M. 2015, ApJ, 812, L5 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Jensen, K. A., Due, M., & Holm, A. 1965, Acta Chem. Scand., 19, 438 [CrossRef] [Google Scholar]
- Kisiel, Z., Pszczółkowski, L., Medvedev, I. R., et al. 2005, J. Mol. Spectr., 233, 231 [NASA ADS] [CrossRef] [Google Scholar]
- López, A., Tercero, B., Kisiel, Z., et al. 2014, A&A, 572, A44 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Marcelino, N., Cernicharo, J., Tercero, B., & Roueff, E. 2009, ApJ, 690, L27 [NASA ADS] [CrossRef] [Google Scholar]
- Marcelino, N., Brünken, S., Cernicharo, J., et al. 2010, A&A, 516, A105 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Neill, J. L., Muckle, M. T., Zaleski, D. P., et al. 2012, ApJ, 755, 153 [NASA ADS] [CrossRef] [Google Scholar]
- Pasinszki, T., & Westwood, N. P. C. 1995, J. Phys. Chem., 99, 1649 [CrossRef] [Google Scholar]
- Pasinszki, T., & Westwood, N. P. C. 2001, J. Phys. Chem. A, 105, 1244 [CrossRef] [Google Scholar]
- Quan, D., Herbst, E., Osamura, Y., & Roueff, E. 2010, ApJ, 725, 2101 [NASA ADS] [CrossRef] [Google Scholar]
- Sakaizumi, T., Mure, H., Ohashi, O., & Yamaguchi, I. 1990, J. Mol. Spectr., 140, 62 [NASA ADS] [CrossRef] [Google Scholar]
- Sakaizumi, T., Sekishita, K., Furuya, K., et al. 1993, J. Mol. Spectr., 161, 114 [NASA ADS] [CrossRef] [Google Scholar]
- Sakaizumi, T., Namikawa, M., & Ohashi, O. 1995, J. Mol. Struct., 345, 189 [NASA ADS] [CrossRef] [Google Scholar]
- Schuurman, M. S., Muir, S. R., Allen, W. D., & Schaefer, H. F. 2004, J. Chem. Phys., 120, 11586 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Tercero, B., Cernicharo, J., Pardo, J. R., & Goicoechea, J. R. 2010, A&A, 517, A96 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Tercero, B., Cernicharo, J., López, A., et al. 2015, A&A, 582, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Watson, J. K. G. 1977, in Vibrational Spectra and Structure, ed. J. R. Durig (Amsterdam: Elsevier), 6, 1 [Google Scholar]
- Wu, Y., Liu, T., & Qin, S.-L. 2014, ApJ, 791, 123 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Methyl cyanate depicted in the principal axis system. |
| In the text | |
 |
Fig. 2 Section of the methyl cyanate rotational spectrum with the assignment of the A-E doublets of the a-type R-branch transitions (ΔJ = + 1, ΔKa = 0, ΔKc = + 1) within the cluster for J′′ = 13. The quantum numbers of the lower energy levels, |
| In the text | |
 |
Fig. 3 Comparison of the predicted positions of the ground torsional state a-type R-branch transitions of methyl cyanate with the experimentally observed spectrum. The blue model shows predictions based on the previous work of Sakaizumi et al. (1995) and Δν quantity provides the difference between the observed and predicted frequencies. Predictions based on the results of this work are represented by the red model. |
| In the text | |
 |
Fig. 4 ALMA SV data of three different positions towards Orion KL at selected frequencies, together with the synthetic spectra obtained using the column densities given in Sect. 4 as upper limits. A vLSR of +9.0 km s-1 is assumed. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.