| Issue |
A&A
Volume 506, Number 3, November II 2009
|
|
|---|---|---|
| Page(s) | 1487 - 1499 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/200912932 | |
| Published online | 27 August 2009 | |
A&A 506, 1487-1499 (2009)
Rotational spectra of isotopic species of methyl cyanide, CH3CN, in their ground vibrational states up to terahertz frequencies
H. S. P. Müller1 - B. J. Drouin2 - J. C. Pearson2
1 - I. Physikalisches Institut, Universität zu Köln,
Zülpicher Str. 77, 50937 Köln, Germany
2 -
Jet Propulsion Laboratory, California Institute of Technology,
Mail Stop 183-301, 4800 Oak Grove Drive,
Pasadena, CA 91011-8099, USA
Received 20 July 2009 / Accepted 15 August 2009
Abstract
Context. Methyl cyanide is an important trace molecule in
star-forming regions. It is one of the more common molecules used to
derive kinetic temperatures in such sources.
Aims. As preparatory work for Herschel, SOFIA, and in particular
ALMA we want to improve the rest frequencies of the main as well as
minor isotopologs of methyl cyanide.
Methods. The laboratory rotational spectrum of methyl cyanide in natural isotopic composition has been recorded up to 1.63 THz.
Results. Transitions with good signal-to-noise ratio could be identified for CH3CN, 13CH3CN, CH313CN, CH3C15N, CH2DCN, and 13CH313CN
in their ground vibrational states up to about 1.2 THz. The main
isotopic species could be identified even in the highest frequency
spectral recordings around 1.6 THz. The highest J' quantum numbers included in the fit are 64 for 13CH313CN
and 89 for the main isotopic species. Greatly improved spectroscopic
parameters have been obtained by fitting the present data together with
previously reported transition frequencies.
Conclusions. The present data will be helpful to identify
isotopologs of methyl cyanide in the higher frequency bands of
instruments such as the recently launched Herschel satellite, the
upcoming airplane mission SOFIA or the radio telescope array ALMA.
Key words: molecular data - methods: laboratory - techniques: spectroscopic - radio lines: ISM - ISM: molecules
1 Introduction
Methyl cyanide, CH3CN, also known as acetonitrile, or cyanomethane,
was among the early molecules to be detected by radio astronomical means.
Solomon et al. (1971) detected it almost 40 years ago toward the massive
star-forming regions Sagittarius A and B close to the Galactic center.
It has been detected in dark clouds such as TMC-1 (Matthews & Sears 1983),
around the low-mass protostar IRAS 16293-2422 (Cazaux et al. 2003),
in the circumstellar shell of the famous carbon-rich star IRC +10216
(Agúndez et al. 2008), in comets, such as Kohoutek
(Ulich & Conklin 1974), and in external galaxies (Mauersberger et al. 1991).
As a strongly prolate symmetric top, meaning ![]() ,
transitions
with different K but the same J occur in fairly narrow
frequency regions, but sample rather different energies.
Therefore, CH3CN is quite commonly used to derive the kinetic
temperatures of dense molecular clouds as already done in Solomon et al. (1971)
or in e.g. Cummins et al. (1983). In less dense clouds the large
dipole moment of 3.922 D (Gadhi et al. 1979) may prevent
thermal equilibration. In that case, the isoelectronic
methyl acetylene, or propyne, CH3CCH, is often used instead.
,
transitions
with different K but the same J occur in fairly narrow
frequency regions, but sample rather different energies.
Therefore, CH3CN is quite commonly used to derive the kinetic
temperatures of dense molecular clouds as already done in Solomon et al. (1971)
or in e.g. Cummins et al. (1983). In less dense clouds the large
dipole moment of 3.922 D (Gadhi et al. 1979) may prevent
thermal equilibration. In that case, the isoelectronic
methyl acetylene, or propyne, CH3CCH, is often used instead.
The 12C/13C ratio in the vicinity of the Galactic center is approximately 20 (Wilson & Rood 1994; Müller et al. 2008). Thus, it is not surprising that the two isotopomers containing 13C were detected fairly soon after the detection of the main isotopolog. CH3 13CN (Cummins et al. 1983) was detected first probably by accident because the isotopic substitution at the central C-atom does not change the spectroscopic parameters much with respect to the main species. Sutton et al. (1985) detected lines of both isotopomers with one 13C in a line survey of Orion. More recently, CH2DCN has been detected in two hot core sources (Gerin et al. 1992), and some lines of CH3C15N were detected in a line survey of Sagittarius B2 (Nummelin et al. 1998).
A plethora of high-resolution spectroscopic investigations have been performed on methyl cyanide. It was actually among the first molecules to be studied by microwave spectroscopy by Ring et al. (1947). Cazzoli & Puzzarini (2006) reviewed the investigations for the main isotopic species and performed high resolution, high accuracy measurements up to 1.2 THz. Pearson & Müller (1996) analogously presented data up to 607 GHz for the singly substituted 13C isotopomers as well as for the species with 15N. Le Guennec et al. (1992) built on the very small data set for CH2DCN by measuring an isotopically enriched sample up to 471 GHz. Finally, there has been only one report on the isotopolog with two 13C which was restricted to frequencies up to 72 GHz (Tam et al. 1988).
We have measured rotational spectra of methyl cyanide in natural isotopic
composition both in wide frequency windows as well as selected lines
up to 1.63 THz to provide new or updated catalog entries for various
isotopologs of methyl cyanide as well as for excited vibrational states.
With rotational temperatures of CH3CN in hot cores reaching at least
150-200 K (Sutton et al. 1985; Belloche et al. 2009), these data
will be of great relevance for the Atacama Large Millimeter Array (ALMA).
The main isotopolog and possibly even the 13C isotopomers or the
![]() excited vibrational state will be seen with the
HIFI instrument (Heterodyne Instrument for the Far-Infrared) on board
of the recently launched Herschel satellite or with SOFIA (Stratospheric
Observatory For Infrared Astronomy).
In the present article we provide the ground state rotational data
obtained for six different isotopic species: CH3CN, 13CH3CN,
CH313CN, CH3C15N, CH2DCN, and 13CH313CN;
as usual, unlabeled atoms refer to 12C and 14N.
Analyses of selected excited vibrational states are currently
under way.
excited vibrational state will be seen with the
HIFI instrument (Heterodyne Instrument for the Far-Infrared) on board
of the recently launched Herschel satellite or with SOFIA (Stratospheric
Observatory For Infrared Astronomy).
In the present article we provide the ground state rotational data
obtained for six different isotopic species: CH3CN, 13CH3CN,
CH313CN, CH3C15N, CH2DCN, and 13CH313CN;
as usual, unlabeled atoms refer to 12C and 14N.
Analyses of selected excited vibrational states are currently
under way.
2 Experimental details
Individual transitions have been recorded with the Cologne Terahertz Spectrometer (CTS) which has been described in detail by Winnewisser et al. (1994). It uses broadband tunable, phase-locked backward-wave oscillators (BWOs) as powerful sources, and a magnetically tuned, liquid helium cooled hot-electron InSb bolometer as detector. In the present case, one BWO was used to record transitions between 249 and 340 GHz.
The pressure of methyl cyanide in the 3 m long absorption cell was around 0.3-0.5 Pa. The measurements were carried out in static mode, and the sample was pumped off and replaced about every half hour.
The accuracies with which lines can be measured with the CTS depend
on the lines shape, mostly on how symmetric the line is, and on the
signal-to-noise (S/N) ratio.
Quite commonly, we reach relative accuracies of 10-8 or
even slightly better (Müller & Brünken 2005; Müller et al. 2008,2007a).
Müller et al. (2000) have shown that similar accuracies can be achieved
for the rare isotopolog SO17O in a rather dense spectrum.
The lines recorded for 13CH3CN, CH313CN, and CH3C15N
had very good S/N ratios and line shapes. Thus, the uncertainties
were judged to be ![]() kHz. The lines of CH2DCN and
13CH313CN were considerably weaker such that uncertainties
of up to 30 kHz were assigned.
kHz. The lines of CH2DCN and
13CH313CN were considerably weaker such that uncertainties
of up to 30 kHz were assigned.
The majority of the data has been extracted from broad frequency scans
taken with the JPL cascaded multiplier spectrometer (Drouin et al. 2005).
Generally, radiation of a multiplier chain source is passed through a 1-2 m pathlength
flow cell and is detected by a silicon bolometer cooled to near 1.7 K.
The cell is filled with a steady flow of reagent grade acetonitrile
and the pressure and modulation are optimized to enable good S/N ratios
with narrow lineshapes.
With a gas with very strong transitions, such as the K < 7 transitions
of the main isotopolog of acetonitrile, the S/N ratio was optimized for
a higher-K transition (e.g. K = 12) such that the lower K transitions
exhibit saturated line profiles.
This procedure enables better dynamic range for the extraction of line positions
for rare isotopologs and highly excited vibrational satellites.
The frequency ranges covered were 440-540, 619-631, 638-648,
770-855, 875-930, 967-1050, 1083-1093, 1100-1159,
1168-1198, 1576-1591, and 1614-1627 GHz. Most of these multiplier sources
were previously described (Drouin et al. 2005).
However, the multiplier chain with frequency range coverage between
967-1050 GHz was not described in that work.
This chain consists of two cascaded triplers after the amplified W-band stage,
the peak output power is near 100 ![]() W.
The efficiency of frequency multipliers usually changes strongly with frequency.
In addition, recording conditions and sensitivities of detectors can have
strong influences on the quality of the spectra.
Particularly good S/N ratios were reached around 630, 900 and around
1100-1200 GHz. The spectra around 500 and around 800 GHz had poorer
S/N ratios. In addition, the S/N ratios changed considerably
within each scan and were usually lower towards the ends.
Uncertainties of 50 kHz were assigned to isolated lines with good to
very good S/N ratios throughout the frequency regions.
Larger uncertainties were assigned to weaker lines or lines
which were not isolated. In the course of the analysis it was observed
that several strong lines had residuals considerably smaller than 50 kHz.
Thus, smaller uncertainties, down to 20 kHz, were assigned to
very strong lines, depending on the symmetry of the line shape.
W.
The efficiency of frequency multipliers usually changes strongly with frequency.
In addition, recording conditions and sensitivities of detectors can have
strong influences on the quality of the spectra.
Particularly good S/N ratios were reached around 630, 900 and around
1100-1200 GHz. The spectra around 500 and around 800 GHz had poorer
S/N ratios. In addition, the S/N ratios changed considerably
within each scan and were usually lower towards the ends.
Uncertainties of 50 kHz were assigned to isolated lines with good to
very good S/N ratios throughout the frequency regions.
Larger uncertainties were assigned to weaker lines or lines
which were not isolated. In the course of the analysis it was observed
that several strong lines had residuals considerably smaller than 50 kHz.
Thus, smaller uncertainties, down to 20 kHz, were assigned to
very strong lines, depending on the symmetry of the line shape.
3 Analysis and discussion
The previously available data set will be described in the following for each isotopolog individually, with the exception of the two singly substituted 13C species because the latter had almost always been investigated together. The extent of lines added in the course of the present work will be given also.
The main isotopolog of methyl cyanide as well as all other ones
investigated in the present work are strongly prolate symmetric rotors
having
![]() symmetry, except for CH2DCN.
Spin-statistics have to be taken into account for the
symmetric rotor isotopologs. Levels having
symmetry, except for CH2DCN.
Spin-statistics have to be taken into account for the
symmetric rotor isotopologs. Levels having
![]() belong to the E symmetry class whereas levels having K = 3n
belong to the A symmetry class;
belong to the E symmetry class whereas levels having K = 3n
belong to the A symmetry class; ![]() in all instances.
The spin-statistical weight of A symmetry levels with K > 0
is twice that of K = 0 and all E symmetry levels.
in all instances.
The spin-statistical weight of A symmetry levels with K > 0
is twice that of K = 0 and all E symmetry levels.
The observed transitions all obey the
![]() selection rule
thus their positions are unaffected by the purely K-dependent parameters.
However, these parameters are not negligible as they affect
the intensities of the
selection rule
thus their positions are unaffected by the purely K-dependent parameters.
However, these parameters are not negligible as they affect
the intensities of the
![]() transitions due
to their contribution to the energies of the rotational levels.
transitions due
to their contribution to the energies of the rotational levels.
In a strongly prolate molecule, such as methyl cyanide,
![]() transitions only gain intensity through centrifugal distortion effects;
they were too weak to be identified unambigously in the present work.
Because of this, the purely K-dependent parameters A, DK, etc.
are usually not determinable for such molecules by means of
rotational spectroscopy.
transitions only gain intensity through centrifugal distortion effects;
they were too weak to be identified unambigously in the present work.
Because of this, the purely K-dependent parameters A, DK, etc.
are usually not determinable for such molecules by means of
rotational spectroscopy.
The dipole moment has been measured both for CH3CN (Gadhi et al. 1979) and CH3C15N (Mito et al. 1984). Isotopic differences in the dipole moments are expected to be small for the symmetric top species. In the case of CH3C15N, a value was obtained that deviated barely significantly (3.9256 (7) D) from the value for the main isotopolog (3.92197 (13) D).
CH2DCN is the only asymmetric top rotor among the isotopologs
dealt with in the present article. It only has CS symmetry,
therefore, no spin-statistics have to be considered, in contrast
to what was assumed in Gerin et al. (1992).
The asymmetry parameter
![]() is -0.9973,
expectedly close to the prolate symmetric top limit of -1.
The small rotation of the inertial axis system caused
by the substitution of one H atom by D gives rise to a small
b-dipole moment component of
is -0.9973,
expectedly close to the prolate symmetric top limit of -1.
The small rotation of the inertial axis system caused
by the substitution of one H atom by D gives rise to a small
b-dipole moment component of ![]() 0.17 D.
0.17 D.
![]() and
and
![]() have to be odd for b-type transitions.
However, most of the previously observed and all of the new transitions
are a-type transitions with
have to be odd for b-type transitions.
However, most of the previously observed and all of the new transitions
are a-type transitions with
![]() and
and
![]() .
Transitions having
.
Transitions having
![]() are allowed also, but are very weak
for asymmetric molecules close to the prolate symmetric limit.
are allowed also, but are very weak
for asymmetric molecules close to the prolate symmetric limit.
Table 1: Lower state quantum numbers of rotational transitionsa of CH3CN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
3.1 CH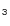 CN
CN
Cazzoli & Puzzarini (2006) carried out extensive high resolution, high accuracy
measurements on CH3CN up to 1.2 THz. Their data set also included
data from Boucher et al. (1977); Kukolich et al. (1973); Kukolich (1982) below 74 GHz.
Simecková et al. (2004) pointed to an interaction between ![]() ,
K = 14
and the lowest excited, doubly degenerate
,
K = 14
and the lowest excited, doubly degenerate
![]() bending mode
at K = 12 in the lower energy l = +1 component causing non-negligible
shifts between
bending mode
at K = 12 in the lower energy l = +1 component causing non-negligible
shifts between
![]() and 48. However, they were only able
to perform a preliminary analysis because they only observed J'' = 38
and 39 of
and 48. However, they were only able
to perform a preliminary analysis because they only observed J'' = 38
and 39 of ![]() .
A more complete analysis has been presented by
Müller et al. (2007b); a manuscript on this work, involving extensive
rotational and rovibrational data for states with
.
A more complete analysis has been presented by
Müller et al. (2007b); a manuscript on this work, involving extensive
rotational and rovibrational data for states with
![]() ,
is in preparation. The perturbation is negligible for all but the small number
of
,
is in preparation. The perturbation is negligible for all but the small number
of ![]() ,
K = 14 transitions. Thus, in the present analysis,
the perturbed transitions were omitted from the final fit.
No evidence for perturbations was found in the
rotational spectra of the less abundant isotopologs.
,
K = 14 transitions. Thus, in the present analysis,
the perturbed transitions were omitted from the final fit.
No evidence for perturbations was found in the
rotational spectra of the less abundant isotopologs.
37 lines have been detected around 1.6 THz covering J'' = 86 to 88
with K up to 15. These data are given in Table 1
together with their assignments, uncertainties, and the residuals
between measured frequencies and those calculated from the
final spectroscopic parameters.
They have been fit together with previous data mentioned above
which extended to K = 21 and J'' = 64.
Also included in the fits were the
![]() ground state energy differences from Anttila et al. (1993)
in order to determine the purely K-dependent terms A and DK.
Tunable far-infrared data from Pavone et al. (1990) were omitted from
the final fits because trial fits with these data included showed
their effect on the spectroscopic parameters to be negligible.
ground state energy differences from Anttila et al. (1993)
in order to determine the purely K-dependent terms A and DK.
Tunable far-infrared data from Pavone et al. (1990) were omitted from
the final fits because trial fits with these data included showed
their effect on the spectroscopic parameters to be negligible.
The considerable extension of the data set in J required two additional
higher order parameters to be included in the fit, the decic centrifugal
distortion parameters PJK and PJJK, as shown in
Table 2. On the other hand, the centrifugal
distortion correction term eQqJ has been omitted from the final
fit because it was not significantly determined,
![]() Hz,
and because it may still be too large in magnitude even though the
absolute value in Cazzoli & Puzzarini (2006) was more than one order of
magnitude smaller than that in Simecková et al. (2004).
If one assumes the ratio of -DJ/B to be a good estimate
for the ratio of eQqJ/eQq then the eQqJ value
in Cazzoli & Puzzarini (2006) is about one order of magnitude too large,
but has presumably the correct (positive) sign; Simecková et al. (2004)
determined a negative value. As the hyperfine parameters
and the lower order distortion terms are mostly determined by
the data published by Cazzoli & Puzzarini (2006) it is not surprising that
there is a good agreement between the parameter values and uncertainties
determined both in that study as well as in the present work.
The increase in J in the present study causes the purely J-dependent
terms (B, ..., LJ) to be improved in accuracy
despite the additional centrifugal distortion terms.
Hz,
and because it may still be too large in magnitude even though the
absolute value in Cazzoli & Puzzarini (2006) was more than one order of
magnitude smaller than that in Simecková et al. (2004).
If one assumes the ratio of -DJ/B to be a good estimate
for the ratio of eQqJ/eQq then the eQqJ value
in Cazzoli & Puzzarini (2006) is about one order of magnitude too large,
but has presumably the correct (positive) sign; Simecková et al. (2004)
determined a negative value. As the hyperfine parameters
and the lower order distortion terms are mostly determined by
the data published by Cazzoli & Puzzarini (2006) it is not surprising that
there is a good agreement between the parameter values and uncertainties
determined both in that study as well as in the present work.
The increase in J in the present study causes the purely J-dependent
terms (B, ..., LJ) to be improved in accuracy
despite the additional centrifugal distortion terms.
The purely K-dependent terms A and DK are determined in the
present study entirely by the
![]() ground state energy
differences from Anttila et al. (1993). Thus, good agreement is not surprising.
Trial fits with HK released yielded a value of (
ground state energy
differences from Anttila et al. (1993). Thus, good agreement is not surprising.
Trial fits with HK released yielded a value of (
![]() Hz
as in Anttila et al. (1993).
Judging by the DK/A ratio, it is too large by about a factor of 3.
Moreover, the parameter has not been determined with significance.
Therefore, HK was fixed in the present analysis to a value
derived from the DK/A ratio. Current results from the more extended
analysis (Müller et al. 2007b) are in support of this value.
Hz
as in Anttila et al. (1993).
Judging by the DK/A ratio, it is too large by about a factor of 3.
Moreover, the parameter has not been determined with significance.
Therefore, HK was fixed in the present analysis to a value
derived from the DK/A ratio. Current results from the more extended
analysis (Müller et al. 2007b) are in support of this value.
The weighted standard deviation of the fit is 0.906, indicating that the data have been reproduced within experimental uncertainties on average. The corresponding value for only the new transition frequencies is 0.670, suggesting the estimates of the uncertainties to be slightly too conservative.
Table 2:
Spectroscopic parametersa (MHz) of methyl cyanide isotopic
species with
![]() symmetry and dimensionless weighted standard
deviation wrms.
symmetry and dimensionless weighted standard
deviation wrms.
![\begin{figure}
\includegraphics[width=9cm,clip]{12932fg1.eps}
\end{figure}](/articles/aa/full_html/2009/42/aa12932-09/Timg30.png)
|
Figure 1:
Section of the submillimeter spectrum of CH3CN.
Transitions of CH3C15N have been labeled with their K
quantum numbers. K = 3 and 6 appear stronger than expected because
of the spin-statistics, see Sect. 3. Also labeled are
K = 10 and 9 of 13CH3CN by |
| Open with DEXTER | |
3.2 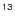 CH
CH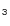 CN and CH
CN and CH
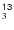 CN
CN
The data set of Pearson & Müller (1996) contained besides their new measurements of the rotational spectra for the two singly substituted 13C isotopomers data from Demaison et al. (1979) for both species, covering 71-184 GHz as well as data for the J = 2 - 1 transitions for CH313CN from Kukolich (1982).
The terrestrial 12C/13C ratio is about 90. Hence, transitions
of these species were easily observable in samples of natural isotopic
composition, see Fig. 1. It is worthwhile mentioning that transitions
of the main isotopolog in excited vibrational states
![]() and 2 and
and 2 and
![]() having the same K are about as strong or even stronger
at room temperature than the transitions of the 13C isotopomers.
having the same K are about as strong or even stronger
at room temperature than the transitions of the 13C isotopomers.
262 and 244 transitions were used in the final fits of 13CH3CN and CH313CN from the broad spectral recordings. J'' was extended to 66 and 64, respectively, near 1.2 THz and K reached 16 and 15, respectively. Some transitions were even identified near 1.6 THz, but they were too few and of too poor S/N to have a signifant impact on the data sets. The weighted standard deviation for the lines measured at JPL are 0.854 and 0.875, respectively, indicating essentially appropriate error estimates on average.
Only five transions each were recorded in Cologne for 13CH3CN and CH313CN at J'' = 18 and 16, respectively, and K = 0 - 3 and 6 in order to improve some of the low order spectroscopic parameters. Only in the process of preparing the manuscript was it noted that the residuals between measured frequencies and those calculated from the final set of spectroscopic parameters were considerably smaller than the assumed uncertainties of 3 kHz. In fact, when these transitions were weighted out the average residuals were only 1.9 and 1.5 kHz, respectively. Therefore, uncertainties of 1 kHz were assigned to these transitions. The resulting weighted standard deviations were then 0.303 and 0.571, respectively. This may indicate still too conservative error estimates, but because of the small number of lines which have much smaller uncertainties than most of the transition frequencies such small weighted standard deviations may not mean much.
The present and previous data were fit together to determine spectroscopic parameters for both isotopomers. Higher order parameters which could not be determined reliably were fixed to values derived from the main isotopic species by scaling the parameters with appropriate powers of B and taking into consideration deviations from this estimate for related lower order parameters. The results are also given in Table 2. The newly measured transition frequencies are given in Tables 4 and 5 together with their assignments, uncertainties, and the residuals between measured frequencies and those calculated from the final spectroscopic parameters.
The rotational and centrifugal distortion parameters have been improved considerably with respect to Pearson & Müller (1996) while the values for eQq are determined entirely from data used in that work. The weighted standard deviations for the whole fits are slightly below 1.0.
3.3 CH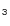 C
C N
N
Pearson & Müller (1996) had in their data set transitions from Bauer et al. (1975) covering 35-143 GHz and from Demaison et al. (1979) covering 214-232 GHz besides their own measurements. In the present fits we have used the J = 1 - 0 transition frequency from Haekel & Mäder (1989).
The terrestrial 14N/15N ratio is about 270, just a factor of three larger than the terrestrial 12C/13C ratio. Thus, transitions of this isotopolog were still quite easily observable in samples of natural isotopic composition, see Fig. 1. Eight transitions, K = 0 - 6 and 12 for J'' = 18 were recorded in Cologne. Additionally, 210 transition frequencies were extracted from the spectra recorded at JPL which extend to J'' = 66 near 1.2 THz and to K = 14.
The present and previous data were fit together to determine spectroscopic parameters. As in Sect 3.2, higher order parameters were estimated and kept fixed. The spectroscopic parameters are given in Table 2, too. The newly measured transition frequencies are given in Table 6 together with their assignments, uncertainties, and the residuals between measured frequencies and those calculated from the final spectroscopic parameters.
The overall weighted standard deviation is 0.692, and the corresponding values for the transitions recorded in Cologne and at JPL are 0.833 and 0.716.
3.4 CH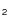 DCN
DCN
Le Guennec et al. (1992) presented extensive measurements of the singly deuterated methyl cyanide molecule between 116 and 471 GHz. They were able to observe several of the very weak b-type transitions because they employed an isotopically enriched sample. Their data set also included three J = 2 - 1 transitions from Thomas et al. (1955).
With transitions of CH3C15N identified almost as frequently
as the singly substituted 13C species it seemed promising to
detect CH2DCN in samples of natural isotopic composition.
The terrestrial H/D ratio is about 6400. The presence of three equivalent
H atoms decreases the H/D ratio by three, the resolved K-doubling
for low ![]() increases it again by two, and the omission of
spin-statistics complicates the situation even more. Altogether, the
lines of CH2DCN are around one order of magnitude weaker than the
lines of CH3C15N.
increases it again by two, and the omission of
spin-statistics complicates the situation even more. Altogether, the
lines of CH2DCN are around one order of magnitude weaker than the
lines of CH3C15N.
10 lines of 16 transitions with
J'' = 15 - 18 and ![]() were recorded
in Cologne. 109 lines of 152 transitions with
were recorded
in Cologne. 109 lines of 152 transitions with
![]() and
and ![]() were extracted from the spectra taken at JPL. With increasing values of J
in the fit it was increasingly difficult to fit the data within experimental
uncertainties. It turned out that omission of one of the two very weak
were extracted from the spectra taken at JPL. With increasing values of J
in the fit it was increasingly difficult to fit the data within experimental
uncertainties. It turned out that omission of one of the two very weak
![]() transitions reported by Le Guennec et al. (1992)
yielded satisfactory fits. Moreover, if one of these transitions
was weighted out it appeared as if a 1 MHz typographical error may
have occured for the other transition. Since the authors of this work
did not have the spectra at their disposal and thus were unable to clarify
this issue we decided to omit both
transitions reported by Le Guennec et al. (1992)
yielded satisfactory fits. Moreover, if one of these transitions
was weighted out it appeared as if a 1 MHz typographical error may
have occured for the other transition. Since the authors of this work
did not have the spectra at their disposal and thus were unable to clarify
this issue we decided to omit both
![]() transitions
from the fit. Without these transitions, DK could not be determined
reliably. Therefore, its value has been estimated by scaling values from
ab initio calculations by the ratios of experimental versus ab initio
values for CH3CN (Table 2) and CHD2CN (Halonen & Mills 1978).
The value of 1.97 MHz is slightly bigger than the 1.83 MHz obtained
in Le Guennec et al. (1992). HK was estimated as DK2/A as done for
CH3CN, see Sect. 3.1. The resulting spectroscopic
parameters are given in Table 3.
The newly measured transition frequencies
are given in Table 7, toward the end of the
manuscript, together with their assignments, uncertainties,
and the residuals between measured frequencies and those
calculated from the final spectroscopic parameters.
transitions
from the fit. Without these transitions, DK could not be determined
reliably. Therefore, its value has been estimated by scaling values from
ab initio calculations by the ratios of experimental versus ab initio
values for CH3CN (Table 2) and CHD2CN (Halonen & Mills 1978).
The value of 1.97 MHz is slightly bigger than the 1.83 MHz obtained
in Le Guennec et al. (1992). HK was estimated as DK2/A as done for
CH3CN, see Sect. 3.1. The resulting spectroscopic
parameters are given in Table 3.
The newly measured transition frequencies
are given in Table 7, toward the end of the
manuscript, together with their assignments, uncertainties,
and the residuals between measured frequencies and those
calculated from the final spectroscopic parameters.
The weighted standard deviation for the whole fit is 0.740, for the Cologne and JPL lines it is 0.533 and 0.792, respectively. In particular the uncertainties of the Cologne lines have been estimated somewhat too conservatively.
Table 3: Spectroscopic parametersa (MHz) of monodeuterated methyl cyanide, CH2DCN and dimensionless weighted standard deviation wrms.
Table 4: Lower state quantum numbers of rotational transitions of 13CH3CN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
3.5 13CH313CN
With even a considerable number of transitions assigned for CH2DCN it seemed promising to assign transitions of the doubly substituted 13C isotopolog, as the lines are weaker by only about a factor of two. This species should be observable fairly readily in Galactic center sources because of the 12C/13C ratio of about 20 in these sources (Wilson & Rood 1994; Müller et al. 2008). Hence, lines of the doubly substituted 13C isotopolog should be only slightly weaker than those of CH3C15N. In fact, inspection of the 3 mm line survey of Sagittarius B2(N) obtained with the IRAM 30 m telecope (Belloche, private communication, 2008; see also Müller et al. 2008; Belloche et al. 2009) revealed that several 13CH313CN lines should have S/N ratios of greater than three. However, all lines were overlapped by stronger lines, frequently belonging to the much stronger 13CH3CN which has fairly similar spectroscopic parameters. At higher frequencies, this overlap will be less severe.
Tam et al. (1988) had reported some transition frequencies for this isotopolog up to 72 GHz. Inspection of the broad spectral recordings however failed to produce any even tentative assignments. Subsequently, all spectroscopic parameters were estimated from the isotopologs with no or only one 13C, and only B was determined. Still, no assignments could be made.
The search for transitions of 13CH313CN was the main reason for the measurements in Cologne. Several tentative assignments could be made near 321 GHz. All of these transitions were about 2.8 MHz higher than predicted, suggesting the assumptions of the centrifugal distortion parameters to be good, but the B value to be slightly too small.
![\begin{figure}
\par\includegraphics[width=7cm,clip]{12932fg2.eps}
\end{figure}](/articles/aa/full_html/2009/42/aa12932-09/Timg38.png)
|
Figure 2:
Section of the lower submillimeter spectrum of CH3CN.
Transitions of 13CH313CN have been labeled with their
K quantum numbers. This isotopolog is less abundant by a factor
of 8000 compared to the main isotopolog.
While the spectrum is rather sparse on the level of the strongest
CH3CN transitions, it is considerably less sparse on the level
of the CH3C15N transition, see Fig. 1, and rather
dense on the level of the 13CH313CN transitions.
One of the lines may be due to a high-K,
|
| Open with DEXTER | |
With an abundance almost four orders of magnitude smaller than that of
the main isotopolog, there are ample chances for overlap.
Not only are there many vibrational states of the main isotopolog up to
2000 cm-1 which are about as strong or even stronger, but also
transitions up to
![]() of the 13C isotopomers, or up
to
of the 13C isotopomers, or up
to
![]() of the 15N species are about as strong.
In addition, many additional species are only slightly weaker,
including those of the isotopomers with 15N and one 13C.
Even though the rotational spectrum of methyl cyanide is very sparse
at the level of the strongest lines of the main isotopolog it is
very rich at the level of the 13CH313CN lines, as can be seen
in Fig. 2. Only the marked lines belonging to this isotopolog
can be assigned unambiguously in this recording. One of the weaker lines
may be due to a high-K,
of the 15N species are about as strong.
In addition, many additional species are only slightly weaker,
including those of the isotopomers with 15N and one 13C.
Even though the rotational spectrum of methyl cyanide is very sparse
at the level of the strongest lines of the main isotopolog it is
very rich at the level of the 13CH313CN lines, as can be seen
in Fig. 2. Only the marked lines belonging to this isotopolog
can be assigned unambiguously in this recording. One of the weaker lines
may be due to a high-K,
![]() line of 13CH3CN,
but because of perturbations by
line of 13CH3CN,
but because of perturbations by
![]() ,
the exact position of
this transition is not certain. All other lines can not be assigned at present.
,
the exact position of
this transition is not certain. All other lines can not be assigned at present.
Altogether, 21 transitions with
J'' = 13 - 18 and ![]() were
recorded in Cologne. Subsequently, 34 transition with
J'' = 25 - 63
and
were
recorded in Cologne. Subsequently, 34 transition with
J'' = 25 - 63
and ![]() could be identified in the spectra taken at JPL. The data
from Tam et al. (1988) were omitted from the final fits because their
large uncertainties cause negligible effects on the spectroscopic
parameters.
could be identified in the spectra taken at JPL. The data
from Tam et al. (1988) were omitted from the final fits because their
large uncertainties cause negligible effects on the spectroscopic
parameters.
Table 5: Lower state quantum numbers of rotational transitions of CH313CN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 6: Lower state quantum numbers of rotational transitions of CH3C15N from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 7: Quantum numbers of rotational transitions of CH2DCN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 8: Lower state quantum numbers of rotational transitions of 13CH313CN, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Five parameters up to sixth order could be determined with significance; several other were kept fixed to estimated values as done further above (see Sect. 3.2). Again, the spectroscopic parameters are listed in Table 2. The measured transition frequencies are given in Table 8, toward the end of the manuscript, together with their assignments, uncertainties, and the residuals between measured frequencies and those calculated from the final spectroscopic parameters.
The weighted standard deviation of the fit is 0.635. It is 0.719 and 0.577 for the data from Cologne and JPL, respectively. The somewhat conservative error estimates can be justified by the possibility that some of the lines may still be affected by overlap to a non-negligible amount. However, the moderately large size of the data set should ensure that such effects are small.
4 Conclusion
Rotational transitions for six astrophysically and astrochemically important isotopologs of methyl cyanide in their ground vibrational states have been recorded and the existing data sets have been extended greatly in most cases. The data should permit reliable predictions to above 2 THz in all instances, sufficient not only for Herschel or ALMA, but very likely also for such missions as SOFIA, CCAT, or others.
Transitions of the even rarer isotopomers containing 15N and one 13C may be observable also, but chance of significant overlap are even higher and the chances to observe these species in space seem vanishingly low.
Predictions of the rotational spectra of the isotopologs studied in the
course of the present investigation will be available in the catalog
section![]() of the Cologne Database for Molecular
Spectroscopy
of the Cologne Database for Molecular
Spectroscopy![]() (Müller et al. 2005,2001). The complete line, parameter and fit files
will be deposited in the spectroscopy data section of the CDMS.
Updated or new JPL catalog (Pickett et al. 1998) entries
(Müller et al. 2005,2001). The complete line, parameter and fit files
will be deposited in the spectroscopy data section of the CDMS.
Updated or new JPL catalog (Pickett et al. 1998) entries![]() will be available also.
will be available also.
H.S.P.M. is very grateful to the Bundesministerium für Bildung und Forschung (BMBF) for financial support aimed at maintaining the Cologne Database for Molecular Spectroscopy, CDMS. This support has been administered by the Deutsches Zentrum für Luft- und Raumfahrt (DLR). A part of the present research has been carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (NASA).
References
- Agúndez, M., Fonfría, J. P., Cernicharo, J., et al. 2008, A&A, 479, 493 [NASA ADS] [CrossRef] [EDP Sciences]
- Anttila, R., Horneman, V.-M., Koivusaari, M., & Paso, R. 1993, J. Mol. Spectrosc., 157, 198 [NASA ADS] [CrossRef]
- Bauer, A., Tarrago, G., & Remy, A. 1975, J. Mol. Spectrosc., 58, 111 [NASA ADS] [CrossRef]
- Belloche, A., Garrod, R. T., Müller, H. S. P., et al. 2009, A&A, 499, 215 [NASA ADS] [CrossRef] [EDP Sciences]
- Boucher, D., Burie, J., Demaison, J., et al. 1977, J. Mol. Spectrosc., 64, 290 [NASA ADS] [CrossRef]
- Cazaux, S., Tielens, A. G. G. M., Ceccarelli, C., et al. 2003, ApJ, 593, L51 [NASA ADS] [CrossRef]
- Cazzoli, G., & Puzzarini, C. 2006, J. Mol. Spectrosc., 240, 153 [NASA ADS] [CrossRef]; Erratum, 2008, J. Mol. Spectrosc., 247, 187 [CrossRef]
- Cummins, S. E., Green, S., Thaddeus, P., & Linke, R. A. 1983, ApJ, 266, 331 [NASA ADS] [CrossRef]
- Demaison, J., Dubrulle, A., & Boucher, D. 1979, J. Mol. Spectrosc., 76, 1 [NASA ADS] [CrossRef]
- Drouin, B. J., Maiwald, F. W., & Pearson, J. C. 2005, Rev. Sci. Instr., 76, Art.-No. 093113
- Gadhi, J., Lahrouni, A., Legrand, J., & Demaison, J. 1995, J. Chem. Phys., 92, 1984
- Gerin, M., Combes, F., Wlodarczak, G. et al. 1992, A&A, 259, L35 [NASA ADS]
- Haekel, J., & Mäder, H. 1989, J. Quant. Spectrosc. Radiat. Transfer, 41, 9 [NASA ADS] [CrossRef]
- Halonen, L., & Mills, I. M. 1978, J. Mol. Spectrosc., 73, 494 [NASA ADS] [CrossRef]
- Kukolich, S. G. 1982, J. Chem. Phys., 76, 97 [NASA ADS] [CrossRef]
- Kukolich, S. G., Ruben, D. J., Wang, J. H. S., & Williams, J. R. 1973, J. Chem. Phys., 58, 3155 [NASA ADS] [CrossRef]
- Le Guennec, M., Wlodarczak, G., Burie, J., & Demaison, J. 1992, J. Mol. Spectrosc., 154, 305 [NASA ADS] [CrossRef]
- Matthews, H. E., & Sears, T. J. 1983, ApJ, 267, L53 [NASA ADS] [CrossRef]
- Mauersberger, R., Henkel, C., Walmsley, C. M., et al. 1991, A&A, 247, 307 [NASA ADS]
- Mito, A., Sakai, J., & Katayama, M. 1984, J. Mol. Spectrosc., 103, 26 [NASA ADS] [CrossRef]
- Müller, H. S. P., & Brünken, S. 2005, J. Mol. Spectrosc., 232, 213 [NASA ADS] [CrossRef]
- Müller, H. S. P., Farhoomand, J., Cohen, E. A., et al. 2000, J. Mol. Spectrosc., 201, 1 [NASA ADS] [CrossRef]
- Müller, H. S. P., Thorwirth, S., Roth, D. A., & Winnewisser, G. 2001, A&A, 370, L49 [NASA ADS] [CrossRef] [EDP Sciences]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct, 742, 215 [NASA ADS] [CrossRef]
- Müller, H. S. P., McCarthy, M. C., Bizzocchi, L., et al. 2007a, Phys. Chem. Chem. Phys., 9, 1579 [CrossRef]
- Müller, H. S. P., Drouin, B. J., Pearson, J. C., et al. 2007b, contribution WG03, presented at the 62nd International Symposium on Molecular Spectroscopy, June 18-22, Columbus, Ohio, USA; see also http://molspect.chemistry.ohio-state.edu/symposium_62/symposium/Abstracts/p074.pdf
- Müller, H. S. P., Belloche, A., Menten, K. M., et. al. 2008, J. Mol. Spectrosc., 251, 319 [NASA ADS] [CrossRef]
- Nummelin, A., Bergman, P., Hjalmarson, Å., et al. 1998, ApJS, 117, 427 [NASA ADS] [CrossRef]
- Pavone, F. S., Zink, L. R., Prevedelli, M., et al. 1990, J. Mol. Spectrosc., 144, 45 [NASA ADS] [CrossRef]
- Pearson, J. C., & Müller, H. S. P. 1996, ApJ, 471, 1067 [NASA ADS] [CrossRef]
- Pickett, H. M., Poynter, R. L., Cohen, E. A., et al. 1998, J. Quant. Spectrosc. Radiat. Transfer, 60, 883 [NASA ADS] [CrossRef]
- Ring, H., Edwards, H., Kessler, M., & Gordy, W. 1947, Phys. Rev., 72, 1262 [NASA ADS] [CrossRef]
- Simecková, M., Urban, S., Fuchs, U., et al. 2004, J. Mol. Spectrosc., 226, 123 [NASA ADS] [CrossRef]
- Solomon, P. M., Jefferts, K. B., Penzias, A. A., & Wilson, R. W. 1971, ApJ, 168, L107 [NASA ADS] [CrossRef]
- Sutton, E. C., Blake, G. A., Masson, C. R., & Phillips, T. G. 1985, ApJS, 58, 341 [NASA ADS] [CrossRef]
- Tam, H., An, I., & Roberts, J. A. 1988, J. Mol. Spectrosc., 129, 202 [NASA ADS] [CrossRef]
- Thomas, L. F., Sherrard, E. J., & Sheridan, J. 1955, Trans. Faraday Soc., 51, 619 [CrossRef]
- Ulich, B. L., & Conklin, E. K. 1974, Nature, 248, 121 [NASA ADS] [CrossRef]
- Wilson, T. L., & Rood, R. 1994, ARA&A, 32, 191 [NASA ADS] [CrossRef]
- Winnewisser, G., Krupnov, A. F., Tretyakov, M. Y., et al. 1994, J. Mol. Spectrosc., 165, 294 [NASA ADS] [CrossRef]
Footnotes
- ...
section
![[*]](/icons/foot_motif.png)
- Website: http://www.astro.uni-koeln.de/cdms/entries/, see also http://www.astro.uni-koeln.de/cdms/catalog/
- ...
Spectroscopy
![[*]](/icons/foot_motif.png)
- Website: http://www.astro.uni-koeln.de/cdms/
- ... entries
![[*]](/icons/foot_motif.png)
- Website: http://spec.jpl.nasa.gov/ftp/pub/catalog/catdir.html, see also http://spec.jpl.nasa.gov/
All Tables
Table 1: Lower state quantum numbers of rotational transitionsa of CH3CN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 2:
Spectroscopic parametersa (MHz) of methyl cyanide isotopic
species with
![]() symmetry and dimensionless weighted standard
deviation wrms.
symmetry and dimensionless weighted standard
deviation wrms.
Table 3: Spectroscopic parametersa (MHz) of monodeuterated methyl cyanide, CH2DCN and dimensionless weighted standard deviation wrms.
Table 4: Lower state quantum numbers of rotational transitions of 13CH3CN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 5: Lower state quantum numbers of rotational transitions of CH313CN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 6: Lower state quantum numbers of rotational transitions of CH3C15N from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 7: Quantum numbers of rotational transitions of CH2DCN from present work, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
Table 8: Lower state quantum numbers of rotational transitions of 13CH313CN, frequencies (MHz), uncertainties unc. (kHz), and residuals o-c (kHz) between observed frequencies and those calculated from the final set of spectroscopic parameters.
All Figures
![\begin{figure}
\includegraphics[width=9cm,clip]{12932fg1.eps}
\end{figure}](/articles/aa/full_html/2009/42/aa12932-09/Timg30.png)
|
Figure 1:
Section of the submillimeter spectrum of CH3CN.
Transitions of CH3C15N have been labeled with their K
quantum numbers. K = 3 and 6 appear stronger than expected because
of the spin-statistics, see Sect. 3. Also labeled are
K = 10 and 9 of 13CH3CN by |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=7cm,clip]{12932fg2.eps}
\end{figure}](/articles/aa/full_html/2009/42/aa12932-09/Timg38.png)
|
Figure 2:
Section of the lower submillimeter spectrum of CH3CN.
Transitions of 13CH313CN have been labeled with their
K quantum numbers. This isotopolog is less abundant by a factor
of 8000 compared to the main isotopolog.
While the spectrum is rather sparse on the level of the strongest
CH3CN transitions, it is considerably less sparse on the level
of the CH3C15N transition, see Fig. 1, and rather
dense on the level of the 13CH313CN transitions.
One of the lines may be due to a high-K,
|
| Open with DEXTER | |
| In the text | |
Copyright ESO 2009
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


