| Issue |
A&A
Volume 562, February 2014
|
|
|---|---|---|
| Article Number | A61 | |
| Number of page(s) | 16 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201322832 | |
| Published online | 06 February 2014 | |
Isotope selective photodissociation of N2 by the interstellar radiation field and cosmic rays
1
Leiden Observatory, Leiden University,
PO Box 9513,
2300 RA
Leiden,
The Netherlands
e-mail:
heays@strw.leidenuniv.nl
2
Department of Astronomy, University of Michigan,
500 Church Street, Ann Arbor, MI
48109-1042,
USA
3
Max-Planck-Institut für Astronomie (MPIA),
Königstuhl 17,
69117
Heidelberg,
Germany
4
Department of Physics and Astronomy, LaserLaB, VU
University, de Boelelaan
1081, 1081 HV
Amsterdam, The
Netherlands
5
Research School of Physics and Engineering, The Australian
National University, ACT,
0200
Canberra,
Australia
6
Max-Planck Institut für Extraterrestrische Physik (MPE),
Giessenbachstraße.
1, 85748
Garching,
Germany
Received:
11
October
2013
Accepted:
16
December
2013
Context. Photodissociation of 14N2 and 14N 15N occurs in interstellar clouds, circumstellar envelopes, protoplanetary discs, and other environments due to ultraviolet radiation originating from stellar sources and the presence of cosmic rays. This source of N atoms initiates the formation of more complex N-bearing species and may influence their isotopic composition.
Aims. We study the photodissociation rates of 14N 15N by ultraviolet continuum radiation and both isotopologues in a field of cosmic ray induced photons. To determine the effect of these on the isotopic composition of more complex molecules.
Methods. High-resolution theoretical photodissociation cross sections of N2 are used from an accurate and comprehensive quantum-mechanical model of the molecule based on laboratory experiments. A similarly high-resolution spectrum of H2 emission following interactions with cosmic rays has been constructed. The spectroscopic data are used to calculate photodissociation rates which are then input into isotopically differentiated chemical models, describing an interstellar cloud and a protoplanetary disc.
Results. The photodissociation rate of 14N 15N in a Draine field assuming 30 K excitation is 1.73 × 10-10 s-1, within 4% of the rate for 14N2, and the rate due to cosmic ray induced photons assuming an H2 ionisation rate of ζ = 10-16 s-1 is about 10-15 s-1, with up to a factor of 10 difference between isotopologues. Shielding functions for 14N15N by 14N2, H2, and H are presented. Incorporating these into an interstellar cloud model, an enhancement of the atomic 15N/14N ratio over the elemental value is obtained due to the self-shielding of external radiation at an extinction of about 1.5 mag. This effect is larger where assumed grain growth has reduced the opacity of dust to ultraviolet radiation. The transfer of photolytic isotopic fractionation of N and N2 to other molecules is demonstrated to be significant in a protoplanetary disc model with grain growth, and is species dependent with 15N enhancement approaching a factor of 10 for HCN. The cosmic ray induced dissociation of CO is revisited employing a more recent photodissociation cross section, leading to a rate that is ~40% lower than previously calculated.
Key words: cosmic rays / ISM: molecules / protoplanetary disks / photon-dominated region (PDR) / molecular processes / radiation mechanisms: non-thermal
© ESO, 2014
1. Introduction
Nitrogen is the fifth most abundance element in the universe and comprises two isotopes with relative populations 14N/15N ≃ 400. The dominant reservoir of elemental N is in the form of atoms and N2, which are essentially unobservable outside of the solar system, the former because its ionic form, N+, is readily removed by charge exchange with neutral hydrogen with the latter lacking a rotational emission spectrum because it is homonuclear. The observation of electronic transitions of N2 is limited to technically-challenging ultraviolet (UV) wavelengths shorter than 1000 Å.
There have been numerous detections of N-derived molecules such as N2H+, CN, HCN, and NH3 in interstellar space, protostellar envelopes and discs, and extra-galactically, (e.g., van Zadelhoff et al. 2001; Bergin et al. 2002; Gerin et al. 2009; Öberg et al. 2010; Tobin et al. 2012), as well as for their isotopologues (e.g., Wannier et al. 1991; Gerin et al. 2009; Adande & Ziurys 2012; Hily-Blant et al. 2013; Bizzocchi et al. 2013; Daniel et al. 2013). The balance of chemically active N and highly stable N2 is an important parameter in the chemistry of these more complex amines and nitriles. The primary driver of this balance in environments exposed to an ultraviolet flux is photodissociation of N2.
The value of 14N/15N in observable species
constitutes an astronomical puzzle. The solar elemental ratio, 440 (Marty et al. 2010), is considered representative of the local
interstellar medium, but differs from the terrestrial value, 270; in the atmospheric of
Titan, 180 (Niemann et al. 2005); and in
interplanetary dust, meteorites and comets, 68–330 (Floss et
al. 2006; Jehin et al. 2009; Aléon 2010; Mumma
& Charnley 2011). Observational surveys have been made of galactic
star-forming regions (Adande & Ziurys 2012)
and dark clouds (Hily-Blant et al. 2013) and also
point to a range of values, as well as variations by source and the molecular species
observed. Some variation of 14N/15N is clearly due to the details of
local nuclear synthesis, with an increasing excess of 15N towards the galactic
centre (Wilson 1999; Adande & Ziurys 2012). Another proposed explanation for the observed
ratios of C14N/C15N,
HC14N/HC15N and
14NH3/15NH3 is
low-temperature isotopic-exchange chemistry. Models considering this mechanism (e.g., Rodgers & Charnley 2008; Wirström et al. 2012; Le Gal et al.
2014) have been successful in introducing strongly non-elemental abundance ratios
under specific conditions. The critical exchange reactions, e.g.,
 rely
on the lower zero-point energy of the heavier molecular species and are exothermic by ≤30 K
(Terzieva & Herbst 2000), limiting their
significance to cold environments. This is in contrast to similar reactions affecting the
H/D abundance in molecules which may proceed at temperatures as high as 170 K.
rely
on the lower zero-point energy of the heavier molecular species and are exothermic by ≤30 K
(Terzieva & Herbst 2000), limiting their
significance to cold environments. This is in contrast to similar reactions affecting the
H/D abundance in molecules which may proceed at temperatures as high as 170 K.
An alternative route to the mass fractionation of molecules is isotope selective photodissociation. For the case of N2 dissociation, self-shielding has been shown to drive the HC14N/HC15N ratio in the atmosphere of Titan (Liang et al. 2007). Additionally, there is strong observational evidence that the fractionation of protostellar oxygen isotopes is assisted by the self-shielding of CO, which has very similar molecular structure to N2 (Sheffer et al. 2002; Lyons & Young 2005; Smith et al. 2009). The investigation of isotope selective N2 photodissociation in interstellar clouds and protoplanetary environments is then highly warranted.
The importance of photodissociation due to the interstellar radiation field (ISRF) or an ultraviolet emitting star will decrease as the radiation is attenuated below the surface of an interstellar cloud, circumstellar envelope, or protoplanetary disc. Li et al. (2013) made a detailed assessment of the dissociation rate of 14N2 and the shielding of ultraviolet radiation by relevant materials (H2, H, 14N2 itself, and dust grains). This work considered in detail the sharply structured absorption spectrum of N2 and shielding species. This was made possible by means of an accurate and comprehensive quantum-mechanical model of N2 photoabsorption and dissociation, which was informed by an extensive history of N2 spectroscopy in the laboratory (e.g., Ajello et al. 1989; Helm et al. 1993; Sprengers et al. 2003, 2004, 2005a; Stark et al. 2008; Lewis et al. 2008b; Heays et al. 2009, 2011) and theoretically (e.g., Spelsberg & Meyer 2001; Lewis et al. 2005b,a, 2008a,c; Haverd et al. 2005; Ndome et al. 2008). This model, detailed in Sect. 2, explicitly accounts for the nuclear masses and so is equally applicable to the case of 14N 15N. In Sect. 3 of this paper, we describe new calculations of 14N 15N photodissociation rates in interstellar and blackbody radiation fields.
The interior regions of interstellar clouds and protoplanetary discs may be completely shielded from external sources of ultraviolet photons. However, collisions between high-energy cosmic rays and H2 produce a weak local UV field that could be important for photodissociation. The cosmic ray induced photoionisation and photodissociation of molecular and atomic species is well established (Gredel et al. 1987, 1989; Dalgarno 2006; Padovani et al. 2009). In Sect. 4 we combine our model of N2 photoabsorption with a similarly thorough theoretical treatment of the H2 line emission associated with cosmic rays. This is, to study in detail for the first time the photodissociation of 14N2 and 14N 15N in cloud and disc interiors. We also revisit calculations of the CO dissociation rate due to cosmic rays (Gredel et al. 1987) taking advantage of an updated photodissociation cross section.
In Sect. 5 of this paper we present the results of chemical models of an interstellar cloud and protoplanetary disc. These include linked chemical networks for species containing 14N and 15N and the photodissociation rates of Li et al. (2013) and Sects. 3 and 4. Using these models we investigate the consequences of isotope selective photodissociation on the abundances of atomic and molecular nitrogen and other N-bearing species observed in clouds and discs.
2. The model of photoabsorption and photodissociation
The photodissociation of N2 first requires excitation into bound states that then rapidly predissociate. Thus, the photoabsorption spectrum contains well separated electronic-rovibrational lines and must be treated at high resolution (~10-3 Å) but over a comparatively large wavelength range (912–1000 Å). Actually, N2 absorbs strongly at considerably shorter wavelengths but the present application is limited to longwards of the atomic H ionisation threshold at 912 Å. Here, a broadband and detailed spectrum is calculated by means of a quantum mechanical model which solves a coupled Schrödinger equation (CSE) for the vibrational wavefunction of the molecule. Properties of the electronic wavefunction are described by potential-energy curves and state coupling parameters optimised with respect to a large database of laboratory line positions, oscillator strengths, and predissociation rates. These empirical parts of the CSE model formulation are independent of molecular mass, and so are unchanged by isotopic substitution of one or both nuclei. Then, the explicit calculation of vibrational wavefunctions models the quantum mixing of electronic states and its dependence on the nuclear masses. A more detailed description of the method, the important electronic states and potential-energy curves used in the model is given in our previous publication (Li et al. 2013), and the chemical physics literature (Lewis et al. 2005b,a, 2008c; Haverd et al. 2005; Heays 2011) with specific discussion of 14N 15N given in Vieitez et al. (2008) and Heays et al. (2011).
Electric-dipole-allowed photoabsorption from the ground state occurs for wavelengths
shorter than 1000 Å and accesses two valence states and several Rydberg series of
 and
and
 symmetry.
Potential-energy curves depicting these states are shown in Fig. 1 with the lowest-energy photoabsorbing state,
b 1Πu, occurring at 12.6 eV.
There is strong electronic coupling between valence and Rydberg states of the same symmetry
which leads to a very perturbed spectrum, including constructive and destructive
interference in the absorption strength and predissociation rates of vibrational bands
(Dressler 1969; Spelsberg & Meyer 2001; Lewis et al.
2005b). Additional rotational interactions between
symmetry.
Potential-energy curves depicting these states are shown in Fig. 1 with the lowest-energy photoabsorbing state,
b 1Πu, occurring at 12.6 eV.
There is strong electronic coupling between valence and Rydberg states of the same symmetry
which leads to a very perturbed spectrum, including constructive and destructive
interference in the absorption strength and predissociation rates of vibrational bands
(Dressler 1969; Spelsberg & Meyer 2001; Lewis et al.
2005b). Additional rotational interactions between
 and
and
 states
lead to strong rotational dependence of line strengths and predissociation rates (or
linewidths) (Stark et al. 2005; Heays et al. 2011).
states
lead to strong rotational dependence of line strengths and predissociation rates (or
linewidths) (Stark et al. 2005; Heays et al. 2011).
 |
Fig. 1 Diabatic-basis potential-energy curves of N2 excited states relevant to
ultraviolet photoabsorption and photodissociation. The energy scale is referenced to
v = 0, J = 0 of the
|
Predissociation occurs through spin-orbit coupling of
 states with a
number of states with
states with a
number of states with  symmetry,
including an unbound level, and one state of
symmetry,
including an unbound level, and one state of  symmetry.
These are shown in Fig. 1 and lead to dissociation into
N atoms in the 4S, 2D, or 2P states. The spin-selection
rules forbid the appearance of N2 triplet states in optical spectra although many
have been observed in the laboratory regardless, due to their coupling with
symmetry.
These are shown in Fig. 1 and lead to dissociation into
N atoms in the 4S, 2D, or 2P states. The spin-selection
rules forbid the appearance of N2 triplet states in optical spectra although many
have been observed in the laboratory regardless, due to their coupling with
 levels (Sprengers et al. 2005a; Lewis et al. 2008b). The majority of excited vibrational states predissociate
after an absorption event with near certainty, that is, they have a predissociation fraction
of ~1. For those states that decay substantially by emission, a reduction of the
CSE-calculated photoabsorption cross section, σabs, has been
made by their less-than-unity predissociation fractions, ηpre,
leading to a photodissociation cross section σpd, so that
levels (Sprengers et al. 2005a; Lewis et al. 2008b). The majority of excited vibrational states predissociate
after an absorption event with near certainty, that is, they have a predissociation fraction
of ~1. For those states that decay substantially by emission, a reduction of the
CSE-calculated photoabsorption cross section, σabs, has been
made by their less-than-unity predissociation fractions, ηpre,
leading to a photodissociation cross section σpd, so that
 (1)
(1)
This provides an example of the utility of the physically-based CSE model. That is, the
b 1Πu(v = 1)
level of 14N2 is known to predissociate significantly less rapidly
than for 14N 15N (Sprengers et al. 2004,
2005b; Wu et al.
2012) because of mass-change-induced shifts of the interacting
 and
and
 levels. These
shifts are evaluated explicitly by the CSE model and calculated predissociation fractions
for b 1Πu(v = 1)
are shown in Fig. 2 for rotational levels with angular
momentum quantum number, J ≤ 30.
levels. These
shifts are evaluated explicitly by the CSE model and calculated predissociation fractions
for b 1Πu(v = 1)
are shown in Fig. 2 for rotational levels with angular
momentum quantum number, J ≤ 30.
 |
Fig. 2 An example of isotopically-dependent predissociation. The predissociation fraction, ηpre, of individual rotational levels, J, of b 1Πu(v = 1) calculated by the CSE model for 14N2 and 14N15N. |
There is a strong dependence on rotational angular momentum, J, for both isotopologues. At the low temperatures of interstellar space only rotational levels with J < 10 are accessible. Then, photons absorbed by 14N 15N and leading to excitation of the b 1Πu(v = 1) level are nearly twice as likely to lead to a dissociation event than for 14N2.
We have calculated 14N 15N photoabsorption and photodissociation cross sections as Li et al. (2013) did for 14N2. A comparison is made in Fig. 3 between transmission functions derived from the two CSE-calculated photoabsorption cross sections. The principal difference is a shift of 14N 15N absorption bands to longer wavelengths which mostly increases with vibrational excitation, that is, with shortening wavelength. However, there are many further differences between the spectra of different N2 isotopologues due to the strong coupling between excited states, such as depicted in Fig. 2 with respect to the b 1Πu(v = 1) predissociation fraction, and these should not be neglected (e.g., Vieitez et al. 2008; Lewis et al. 2008b; Heays et al. 2011).
 |
Fig. 3 Upper panels: representative transmission functions
calculated from theoretical photoabsorption cross sections of important molecular
species, with column densities: N(H) = 1022,
N(H2) = 1020, and
|
The wavelengths of the majority of the modelled rovibrational lines are accurate to within
0.01 Å when compared with laboratory measurements (Heays
2011). The magnitudes of the calculated cross sections are accurate to about 10%,
with their principal uncertainty arising from the absolute calibration of the laboratory
absorption oscillator strengths used to constrain the model electronic transition moments
(Stark et al. 2005, 2008; Heays et al. 2009). The
predissociation fractions pertaining to the longest wavelength bands are well known and
modelled (Lewis et al. 2005b) but are less certain at
shorter wavelengths. For these, a predissociation fraction of 1 is assumed. This is a
reliable assumption with respect to  states, many of
which are known to exhibit significant predissociation broadening (Stark et al. 2008; Heays et al.
2011). It is less certain that all
states, many of
which are known to exhibit significant predissociation broadening (Stark et al. 2008; Heays et al.
2011). It is less certain that all  levels
responsible for absorption at shorter wavelengths are completely predissociative. Further
practical details regarding the calculation of N2 cross sections and dissociation
fractions at different temperatures may be found in Li et
al. (2013).
levels
responsible for absorption at shorter wavelengths are completely predissociative. Further
practical details regarding the calculation of N2 cross sections and dissociation
fractions at different temperatures may be found in Li et
al. (2013).
Also shown in Fig. 3 are transmission functions representing further line-absorbing species relevant to the following treatment of the shielding of N2 absorption in astrophysical environments. For these, photoabsorption cross sections for H2 are synthesised from data obtained from the Meudon PDR code website (Le Petit et al. 2006), and those for CO are taken from the model of Visser et al. (2009).
3. Photodissociation rates and shielding functions
The photodissociation rate, k0, of N2 exposed to UV
radiation can be calculated according to  (2)where
σpd(λ) is the N2 photodissociation
cross section described in Sect. 2, I
is the intensity of the radiation field, and λ the wavelength. The
unattenuated interstellar radiation field (ISRF) of Draine
(1978), in units of photons cm-2 s-1
Å-1 sr-1, is used in most of the following calculations and is given
by
(2)where
σpd(λ) is the N2 photodissociation
cross section described in Sect. 2, I
is the intensity of the radiation field, and λ the wavelength. The
unattenuated interstellar radiation field (ISRF) of Draine
(1978), in units of photons cm-2 s-1
Å-1 sr-1, is used in most of the following calculations and is given
by  (3)with
λ in Å. In the following we employ an angle-integrated intensity,
(3)with
λ in Å. In the following we employ an angle-integrated intensity,
 (4)with an additional
scaling factor relative to the Draine field, χ.The integral in Eq. (2) must be computed between 912 and 1000 Å. This
range is defined by the long-wavelength onset of the N2 absorption spectrum and
the complete suppression of ultraviolet radiation for wavelengths shorter than the atomic H
ionisation limit.
(4)with an additional
scaling factor relative to the Draine field, χ.The integral in Eq. (2) must be computed between 912 and 1000 Å. This
range is defined by the long-wavelength onset of the N2 absorption spectrum and
the complete suppression of ultraviolet radiation for wavelengths shorter than the atomic H
ionisation limit.
The photodissociation rates of 14N2 and
 calculated from
Eq. (2) are given in Table 1 assuming several different radiation fields. In all
cases the difference between 14N2 and
calculated from
Eq. (2) are given in Table 1 assuming several different radiation fields. In all
cases the difference between 14N2 and
 rates is below 5%,
and the calculated dependences on the N2 ground state excitation temperature and
the form of the incident field (a Draine ISRF, or blackbody radiation) are similar to those
calculated by Li et al. (2013) for
14N2.
rates is below 5%,
and the calculated dependences on the N2 ground state excitation temperature and
the form of the incident field (a Draine ISRF, or blackbody radiation) are similar to those
calculated by Li et al. (2013) for
14N2.
For N2 embedded in an externally irradiated cloud or disc, the intervening
gaseous and granular material acts as a shield and leads to a reduced photodissociation
rate, k < k0. The
precise nature of shielding by H, H2, 14N2, and
 is influenced by
the wavelength dependence of their absorption cross sections, but is more usefully applied
as a wavelength-integrated ratio of shielded and unshielded dissociation rates. That is, the
shielding function given by
is influenced by
the wavelength dependence of their absorption cross sections, but is more usefully applied
as a wavelength-integrated ratio of shielded and unshielded dissociation rates. That is, the
shielding function given by ![\begin{eqnarray} \notag \theta &=& \frac{k}{k_0} \\ \label{eq:def shielding function} &=& \frac{ \int I(\lambda) \exp\left[-\sum_{X} N_{X}\sigma^\mathrm{abs}_{X}(\lambda)\right] \exp\left( -\gamma_\mathrm{dust} A_V \right) \sigma^\mathrm{pd}(\lambda)\,{\rm d}\lambda} {\int I(\lambda)\sigma^\mathrm{pd}(\lambda)\,{\rm d}\lambda}\cdot \end{eqnarray}](/articles/aa/full_html/2014/02/aa22832-13/aa22832-13-eq62.png) (5)Here,
(5)Here,
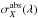 , is the absorption cross section
of shielding species X and NX
its shielding column density.
, is the absorption cross section
of shielding species X and NX
its shielding column density.
The unattenuated photodissociation rates (k0, s-1) of N2 exposed to a completely-surrounding Draine interstellar radiation field for various excitation temperatures (Tex), and for blackbody radiation with temperature Tbb and Tex = 30 K.
The term exp(−γdustAV) in Eq. (5) describes shielding of the UV flux by dust grains, where the visual (5500 Å) extinction is assumed proportional to the column density of hydrogen nuclei, NH, according to AV = NH/1.6 × 1021 (Savage et al. 1977). In principle, this is a wavelength dependent quantity which depends on the distribution of dust grain sizes, their composition, and their geometries. The use of a simple parameterisation of declining intensity with increasing visual extinction is warranted by the significant uncertainties in these parameters. Radiative transfer calculations considering the destruction and scattering of photons by a realistic distribution of interstellar dust grains were made by Roberge et al. (1981, 1991). The ultraviolet extinction relevant to various molecules was parameterised by van Dishoeck et al. (2006) as a decaying exponential like that in Eq. (5) using the the dust optical properties of Roberge et al. (1991). Their extinction calculated for the wavelength range appropriate to CO is adopted here for N2, that is with γdust = 3.53. Further calculations were made by van Dishoeck et al. (2006) assuming larger dust grains (>1 μm in size) as identified in protoplanetary discs (Li & Lunine 2003b; Jonkheid et al. 2006). For this case γdust = 0.6.
 |
Fig. 4 Top left: self-shielding functions for
|
Detailed shielding functions for 14N2 were given in Li et al. (2013) and the associated on-line material.
Here, new calculations are made for  using Eq. (5), with some representative results plotted in
Fig. 4, and full details are available from the Leiden
photodissociation database1. The data in Fig. 4 assume that the lines of shielding H and H2
are Doppler broadened by b = 1 and 3 km s-1, respectively.
The N2 lines are assigned thermal Doppler widths according to their excitation
temperatures but are also naturally broadened by the predissociation process, in some cases
by a few km s-1.
using Eq. (5), with some representative results plotted in
Fig. 4, and full details are available from the Leiden
photodissociation database1. The data in Fig. 4 assume that the lines of shielding H and H2
are Doppler broadened by b = 1 and 3 km s-1, respectively.
The N2 lines are assigned thermal Doppler widths according to their excitation
temperatures but are also naturally broadened by the predissociation process, in some cases
by a few km s-1.
Unsurprisingly, the similarity of the two isotopologues leads to very similar shielding
functions. There is some difference evident in Fig. 4
with respect to shielding by atomic hydrogen. That is, the shielding of
 by a
N(H) = 1020 cm-2 column is somewhat more
effective than for 14N2. This is the result of a nearer alignment of
the
by a
N(H) = 1020 cm-2 column is somewhat more
effective than for 14N2. This is the result of a nearer alignment of
the  absorption band of
14N2 and the line-centre of the shielding 4p ← 1s transition of
atomic hydrogen. The relevant 14N2, 14N 15N, and H features
are plotted in Fig. 3 near 972 Å. For a larger H
column, like that in Fig. 3, with heavily saturated and
broadened H lines, the misalignment of H and 14N2 transitions near 972
Å loses its significance.
absorption band of
14N2 and the line-centre of the shielding 4p ← 1s transition of
atomic hydrogen. The relevant 14N2, 14N 15N, and H features
are plotted in Fig. 3 near 972 Å. For a larger H
column, like that in Fig. 3, with heavily saturated and
broadened H lines, the misalignment of H and 14N2 transitions near 972
Å loses its significance.
Also shown in Fig. 4 is the shielding of 14N 15N photodissociation by 14N2. This rivals 14N 15N self-shielding because of the much greater abundance of 14N2. This is particularly true at higher temperatures where the wavelength offset between isotopologues is compensated for by their broader rotational distributions and Doppler widths. A discussion of the relative importance of different species in mixed shielding media is given in Sect. 5 with respect to an example protoplanetary disc.
Shielding functions calculated according to Eq. (5) describe the reduction in photodissociation rate due to the attenuation of
unidirectional radiation. A related quantity,
θiso(N), describes this reduction as a
function of column density for an extended medium illuminated isotropically at its surface.
The resultant non-perpendicular rays penetrate less deeply and
θiso(N) is smaller than the shielding
function in the unidirectional case, θ(N) (Röllig et al. 2007). For a plane-parallel shielding
medium with N defined perpendicular to its surface, the two shielding
functions are related geometrically by the formula  (6)
(6)
4. Photodissociation due to cosmic-rays
4.1. Cosmic-ray induced emission and photodissociation model
The ionisation of H2 by cosmic ray collisions leads to the generation of free electrons. These may collisionally excite (or ionise) further H2 whose radiative relaxation generates an ultraviolet flux (Prasad & Tarafdar 1983; Cecchi-Pestellini & Aiello 1992). Sternberg et al. (1987), Gredel et al. (1987), and Gredel et al. (1989) estimated the photodissociation properties of several small molecules due to ultraviolet photons arising from this process. Similar calculations are made here for the dissociation of N2 including the detailed effects of line absorption as modelled by the CSE dissociation cross sections.
The H2 emission flux between 912 and 1000 Å is dominated by line transitions.
This emission occurs primarily through the Lyman and Werner bands connecting the ground
state with the  and
C 1Πu excited states (Sternberg et al. 1987), and with a smaller contribution
arising from other excited states (Gredel et al.
1989). Here, we use a model simulating the excitation of 9 electronic states of
H2 by cosmic-ray generated electrons and the resulting emission, developed
and expounded in detail by Gredel et al. (1987,
1989). This model includes the effects of
cascading from sufficiently high-energy states into
and
C 1Πu excited states (Sternberg et al. 1987), and with a smaller contribution
arising from other excited states (Gredel et al.
1989). Here, we use a model simulating the excitation of 9 electronic states of
H2 by cosmic-ray generated electrons and the resulting emission, developed
and expounded in detail by Gredel et al. (1987,
1989). This model includes the effects of
cascading from sufficiently high-energy states into
 and competing
predissociative decay.
and competing
predissociative decay.
The final result of the H2 emission model is a spectrum, P(λ), describing the number of photons generated per unit spectral density per H2 ionisation event. A plot of P(λ) is shown in Fig. 3 and demonstrates a large number of narrow emission lines which actually occupy very little of the wavelength interval shown. The widths of these lines are Doppler limited and assigned full-width half-maxima of 0.005 Å, which is appropriate for a typical dark-cloud non-thermal Doppler broadening of b = 1 km s-1. This is comparable to the widths of N2 absorption lines, also shown in Fig. 3, so model spectra of both species are treated at similarly high resolution. Spectra are shown in Fig. 3 assuming limiting high- and low-temperature ratios for the ortho:para H2 populations, o−H2:p−H2 = 3:1 and 0:1, respectively. The difference is quite apparent and is significant in the following calculations of N2 photodissociation. Despite the 170 K energy separation of o−H2 and p−H2 ground-state levels it is not evident that their low-temperature equilibrium populations are always attained in real molecular clouds (Tielens 2013). Thus, we choose to contrast high and low temperature limits in the present study.
The rate of ultraviolet photons generated by cosmic ray collisions per unit spectral
density per hydrogen nucleus is given by  (7)
(7)
where xH2 = n(H2)/[n(H) + 2n(H2)] is the relative abundance of molecular hydrogen with respect to H nuclei and ζ is the cosmic-ray-induced ionisation rate per H2 molecule. A value of ζ = 10-16 s-1 per H2 is adopted for the following calculations and falls within the broad range of rates estimated from observations of ions in diffuse and translucent clouds (Dalgarno 2006; Padovani et al. 2009; Indriolo & McCall 2012; Rimmer et al. 2012). This collection of observations suggest a rate as high as 10-15 s-1 at the edge of an interstellar cloud, which decreases with extinction to ~10-16 s-1 for AV = 10 mag as the lowest-energy cosmic rays are attenuated. Our adopted value is higher than observed in the interiors of the densest clouds with ζ < 10-17 s-1 (Hezareh et al. 2008). Theoretical consideration of a range of source and attenuation mechanisms influencing the cosmic ray flux (Padovani et al. 2009; Cleeves et al. 2013) suggest a smaller rate ζ than 10-16 s-1 may be expected in circumstellar environments, because of the possible influence of any stellar wind and magnetic field. The photodissociation results presented here may be adapted to ionisation rates other than ζ = 10-16 s-1 by a simple scaling.
The cosmic-ray generated photons may be absorbed by dust grains and by abundant
line-absorbing species: H2, H, N2, and CO. The idea of an absorbing
column density is inappropriate because of the local nature of the photon source. Instead,
the fraction of ultraviolet radiation absorbed by a particular species,
X, and leading to its dissociation is determined from its relative
abundance xX, according to  (8)Here,
xi and
(8)Here,
xi and
 are the abundances and
photoabsorption cross sections of various species,
xdustσdust represents dust
absorption, and
are the abundances and
photoabsorption cross sections of various species,
xdustσdust represents dust
absorption, and  is a photodissociation cross
section.
is a photodissociation cross
section.
An important term in the summation in Eq. (8) arises from H2. For the cases of X being 14N2 or CO, self-shielding is important and must also be included. Self-shielding and shielding by 14N2 are both important for the case of 14N 15N photodissociation. Atomic hydrogen is also a source of UV shielding at the edge of a cloud or disc but is assumed here to have too low abundance (about 10-4 relative to H2) to be important in fully shielded regions. Consequently, the common assumption that all radiation shortwards of 912 Å is completely consumed by H ionisation does not apply here. However, the N2 photodissociation cross section at shorter wavelengths and approaching the photoionisation limit at 800 Å contributes less than 10% to its total photodissociation rate, and is neglected here.
Shielding of a penetrating interstellar radiation field by dust grains was discussed in
Sect. 3. The extinction of cosmic-ray induced radiation is
treated differently. In this case, all photons are assumed absorbed before traversing a
significant fraction of the dark interior of the interstellar cloud or disk where they are
generated. Then, the detailed angular scattering of photons by dust grains is
inconsequential and only the absorption cross section need be considered. The dust
absorption cross section is related to its extinction cross section by  (9)
(9)
where ω is the grain albedo. We adopt a commonly used value derived from
observations (Savage et al. 1977; Bohlin et al. 1978) for the dust extinction cross
section per hydrogen nucleus of 2 × 10-21 cm2 and assume an albedo
of ω = 0.5. Then,  in Eq.
(8).
in Eq.
(8).
The assumption of locally-absorbed UV photons is only appropriate for a sufficiently large and homogeneous medium. The mean-free-path of cosmic-ray generated photons in an interstellar cloud of density nH = 103 cm-3 is 30 000 AU, assuming the UV-to-visual extinction factor of 3.53 discussed in Sect. 3. For the larger densities found near the midplane of a protoplanetary disc (Jonkheid et al. 2006) this distance is accordingly reduced, e.g., nH = 108 cm-3 and ~1 AU. In principle, the various cosmic-ray induced photodissociation rates calculated below will be overestimated for objects which are not significantly larger than this mean-free-path, due to the escape of some UV photons. However, the significance of cosmic-ray induced photon fluxes is generally limited to regions fully-shielded from external UV radiation, and such regions also necessarily shield the escape of internally-generated photons.
The dust grains in a protoplanetary disc are likely larger than those in interstellar
space, which have radii <1 μm (Mathis et al. 1977). Li & Lunine
(2003b) deduced properties of the dust grains in one protoplanetary system,
HD 4796A, by reference to observed infrared and sub-millimetre wavelength emission. Their
modelling required a lower limit of 1 μm on the grain size distribution.
The N2-dissociating cosmic-ray induced UV flux considered here has wavelengths
shorter than 1000 Å and will not be as strongly absorbed by grains larger than this.
However, a population of polycyclic aromatic hydrocarbons (PAHs) has been inferred in a
number of discs (e.g., Li & Lunine 2003a;
Geers et al. 2006, 2007; Maaskant et al. 2013) and
will absorb extra UV radiation (Siebenmorgen &
Krügel 2010). Chaparro Molano & Kamp
(2012) assumed a dust distribution with a minimum grain size of
0.1 μm in their study of cosmic-ray induced photodissociation in a
model disc. Following from this assumption they deduced a value of
 ,
significantly below the commonly-adopted interstellar value. Here, we explore an even
lower value,
,
significantly below the commonly-adopted interstellar value. Here, we explore an even
lower value,  , in
order to contrast a more extreme case of grain growth in a protoplanetary disc with
interplanetary dust.
, in
order to contrast a more extreme case of grain growth in a protoplanetary disc with
interplanetary dust.
Finally, the photodissociation rate of species X (per molecule
X) due to cosmic ray induced photons may be calculated according to
 (10)In some previous
descriptions of cosmic-ray induced photodissociation (Gredel et al. 1989; McElroy et al. 2013)
an efficiency is defined by factoring the H2 ionisation rate and dust grain
albedo from Eq. (10). In this case, the
importance of molecular line absorption in Eq. (8) prevents the factoring of dust properties.
(10)In some previous
descriptions of cosmic-ray induced photodissociation (Gredel et al. 1989; McElroy et al. 2013)
an efficiency is defined by factoring the H2 ionisation rate and dust grain
albedo from Eq. (10). In this case, the
importance of molecular line absorption in Eq. (8) prevents the factoring of dust properties.
4.2. Cosmic-ray induced photodissociation of N2
Photodissociation rate per molecule due to cosmic-ray induced radiation.
The rate of dissociation of 14N2 and 14N 15N due to cosmic-ray induced ultraviolet radiation has been calculated and listed in Table 2. This rate is dependent on the local physical conditions, and a set of base conditions is chosen which plausibly simulate the interior of a dark interstellar cloud:
-
The assumed abundances of 14N2 and CO relative to nH = n(H) + 2n(H2) are 10-5 and 7 × 10-5, respectively, and reflect typical dense cloud abundances (Aikawa et al. 2008).
-
A
 ratio of
225 is chosen, appropriate for the solar elemental ratio of 450 (Marty et al. 2010).
ratio of
225 is chosen, appropriate for the solar elemental ratio of 450 (Marty et al. 2010). -
All molecular species are assumed to have a thermal excitation of Tex = 20 K and be turbulently broadened with Doppler velocity b = 1 km s-1.
-
Dust grains are assumed to have a scattering cross section per H nucleus of
 .
.
-
The high temperature limit, 3:1, is assumed for the ortho:para ratio of emitting H2.
Further calculations listed in Table 2 probe variations on this set of parameters.
For the base set of parameters considered in Table 2, the 14N2 photodissociation rate is 14 × 10-16 s-1. This is 5 orders of magnitude below the rate calculated for a standard interstellar radiation field in Sect. 3. The cosmic-ray induced photodissociation rates calculated by Gredel et al. (1989) for a large number of other molecular species are generally larger than the N2 value determined here, in many cases by multiple orders of magnitude. For example, assuming ζ = 10-16 s-1 and a grain albedo of ω = 0.5, their photodissociation rates for H2O and CN are 2.0 × 10-14 and 1.8 × 10-13 s-1, respectively.
The self-shielding of 14N2 cannot be neglected because of the
resonant nature of its spectrum. For this reason, decreasing the
14N2 density rapidly increases the dissociation rate, until the
abundance relative to H nuclei,  ,
falls below about 10-7, as is evident from the values in Table 2. Line absorption of H2 also effectively
shields N2 dissociation, reducing its rate by a factor of 2. Dust opacity
reduces the
,
falls below about 10-7, as is evident from the values in Table 2. Line absorption of H2 also effectively
shields N2 dissociation, reducing its rate by a factor of 2. Dust opacity
reduces the  dissociation
rate by a further 35%, and shielding by CO less than 5%.
dissociation
rate by a further 35%, and shielding by CO less than 5%.
 |
Fig. 5 Modelled molecular absorption lines for two different excitation temperatures, 20
and 100 K, and cosmic-ray induced H2 emission. Otherwise calculated as
for Fig. 3. Left: overlap of
an o-H2 emission line with the
|
The effective dissociation of 14N2 arises from relatively few
overlaps between its absorption lines and H2 emission lines. These are
indicated by steps in an accumulation of the integrated dissociation rate with wavelength,
as shown in Fig. 6. This accumulation is dominated by
a line overlap occurring at 960.45 Å, plotted in detail in Fig. 5. This corresponds to the P(4) line of the
 absorption band of
14N2 and the P(3) line of the
absorption band of
14N2 and the P(3) line of the
 H2 Lyman emission
band. The significance of this overlap is dependent on the assumed excitation temperatures
of absorbing 14N2 and shielding H2. When
Tex = 20 K, also indicated in Figs. 5 and 6, the fraction of
N2 ground state molecules in the J = 4 rotational level is
low and the significance of the 960.45 Å overlap is small. For
Tex = 100 K, the J = 3 level population of
ground state H2 becomes significant and P(3) absorption
effectively shields 14N2 photodissociation. Thus, the largest
14N2 photodissociation rate occurs at intermediate temperature.
H2 Lyman emission
band. The significance of this overlap is dependent on the assumed excitation temperatures
of absorbing 14N2 and shielding H2. When
Tex = 20 K, also indicated in Figs. 5 and 6, the fraction of
N2 ground state molecules in the J = 4 rotational level is
low and the significance of the 960.45 Å overlap is small. For
Tex = 100 K, the J = 3 level population of
ground state H2 becomes significant and P(3) absorption
effectively shields 14N2 photodissociation. Thus, the largest
14N2 photodissociation rate occurs at intermediate temperature.
Only small increases in the 14N2 photodissociation rate occur for increased turbulent broadening, b. Greater sensitivity is found with respect to the assumed o−H2:p−H2 ratio. This is limited to 0:1 and 3:1 at high and low temperatures, respectively, given sufficiently high densities for thermal equilibrium to be maintained. The 14N2 photodissociation rate is approximately halved when the low temperature limit is adopted, primarily because the H2 emission at 960.45 Å arises from an excited o−H2 level.
Table 2 also includes photodissociation rates
assuming reduced absorption by dust grains, in order to simulate the interior of a
protoplanetary disc. The adopted value of  effectively
removes dust from the competitive absorption of photons. The N2
photodissociation rate is then somewhat higher than for an interstellar cloud, but follows
the same trends with respect to temperature and the
o−H2:p−H2 ratio.
effectively
removes dust from the competitive absorption of photons. The N2
photodissociation rate is then somewhat higher than for an interstellar cloud, but follows
the same trends with respect to temperature and the
o−H2:p−H2 ratio.
Calculations of the cosmic-ray induced photodissociation of
 are also
presented in Table 2. The resultant rate assuming
the base conditions designed to represent a typical dark interstellar cloud is
21 × 10-16 s-1. This is slightly larger than for
14N2 and is the result of a different collection of overlapping
N2 absorption and H2 emission lines. The 14N 15N
dissociation rate is found to be much larger than for 14N2 in the
cold-temperature limit
o−H2:p−H2 = 0:1 and for
T ≃ 30 K. This is due to a strong overlap of H2 emission
lines near 913.1 and 913.3 Å with absorption lines corresponding to low rotational levels
of the
are also
presented in Table 2. The resultant rate assuming
the base conditions designed to represent a typical dark interstellar cloud is
21 × 10-16 s-1. This is slightly larger than for
14N2 and is the result of a different collection of overlapping
N2 absorption and H2 emission lines. The 14N 15N
dissociation rate is found to be much larger than for 14N2 in the
cold-temperature limit
o−H2:p−H2 = 0:1 and for
T ≃ 30 K. This is due to a strong overlap of H2 emission
lines near 913.1 and 913.3 Å with absorption lines corresponding to low rotational levels
of the  transition of
14N 15N, plotted in Fig. 5. The influence
of these overlaps dominate the accumulated dissociation rate shown in Fig. 6. The isotopologue difference between dissociation rates
is increased significantly when the reduced dust shielding in a protoplanetary disc is
accounted for and approaches a factor of 8. Thus, from Table 2, the most likely candidate for isotopic fractionation and an
enhancement of atomic 15N due to cosmic ray induced photodissociation occurs in
disc material around 30 K and with a small
o−H2/p−H2
ratio. The difference between 14N2 and 14N 15N
dissociation rates diminishes for temperatures below 30 K, and is negligible at 10 K when
assuming a low-temperature limit for
o−H2/p−H2.
transition of
14N 15N, plotted in Fig. 5. The influence
of these overlaps dominate the accumulated dissociation rate shown in Fig. 6. The isotopologue difference between dissociation rates
is increased significantly when the reduced dust shielding in a protoplanetary disc is
accounted for and approaches a factor of 8. Thus, from Table 2, the most likely candidate for isotopic fractionation and an
enhancement of atomic 15N due to cosmic ray induced photodissociation occurs in
disc material around 30 K and with a small
o−H2/p−H2
ratio. The difference between 14N2 and 14N 15N
dissociation rates diminishes for temperatures below 30 K, and is negligible at 10 K when
assuming a low-temperature limit for
o−H2/p−H2.
4.3. Cosmic-ray induced photodissociation of CO
We also combined the high-resolution H2 cosmic-ray induced emission model with the CO photodissociation cross sections of Visser et al. (2009). There is an example CO transmission spectrum plotted in Fig. 3 which exhibits similar band structure as for N2. The reduced symmetry of heteronuclear CO allows for greater perturbation of its excited states and leads to more rapid predissociation on average than for N2. The CO rotational absorption lines are then somewhat broader than for N2.
The CO photodissociation rates in a cosmic-ray induced UV field assuming various physical parameters are listed in Table 2 and are of a similar magnitude to the N2 rates. The on-average broader absorption lines of CO overlaps with H2 emission lines more frequently than for N2, and the resulting photodissociation is then less sensitive to variations in the assumed excitation temperature and o−H2:p−H2 ratio.
The calculated CO photodissociation rate in Table 2 for the base conditions representing an interstellar cloud is 8.1 × 10-16 s-1. This is somewhat less than the value calculated by Gredel et al. (1987), 13 × 10-16 s-1 (assuming ω = 0.5 and ζ = 10-16 s-1), who used a less sophisticated model of CO photodissociation.
5. Chemical models including photodissociation
5.1. Interstellar cloud model
A chemical model simulating an interstellar cloud was studied in order to explore the
details of  photodissociation due to an interstellar radiation field impinging on its surface and a
flux of cosmic rays within its interior. The model is similar to that of Li et al. (2013). Here, we used a modified version of
the UMIST 2012 chemical network (McElroy et al.
2013). The network was stripped down to species containing only H, He, C, N and
O; and a maximum of two C, N or O atoms. Supra-thermal chemistry was included to enhance
the formation of CH+ (and thus also CO) at low
AV, following Visser et al. (2009). This boosts the rate of ion-neutral reactions
after setting the Alfvén speed to 3.3 km s-1 for column densities less than
4 × 1020 cm-2.
photodissociation due to an interstellar radiation field impinging on its surface and a
flux of cosmic rays within its interior. The model is similar to that of Li et al. (2013). Here, we used a modified version of
the UMIST 2012 chemical network (McElroy et al.
2013). The network was stripped down to species containing only H, He, C, N and
O; and a maximum of two C, N or O atoms. Supra-thermal chemistry was included to enhance
the formation of CH+ (and thus also CO) at low
AV, following Visser et al. (2009). This boosts the rate of ion-neutral reactions
after setting the Alfvén speed to 3.3 km s-1 for column densities less than
4 × 1020 cm-2.
 |
Fig. 6 Dissociation rate of N2 due to cosmic-ray induced H2 emission under interstellar cloud conditions, integrated cumulatively with increasing wavelength. Rates for 14N2 are plotted with for several temperatures (solid curves), for and Tex = 20 K (black dotted curve), and a single case is included for 14N 15N (red dotted curve). |
Freeze-out and thermal evaporation for all neutral species were added to the gas-phase only UMIST network as well as the grain-surface hydrogenation reactions used by Visser et al. (2011). The latter include H2 formation and the conversion of C to CH4, N to NH3, O to H2O, and S to H2S.Photodesorption due to the UV field generated from cosmic-ray ionisation and the attenuated ISRF were included, but direct cosmic-ray desorption was not.
The wavelength dependence of the Draine ISRF was adopted for a unidirectional radiation field perpendicularly incident on the model cloud’s edge. This was scaled by a factor of χ = 2 so that one-sided irradiation of the cloud leads to the same unshielded dissociation rates at its edge as those given in Table 1. The cloud is assumed sufficiently thick that no radiation penetrates from the far side. Self-shielding of CO is computed using the shielding functions of Visser et al. (2009), for 14N2 we use those calculated by Li et al. (2013) at 30 K, and the presently calculated shielding functions are used for 14N 15N. The scattering of UV radiation out of the incident beam is not included in our model.
The wavelength dependence of the Draine ISRF was designed to simulate an isotropic field generated from a remote stellar population (Draine 1978), and some previous interstellar cloud models also assume isotropic radiation (Röllig et al. 2007). The transition between atomic and molecular H2 was shown by Röllig et al. (2007) to differ between irradiation geometries, occurring at lower AV for the isotropic case relative to unidirectional radiation. The transition for N2 occurring in our unidirectional model would be similarly shifted to shallower depth if the incident radiation were isotropic.
The elemental abundances relative to H are those of Aikawa et al. (2008): 0.0975 for He, 7.86 × 10-5 for C, 2.47 × 10-5 for N, and 1.80 × 10-4 for O. The elemental 14N:15N ratio was fixed to the protosolar value, 450:1. The rate of H2 ionisation due to cosmic rays was set to 5 × 10-17 s-1. The abundances of N, N2 and CO reach steady state after ~1 Myr, regardless of whether the gas starts in atomic or molecular form.
The cloud was assumed to have a constant hydrogen-nuclei density of nH = n(H) + 2n(H2) = 103 cm-3 and a temperature of 30 K. The turbulent broadening, b, of CO, H2, and H were set to 0.3, 3, and 5 km s-1; respectively; and N2 lines were assigned linewidths equivalent to their thermal Doppler width at 30 K, in an identical fashion to the similar model of Li et al. (2013).
The dust population was assigned a coefficient γdust = 3.53 in Eq. (5) to simulate grains with an interstellar size distribution, with radius ~0.1 μm size. A value for the cosmic-ray induced photodissociation rate of N2 was adopted corresponding to the low temperature o−H2:p−H2 ratio, 0:1.
The nitrogen reaction network was augmented so that separate accounting of pure 14N and single-15N containing species was possible. For this, all N-containing species and reactions were cloned and a 14N to 15N substitution made. Two kinds of isotope-differentiating reactions are included in our model chemical network: photodissociation and low-temperature ion-molecule exchange reactions. The selective effects of photodissociation are encoded in the shielding functions and cosmic-ray induced photodissociation rates presented in Sects. 3 and 4.2. Exchange reactions were added to our network using the rates listed in Table 2 of Terzieva & Herbst (2000). These reactions exothermically favour increased abundances of 15N in more complex species because of the lower zero-point energy of molecules with heavier nuclei, with typical excess energies of 10 to 30 K.
 |
Fig. 7 Top: abundances relative to H nuclei and left-to-right accumulated column densities of the isotopologues of N and N2 in the modelled interstellar cloud. Bottom: ratios of abundances and column densities of 14N- and 15N-bearing species. Also indicated are the adopted elemental ratios of 14N / 15N and 14N2 / 14N 15N (dashed lines). |
 |
Fig. 8 Top: abundances relative to H nuclei and right-to-left
accumulated column densities of atomic and molecular nitrogen, and
N2 ice in the modelled protoplanetary disc as a function of height
above the midplane at a radius of 105 AU. Also shown for species with a single
15N substitution i.e., 15N and 14N 15N (dotted
curves). Bottom: ratios of abundances and column densities of
14N- and 15N-bearing species. In some cases the ice and
gaseous N2 curves coincide. Also indicated is the elemental
|
 |
Fig. 9 Shielding functions for 14N2 and
|
Figure 7 shows the calculated abundances of
14N, 15N, 14N2, and
 as well as their
accumulated column densities, i.e.,
as well as their
accumulated column densities, i.e.,  (11)where
n(X) is the absolute abundance of species
X and
z(AV) is the distance from
the clouds edge for a particular extinction. The trend of these quantities as a function
of AV is qualitatively very similar for
either isotopologue. Nitrogen is preserved in atomic form near the edge of the cloud due
to the intense photodissociating interstellar radiation field. Increasing shielding with
depth into the cloud permits a significant population in molecular form beginning around
AV = 1.5.
(11)where
n(X) is the absolute abundance of species
X and
z(AV) is the distance from
the clouds edge for a particular extinction. The trend of these quantities as a function
of AV is qualitatively very similar for
either isotopologue. Nitrogen is preserved in atomic form near the edge of the cloud due
to the intense photodissociating interstellar radiation field. Increasing shielding with
depth into the cloud permits a significant population in molecular form beginning around
AV = 1.5.
The relative abundances of 14N/15N and
14N2/ calculated by
the model are shown in Fig. 7. Any fractionation
process will alter the isotopic abundances from the assumed elemental ratios
14N/15N = 450 and
14N2/
calculated by
the model are shown in Fig. 7. Any fractionation
process will alter the isotopic abundances from the assumed elemental ratios
14N/15N = 450 and
14N2/ .
There is indeed an enhancement of 15N in atomic form for
AV between 1 and 3 mag, and its
concurrent depletion in the form of N2. This range of extinction corresponds to
a column-density of 14N2 sufficiently large to significantly
self-shield the ISRF and slow its rate of photodissociation. The lower column of
14N15N is not as effectively self-shielding and leads to a larger
14N15N photodissociation rate and the calculated excess of
15N atoms.
.
There is indeed an enhancement of 15N in atomic form for
AV between 1 and 3 mag, and its
concurrent depletion in the form of N2. This range of extinction corresponds to
a column-density of 14N2 sufficiently large to significantly
self-shield the ISRF and slow its rate of photodissociation. The lower column of
14N15N is not as effectively self-shielding and leads to a larger
14N15N photodissociation rate and the calculated excess of
15N atoms.
A modified model was also run neglecting ion-molecule exchange reactions in order to test their contribution to the calculated fractionation. The modelled abundances were not significantly altered by this modification and the fractionation between AV = 1 and 3 mag is completely dominated by isotope-selective photodissociation due to self-shielding, at least at the adopted temperature of 30 K.In contrast, significant enhancements of 15N in NH3 and HCN were produced in the models of Charnley & Rodgers (2002), Rodgers & Charnley (2004), and Wirström et al. (2012) which simulate dense protostellar cores. These models included isotope-exchange reactions similar to those adopted here and neglected isotope-selective photodissociation. They also simulated somewhat colder conditions than ours (10 versus 30 K), enhancing the rate of the fractionating exchange reactions, and assumed significant depletion of CO relative to N2 by condensation onto grains, slowing the effect of fractionation-reducing reprocessing to N2. It is then possible that the observed fractionation of N-isotopes may have a combined chemical and photolytic origin.
 |
Fig. 10 Top: abundances relative to H nuclei and right-to-left
accumulated column densities of CN, HCN, and their ices in the modelled
protoplanetary disc as a function of height above the midplane at a radius of
105 AU. Also shown for species with a single 15N substitution (dotted
curves). Bottom: ratios of abundances and column densities of
14N- and 15N-bearing species. Also indicated is the
elemental |
There is no indication in Fig. 7 for fractionation
in the abundance of N2 or N in the completely shielded interior of the
interstellar cloud model (AV ≳ 3). This is
despite the inclusion of isotope-selective ion-molecule exchange reactions and a 40%
faster rate of cosmic ray induced photodissociation for 14N 15N relative to
14N2 given the physical parameters selected for this cloud model.
Ultimately, cosmic-ray induced photodissociation is rendered irrelevant by faster and
isotopologue-independent destruction mechanisms included in our model:  (12)and
(12)and  (13)The combined
destruction rate due to these processes exceeds 10-13 s-1 and is two
orders of magnitude faster than cosmic-ray induced photodissociation.
(13)The combined
destruction rate due to these processes exceeds 10-13 s-1 and is two
orders of magnitude faster than cosmic-ray induced photodissociation.
The total-column fractionation of N and N2 depends on the assumed depth of the modelled cloud, and is also plotted in Fig. 7 as a function of AV. For our plane-parallel single-side-illuminated model this is maximal for AV between 1.5 and 2 mag, but never exceeds a factor of two for atomic or molecular species.
The minor contribution of cosmic-ray induced photodissociation to the nitrogen chemistry in our model does not presuppose a similar conclusion for other molecules, some of which have significantly higher cosmic-ray induced predissociation rates (Gredel et al. 1989).
5.2. Protoplanetary disc model
We have run a second chemical model for a vertical slice through a circumstellar disc using the same network of reactions as in Sect. 5.1. The model setup is identical to that of Visser et al. (2009) and Li et al. (2013). Briefly, we take the disc geometry, dust density and temperature distributions directly from the previous model of D’Alessio et al. (1999) without modification. This simulates a disc of mass of 0.07 M⊙ and outer radius 400 AU surrounding a T Tauri star of mass 0.5 M⊙ and radius 2 R⊙. The simulated vertical slice is located at a radius of 105 AU.
A radiation field with the Draine wavelength dependence is perpendicularly incident on the surface of the slice, 120 AU above the midplane. The use of a scaled Draine field to represent the combined UV flux from the central star and the ISRF has been previously investigated in detail for the same disc geometry employed here (van Zadelhoff et al. 2003; Jonkheid et al. 2004). From these studies, a Draine field with χ = 516 is a satisfactory proxy for a more realistic field including the details of the stellar spectrum at a disc radius of 105 AU. Dust grains of radius 1 μm are assumed to populate the disc which leads to weaker shielding and a greater penetration of ultraviolet radiation than for the interstellar cloud model of Sect. 5.1.
The abundances and cumulative column densities of atomic and molecular nitrogen in the
disc model are plotted in Fig. 8. The abundance of
N2 becomes significant about 40 AU above the midplane and a rapid freeze-out
of gaseous N2 onto dust grains occurs 20 AU above the midplane, where the
temperature falls below 20 K. The effect of photodissociative fractionation in the
intervening 20 to 40 AU region is evident in the abundance ratios plotted in Fig. 8, where the atomic ratio of
 dips by a
factor of 10 below the elemental ratio. However, fractionation of the total atomic N
column is negligible because of its low abundance in this region. As for the interstellar
cloud model, cosmic-ray induced photodissociation of N2 is found to be
negligible throughout the disc slice, and does not contribute to isotopic fractionation.
Similarly, the contribution of ion-molecule exchange reactions to the calculated chemical
abundances was found to be negligible following comparison with an alternative model where
these are neglected. That is, the ice-phase predominance of nitrogen species in our model
for disc heights with sufficiently low-temperature for chemical fractionation to occur
suppresses these reactions.
dips by a
factor of 10 below the elemental ratio. However, fractionation of the total atomic N
column is negligible because of its low abundance in this region. As for the interstellar
cloud model, cosmic-ray induced photodissociation of N2 is found to be
negligible throughout the disc slice, and does not contribute to isotopic fractionation.
Similarly, the contribution of ion-molecule exchange reactions to the calculated chemical
abundances was found to be negligible following comparison with an alternative model where
these are neglected. That is, the ice-phase predominance of nitrogen species in our model
for disc heights with sufficiently low-temperature for chemical fractionation to occur
suppresses these reactions.
The contribution of different species to the shielding of 14N2 and 14N 15N photodissociation were calculated as a function of height and AV and are plotted in Fig. 9. These calculations include all shielding species in an integral with the form in Eq. (5) by adopting column densities output from the disc model. From this, it is evident that shielding by 1 μm dust grains (approximated for Fig. 9 by assuming γdust = 0.6) and the hydrogen column are comparably effective. The shielding by CO is quite negligible because of the rarity of its overlaps with N2, evident in Fig. 3. The significance of photodissociation-induced isotopic fractionation of atomic N relies on the relative importance of self-shielding of 14N2 and 14N 15N. For 14N2 this becomes important around 40 AU above the midplane and is comparable to dust and hydrogen shielding between 20 and 35 AU. For 14N 15N, self-shielding never contributes more than 10% of the total shielding and is about equivalent to its shielding by 14N2.
The appearance of N and N2 fractionation between 20 and 40 AU in Fig. 8 has consequences for other nitrogen bearing species.
For example, Fig. 10 shows the abundances, column
densities, and isotopic ratios of CN, HCN, and their ices; which are formed primarily from
atomic N via the reactions  (14)and
(14)and  (15)Additionally, CN
and HCN are themselves susceptible to photodissociation and do not survive far from the
midplane. Then, their maximum abundances occur within the region of enhanced
15N atoms and this enhancement is mirrored in their integrated column
densities. The isotopic fractionation of HCN gas and ice columns is plotted in Fig. 10 and approaches a factor of 10, whereas the
fractionation of gaseous CN is somewhat less because a major portion of its column forms
above 40 AU and outside the region of photo-fractionated N.
(15)Additionally, CN
and HCN are themselves susceptible to photodissociation and do not survive far from the
midplane. Then, their maximum abundances occur within the region of enhanced
15N atoms and this enhancement is mirrored in their integrated column
densities. The isotopic fractionation of HCN gas and ice columns is plotted in Fig. 10 and approaches a factor of 10, whereas the
fractionation of gaseous CN is somewhat less because a major portion of its column forms
above 40 AU and outside the region of photo-fractionated N.
 |
Fig. 11 Top: abundances relative to H nuclei and right-to-left
accumulated column densities of NH3 and NH3 ice in
the modelled protoplanetary disc as a function of height above the midplane at a
radius of 105 AU. Also shown for species with a single 15N substitution
(dotted curves). Bottom: ratios of abundances and column densities
of 14N- and 15N-bearinge species. Also indicated is the
elemental |
A different isotope fractionation pattern is evident in Fig. 11 for NH3. The majority of the NH3 population is
frozen onto grain surfaces and its abundance has a sharp maximum at the inner edge of the
15N / 14N fractionation zone, near 20 AU. We do not compute much
difference between the integrated column densities of 14NH3 and
15NH3 in either gas or ice phases but the local
15NH3 abundance is significantly enhanced and depleted in the
ranges 20–25 and 25–40 AU, respectively. The pattern of NH3 photolytic
fractionation is more complex than for CN and HCN due to a more complex formation
mechanism. There are two pathways leading to NH3 in our model, the first
occurring entirely in the gas phase and initiated from molecular N2, that is,
 (16)and the second
follows from the hydrogenation of atomic N after its condensation onto ice grains,
(16)and the second
follows from the hydrogenation of atomic N after its condensation onto ice grains,
 (17)Intermediate species
NH and NH2 in the reaction series summarised by Eq. (17) are also allowed to evaporate in our model
and contribute to the gaseous NH3 beginning with their ionisation by
H+, e.g.,
(17)Intermediate species
NH and NH2 in the reaction series summarised by Eq. (17) are also allowed to evaporate in our model
and contribute to the gaseous NH3 beginning with their ionisation by
H+, e.g.,  (18)The balance between
formation routes given in Eqs. (16)–(18) dictates the height-dependent isotopic
fractionation of NH3 in sympathy with the fractionation of their respective
precursors, N2 or N. The occurrence of formation route (16) is limited to approximately 20 to 40 AU
above the midplane, where there is an abundance of gaseous N2. The process in
Eq. (17) requires condensed atomic N and
is even more restricted. That is, sufficiently low temperatures only occur within 25 AU of
the midplane in our model, whereas the necessary source of gaseous N is limited to above
20 AU. Then, the NH3 fractionation pattern resembles that of atomic N between
20 and 25 AU, and N2 above this.
(18)The balance between
formation routes given in Eqs. (16)–(18) dictates the height-dependent isotopic
fractionation of NH3 in sympathy with the fractionation of their respective
precursors, N2 or N. The occurrence of formation route (16) is limited to approximately 20 to 40 AU
above the midplane, where there is an abundance of gaseous N2. The process in
Eq. (17) requires condensed atomic N and
is even more restricted. That is, sufficiently low temperatures only occur within 25 AU of
the midplane in our model, whereas the necessary source of gaseous N is limited to above
20 AU. Then, the NH3 fractionation pattern resembles that of atomic N between
20 and 25 AU, and N2 above this.
Overall, the complexity of photodissociative fractionation of NH3 deduced here suggests the induced 14NH3 / 15NH3 column density ratio in a particular environment will depend sensitively on its temperature and density profiles. The photodissociative fractionation of CN and HCN is likely more robust because the formation routes of these molecules are relatively simple. The enhancement of 15N in CN and HCN within the self-shielding zone of our model disc (10 ×) is actually larger than for observations of cometary CN and HCN (2–3 ×) (Jehin et al. 2009; Mumma & Charnley 2011). This supports the feasibility of photodissociation as a mechanism for cometary fractionation. The greater fractionation of 15N and HCN relative to NH3 calculated for our particular protoplanetary disc also suggests that photodissociation may contribute to the explanation of a similar difference observed in the cold prestellar core L1544 (Hily-Blant et al. 2013; Bizzocchi et al. 2013), but not necessarily elsewhere in the galaxy (Daniel et al. 2013).
6. Conclusions
We used a high-resolution theoretical spectroscopic model of 14N 15N to calculate its dissociation rate in interstellar space due to ultraviolet radiation, as well as the shielding of this radiation by dust grains and gas phase species. We obtain an unshielded rate of 1.73 × 10-10 s-1 assuming a Draine ISRF and an excitation temperature of 30 K, which is very near to the rate for 14N2 obtained in our previous study (Li et al. 2013). Comprehensive tabulated shielding functions for 14N2 and 14N 15N are available online2.
To calculate the rate of photodissociation in regions fully shielded from external radiation, the model of 14N2 and 14N 15N was combined with a detailed theoretical spectrum of H2 emission arising from cosmic rays. Assuming ζ = 10-16 s-1, a variable rate of ~10-15 s-1 was found to depend on the assumed details of the local environment, most significantly, the excitation temperature of N2 and shielding species, the o−H2:p−H2 ratio, and the dust opacity at ultraviolet wavelengths. Isotope-dependent cosmic-ray induced photodissociation was also investigated, with the most extreme difference occurring when assuming 30 K, o−H2:p−H2 = 0:1, and a low dust absorption cross section appropriate to a protoplanetary disc experiencing grain growth. Then, the dissociation rate of 14N 15N exceeds that of 14N2 by a factor of 8. This large isotopic difference does not occur at a lower excitation temperature of 10 K. We also calculated the rate of CO dissociation by cosmic-ray induced photons previously determined by Gredel et al. (1987) using more accurate photodissociation cross sections, and deduced a ~40 % lower value.
Chemical models were run simulating an interstellar cloud and a protoplanetary disc to test the importance of self-shielding of external radiation and cosmic ray initiated photodissociation to the balance of 14N- and 15N-bearing species. An enhancement of atomic 15N relative to 14N is found at extinctions with 1 ≲ AV ≲ 3, and is due to more effective self-shielding of the ISRF by 14N2 than by 14N 15N. This fractionation is larger for our disc model where weaker dust shielding was assumed to simulate the growth and depletion of small dust grains. The photodissociation due to cosmic-ray induced ultraviolet radiation is found to be too slow to significantly influence the chemistry in the dark interiors of our models.
The isotopically-dependent photodissociation of N2 in our protoplanetary disc
model leads to a significant enhancement in the column densities of HC15N
relative to HC14N. Our model predicts a value of
HC14N/HC15N ≃ 100, when assuming an elemental nitrogen abundance ratio
of 14N/15N = 450. This corresponds to an isotopic
fractionation in δ-notation of  ≃
-800 %0. This enhancement mirrors the fractionation of atomic N induced by
photodissociation. Our model predicts a lower fractionation ratio for CN, with
C14N/C15N ≃ 250. This is because the region where CN forms does not
coincide as neatly with the peak of N2 isotope dependent photodissociation. The
formation chemistry of NH3 is more complex and can be initiated from both
N2 and atomic N. This species is then less fractionated in our model.
≃
-800 %0. This enhancement mirrors the fractionation of atomic N induced by
photodissociation. Our model predicts a lower fractionation ratio for CN, with
C14N/C15N ≃ 250. This is because the region where CN forms does not
coincide as neatly with the peak of N2 isotope dependent photodissociation. The
formation chemistry of NH3 is more complex and can be initiated from both
N2 and atomic N. This species is then less fractionated in our model.
Many assumptions must be made when modelling the chemistry of complex and remote objects such as protoplanetary discs. However, the calculations underpinning our predictions of photodissociative fractionation are based on reliable laboratory and theoretical spectroscopy and may be resilient to many structural details of astrophysical objects.
Acknowledgments
Astrochemistry in Leiden is supported by the Netherlands Research School for Astronomy (NOVA), by a Spinoza grant and grant 648.000.002 from the Netherlands Organisation for Scientific Research (NWO) via the Dutch Astrochemistry Network, and by the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreements 291141 (CHEMPLAN) and 238258 (LASSIE). Calculations of the N2 photodissociation cross sections were supported by the Australian Research Council Discovery Program, through Grant Nos. DP0558962 and DP0773050. We would also like to thank Simon Bruderer and Xiaohu Li for discussions on the optical properties of protoplanetary dust grains and 14N2 photodissociation, respectively.
References
- Adande, G. R., & Ziurys, L. M. 2012, ApJ, 744, 194 [NASA ADS] [CrossRef] [Google Scholar]
- Aikawa, Y., Wakelam, V., Garrod, R. T., & Herbst, E. 2008, ApJ, 674, 984 [NASA ADS] [CrossRef] [Google Scholar]
- Ajello, J. M., James, G. K., Franklin, B. O., & Shemansky, D. E. 1989, Phys. Rev. A, 40, 3524 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Aléon, J. 2010, ApJ, 722, 1342 [NASA ADS] [CrossRef] [Google Scholar]
- Bergin, E. A., Alves, J., Huard, T., & Lada, C. J. 2002, ApJ, 570, L101 [NASA ADS] [CrossRef] [Google Scholar]
- Bizzocchi, L., Caselli, P., Leonardo, E., & Dore, L. 2013, A&A, 555, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bohlin, R. C., Savage, B. D., & Drake, J. F. 1978, ApJ, 224, 132 [NASA ADS] [CrossRef] [Google Scholar]
- Cecchi-Pestellini, C., & Aiello, S. 1992, MNRAS, 258, 125 [NASA ADS] [CrossRef] [Google Scholar]
- Chaparro Molano, G., & Kamp, I. 2012, A&A, 537, A138 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Charnley, S. B., & Rodgers, S. D. 2002, ApJ, 569, L133 [NASA ADS] [CrossRef] [Google Scholar]
- Cleeves, L. I., Adams, F. C., & Bergin, E. A. 2013, ApJ, 772, 5 [NASA ADS] [CrossRef] [Google Scholar]
- D’Alessio, P., Calvet, N., Hartmann, L., Lizano, S., & Cantó, J. 1999, ApJ, 527, 893 [NASA ADS] [CrossRef] [Google Scholar]
- Dalgarno, A. 2006, Pro. Natl. Acad. Sci., 103, 12269 [NASA ADS] [CrossRef] [Google Scholar]
- Daniel, F., Gerin, M., Roueff, E., et al. 2013, A&A, 560, A3 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Draine, B. T. 1978, ApJSS, 36, 595 [NASA ADS] [CrossRef] [Google Scholar]
- Dressler, K. 1969, Can. J. Phys., 47, 547 [NASA ADS] [CrossRef] [Google Scholar]
- Floss, C., Stadermann, F. J., Bradley, J. P., et al. 2006, Geochim. Cosmochim. Acta, 70, 2371 [NASA ADS] [CrossRef] [Google Scholar]
- Geers, V. C., Augereau, J.-C., Pontoppidan, K. M., et al. 2006, A&A, 459, 545 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Geers, V. C., Pontoppidan, K. M., van Dishoeck, E. F., et al. 2007, A&A, 469, L35 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Gerin, M., Marcelino, N., Biver, N., et al. 2009, A&A, 498, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Gredel, R., Lepp, S., & Dalgarno, A. 1987, ApJ, 323, L137 [NASA ADS] [CrossRef] [Google Scholar]
- Gredel, R., Lepp, S., Dalgarno, A., & Herbst, E. 1989, ApJ, 347, 289 [NASA ADS] [CrossRef] [Google Scholar]
- Haverd, V. E., Lewis, B. R., Gibson, S. T., & Stark, G. 2005, J. Chem. Phys., 123, 214304 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Heays, A. N. 2011, Ph.D. thesis, The Australian National University [Google Scholar]
- Heays, A. N., Lewis, B. R., Stark, G., et al. 2009, J. Chem. Phys., 131, 194308 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Heays, A. N., Dickenson, G. D., Salumbides, E. J., et al. 2011, J. Chem. Phys., 135, 244301 [CrossRef] [PubMed] [Google Scholar]
- Helm, H., Hazell, I., & Bjerre, N. 1993, Phys. Rev. A, 48, 2762 [NASA ADS] [CrossRef] [Google Scholar]
- Hezareh, T., Houde, M., McCoey, C., Vastel, C., & Peng, R. 2008, ApJ, 684, 1221 [NASA ADS] [CrossRef] [Google Scholar]
- Hily-Blant, P., Bonal, L., Faure, A., & Quirico, E. 2013, Icarus, 223, 582 [Google Scholar]
- Indriolo, N., & McCall, B. J. 2012, ApJ, 745, 91 [NASA ADS] [CrossRef] [Google Scholar]
- Jehin, E., Manfroid, J., Hutsemékers, D., Arpigny, C., & Zucconi, J.-M. 2009, Earth Moon and Planets, 105, 167 [CrossRef] [Google Scholar]
- Jonkheid, B., Faas, F., van Zadelhoff, G., & van Dishoeck, E. 2004, A&A, 428, 511 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Jonkheid, B., Kamp, I., Augereau, J.-C., & van Dishoeck, E. F. 2006, A&A, 453, 163 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Le Gal, R., Hily-Blant, P., Faure, A., et al. 2014, A&A, in press, DOI: 10.1051/0004-6361/201322386 [Google Scholar]
- Le Petit, F., Nehmé, C., Le Bourlot, J., & Roueff, E. 2006, ApJS, 164, 506 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Lewis, B. R., Gibson, S. T., Sprengers, J. P., et al. 2005a, J. Chem. Phys., 123, 236101 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lewis, B. R., Gibson, S. T., Zhang, W., Lefebvre-Brion, H., & Robbe, J. M. 2005b, J. Chem. Phys., 122, 144302 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lewis, B. R., Baldwin, K. G. H., Heays, A. N., et al. 2008a, J. Chem. Phys., 129, 204303 [NASA ADS] [CrossRef] [Google Scholar]
- Lewis, B. R., Baldwin, K. G. H., Sprengers, J. P., et al. 2008b, J. Chem. Phys., 129, 164305 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Lewis, B. R., Heays, A. N., Gibson, S. T., Lefebvre-Brion, H., & Lefebvre, R. 2008c, J. Chem. Phys., 129, 164306 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Li, A., & Lunine, J. I. 2003a, ApJ, 594, 987 [NASA ADS] [CrossRef] [Google Scholar]
- Li, A., & Lunine, J. I. 2003b, ApJ, 590, 368 [NASA ADS] [CrossRef] [Google Scholar]
- Li, X., Heays, A. N., Visser, R., et al. 2013, A&A, 555, A14 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Liang, M.-C., Heays, A. N., Lewis, B. R., Gibson, S. T., & Yung, Y. L. 2007, ApJ, 664, L115 [NASA ADS] [CrossRef] [Google Scholar]
- Lyons, J., & Young, E. 2005, Nature, 435, 317 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Maaskant, K. M., Honda, M., Waters, L. B. F. M., et al. 2013, A&A, 555, A64 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Marty, B., Zimmermann, L., Burnard, P. G., et al. 2010, Geochim. Cosmochim. Acta, 74, 340 [NASA ADS] [CrossRef] [Google Scholar]
- Mathis, J. S., Rumpl, W., & Nordsieck, K. H. 1977, ApJ, 217, 425 [NASA ADS] [CrossRef] [Google Scholar]
- McElroy, D., Walsh, C., Markwick, A. J., et al. 2013, A&A, 550, A36 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Mumma, M. J., & Charnley, S. B. 2011, ARA&A, 49, 471 [NASA ADS] [CrossRef] [Google Scholar]
- Ndome, H., Hochlaf, M., Lewis, B. R., et al. 2008, J. Chem. Phys., 129, 164307 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Niemann, H. B., Atreya, S. K., Bauer, S. J., et al. 2005, Nature, 438, 779 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Öberg, K. I., Qi, C., Fogel, J. K. J., et al. 2010, ApJ, 720, 480 [NASA ADS] [CrossRef] [Google Scholar]
- Padovani, M., Galli, D., & Glassgold, A. E. 2009, A&A, 501, 619 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Prasad, S. S., & Tarafdar, S. P. 1983, ApJ, 267, 603 [NASA ADS] [CrossRef] [Google Scholar]
- Rimmer, P. B., Herbst, E., Morata, O., & Roueff, E. 2012, A&A, 537, A7 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Roberge, W. G., Dalgarno, A., & Flannery, B. P. 1981, ApJ, 243, 817 [NASA ADS] [CrossRef] [Google Scholar]
- Roberge, W. G., Jones, D., Lepp, S., & Dalgarno, A. 1991, ApJS, 77, 287 [NASA ADS] [CrossRef] [Google Scholar]
- Rodgers, S. D., & Charnley, S. B. 2004, MNRAS, 352, 600 [NASA ADS] [CrossRef] [Google Scholar]
- Rodgers, S. D., & Charnley, S. B. 2008, ApJ, 689, 1448 [NASA ADS] [CrossRef] [Google Scholar]
- Röllig, M., Abel, N. P., Bell, T., et al. 2007, A&A, 467, 187 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Savage, B. D., Bohlin, R. C., Drake, J. F., & Budich, W. 1977, ApJ, 216, 291 [NASA ADS] [CrossRef] [Google Scholar]
- Sheffer, Y., Lambert, D. L., & Federman, S. R. 2002, ApJ, 574, L171 [NASA ADS] [CrossRef] [MathSciNet] [Google Scholar]
- Siebenmorgen, R., & Krügel, E. 2010, A&A, 511, A6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Smith, R. L., Pontoppidan, K. M., Young, E. D., Morris, M. R., & van Dishoeck, E. F. 2009, ApJ, 701, 163 [NASA ADS] [CrossRef] [Google Scholar]
- Spelsberg, D., & Meyer, W. 2001, J. Chem. Phys., 115, 6438 [NASA ADS] [CrossRef] [Google Scholar]
- Sprengers, J. P., Ubachs, W., Baldwin, K. G. H., Lewis, B. R., & Tchang-Brillet, W.-Ü. L. 2003, J. Chem. Phys., 119, 3160 [NASA ADS] [CrossRef] [Google Scholar]
- Sprengers, J. P., Ubachs, W., Johansson, A., et al. 2004, J. Chem. Phys., 120, 8973 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Sprengers, J. P., Reinhold, E., Ubachs, W., Baldwin, K. G. H., & Lewis, B. R. 2005a, J. Chem. Phys., 123, 144315 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Sprengers, J. P., Ubachs, W., & Baldwin, K. G. H. 2005b, J. Chem. Phys., 122, 144301 [NASA ADS] [CrossRef] [Google Scholar]
- Stark, G., Huber, K. P., Yoshino, K., Smith, P. L., & Ito, K. 2005, J. Chem. Phys., 123, 214303 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Stark, G., Lewis, B. R., Heays, A. N., et al. 2008, J. Chem. Phys., 128, 114302 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Sternberg, A., Dalgarno, A., & Lepp, S. 1987, ApJ, 320, 676 [NASA ADS] [CrossRef] [Google Scholar]
- Terzieva, R., & Herbst, E. 2000, MNRAS, 317, 563 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. G. G. M. 2013, Rev. Mod. Phys., 85, 1021 [NASA ADS] [CrossRef] [Google Scholar]
- Tobin, J. J., Hartmann, L., Bergin, E., et al. 2012, ApJ, 748, 16 [Google Scholar]
- van Dishoeck, E. F., Jonkheid, B., & van Hemert, M. C. 2006, Faraday Discuss., 133, 231 [Google Scholar]
- van Zadelhoff, G.-J., van Dishoeck, E. F., Thi, W.-F., & Blake, G. A. 2001, A&A, 377, 566 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- van Zadelhoff, G.-J., Aikawa, Y., Hogerheijde, M., & van Dishoeck, E. 2003, A&A, 397, 789 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Vieitez, M. O., Ivanov, T. I., de Lange, C. A., et al. 2008, J. Chem. Phys., 128, 134313 [NASA ADS] [CrossRef] [Google Scholar]
- Visser, R., van Dishoeck, E. F., & Black, J. H. 2009, A&A, 503, 323 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Visser, R., Doty, S. D., & van Dishoeck, E. F. 2011, A&A, 534, A132 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wannier, P. G., Andersson, B.-G., Olofsson, H., Ukita, N., & Young, K. 1991, ApJ, 380, 593 [NASA ADS] [CrossRef] [Google Scholar]
- Wilson, T. L. 1999, Rep. Prog. Phys., 62, 143 [Google Scholar]
- Wirström, E. S., Charnley, S. B., Cordiner, M. A., & Milam, S. N. 2012, ApJ, 757, L11 [NASA ADS] [CrossRef] [Google Scholar]
- Wu, C. Y. R., Judge, D. L., Tsai, M.-H., et al. 2012, J. Chem. Phys., 136, 044301 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
The unattenuated photodissociation rates (k0, s-1) of N2 exposed to a completely-surrounding Draine interstellar radiation field for various excitation temperatures (Tex), and for blackbody radiation with temperature Tbb and Tex = 30 K.
All Figures
 |
Fig. 1 Diabatic-basis potential-energy curves of N2 excited states relevant to
ultraviolet photoabsorption and photodissociation. The energy scale is referenced to
v = 0, J = 0 of the
|
| In the text | |
 |
Fig. 2 An example of isotopically-dependent predissociation. The predissociation fraction, ηpre, of individual rotational levels, J, of b 1Πu(v = 1) calculated by the CSE model for 14N2 and 14N15N. |
| In the text | |
 |
Fig. 3 Upper panels: representative transmission functions
calculated from theoretical photoabsorption cross sections of important molecular
species, with column densities: N(H) = 1022,
N(H2) = 1020, and
|
| In the text | |
 |
Fig. 4 Top left: self-shielding functions for
|
| In the text | |
 |
Fig. 5 Modelled molecular absorption lines for two different excitation temperatures, 20
and 100 K, and cosmic-ray induced H2 emission. Otherwise calculated as
for Fig. 3. Left: overlap of
an o-H2 emission line with the
|
| In the text | |
 |
Fig. 6 Dissociation rate of N2 due to cosmic-ray induced H2 emission under interstellar cloud conditions, integrated cumulatively with increasing wavelength. Rates for 14N2 are plotted with for several temperatures (solid curves), for and Tex = 20 K (black dotted curve), and a single case is included for 14N 15N (red dotted curve). |
| In the text | |
 |
Fig. 7 Top: abundances relative to H nuclei and left-to-right accumulated column densities of the isotopologues of N and N2 in the modelled interstellar cloud. Bottom: ratios of abundances and column densities of 14N- and 15N-bearing species. Also indicated are the adopted elemental ratios of 14N / 15N and 14N2 / 14N 15N (dashed lines). |
| In the text | |
 |
Fig. 8 Top: abundances relative to H nuclei and right-to-left
accumulated column densities of atomic and molecular nitrogen, and
N2 ice in the modelled protoplanetary disc as a function of height
above the midplane at a radius of 105 AU. Also shown for species with a single
15N substitution i.e., 15N and 14N 15N (dotted
curves). Bottom: ratios of abundances and column densities of
14N- and 15N-bearing species. In some cases the ice and
gaseous N2 curves coincide. Also indicated is the elemental
|
| In the text | |
 |
Fig. 9 Shielding functions for 14N2 and
|
| In the text | |
 |
Fig. 10 Top: abundances relative to H nuclei and right-to-left
accumulated column densities of CN, HCN, and their ices in the modelled
protoplanetary disc as a function of height above the midplane at a radius of
105 AU. Also shown for species with a single 15N substitution (dotted
curves). Bottom: ratios of abundances and column densities of
14N- and 15N-bearing species. Also indicated is the
elemental |
| In the text | |
 |
Fig. 11 Top: abundances relative to H nuclei and right-to-left
accumulated column densities of NH3 and NH3 ice in
the modelled protoplanetary disc as a function of height above the midplane at a
radius of 105 AU. Also shown for species with a single 15N substitution
(dotted curves). Bottom: ratios of abundances and column densities
of 14N- and 15N-bearinge species. Also indicated is the
elemental |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.






