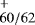| Issue |
A&A
Volume 623, March 2019
|
|
|---|---|---|
| Article Number | A102 | |
| Number of page(s) | 5 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/201834847 | |
| Published online | 11 March 2019 | |
Laboratory formation of large molecules in the gas phase
1
CAS Key Laboratory for Research in Galaxies and Cosmology, Department of Astronomy, University of Science and Technology of China,
Hefei
230026,
PR China
2
School of Astronomy and Space Science, University of Science and Technology of China,
Hefei
230026,
PR China
e-mail: jfzhen@ustc.edu.cn
Received:
13
December
2018
Accepted:
25
January
2019
We report the experimental study on the formation process of large molecules (e.g. a family group of molecular clusters and graphene) in the gas phase. The experiment was carried out using a quadrupole ion trap in combination with time-of-flight mass spectrometry. As the initial molecular precursor, dicoronylene (DC, C48H20)/anthracene (C14H10) cluster cations, the results show that large PAH cluster cations (e.g., (C14H10)C48Hn+, n = [1–19] and (C14H10)C62Hm+, m = [1–25]) and PAH-graphene cluster cations (e.g., (C14H10)nC48+, n = 0, 1, 2, 3 and (C14H10)mC62+, m = 0, 1, 2) are formed by gas-phase condensation under laser irradiation conditions. We infer that these results present in here provide a formation route for interstellar large molecules under the influence of a strong radiation field in the ISM. The relevance of newly formed species to the nanometer-sized dust grain in space is briefly discussed.
Key words: astrochemistry / molecular processes / methods: laboratory: molecular / ultraviolet: ISM / evolution
© ESO 2019
1. Introduction
Mid-infrared (IR) spectra of the interstellar medium (ISM) are dominated by broad features at 3.3, 6.2, 7.7, 8.6 and 11.2 μm, these features are generally attributed to IR fluorescence of large (~50–100 C-atom) polycyclic aromatic hydrocarbon (PAH) molecules pumped by ultraviolet (UV) photons (Sellgren 1984; Puget & Leger 1989; Allamandola et al. 1989; Croiset et al. 2016). Interstellar IR spectra also show evidence of the presence of PAH clusters and very small dust grains (Van Diedenhoven et al. 2004; Rapacioli et al. 2005; Berné et al. 2007; Pilleri et al. 2015). In the ISM, PAH clusters or PAH related complex species are believed to be the self-assembled intermediaries between free gas-phase PAHs and amorphous carbon particles, PAH radicals formed by photo-dehydrogenation are discussed to be the driving force for the cluster formation (Henning & Salama 1998; Richter et al. 2004; Rapacioli et al. 2006; Rhee et al. 2007).
Photo-dissociation regions (PDRs) provide a natural laboratory for the study of the interaction of UV photons with carbonaceous species (Pety et al. 2005; Rapacioli et al. 2005; Hollenbach & Tielens 1999; Micelotta et al. 2010). Observations with the Spitzer Space Telescope and the Herschel Space Observatory of the prototypical PDR, NGC 7023, have revealed the important role of photochemistry in the destruction of interstellar PAHs (Malloci et al. 2008; Berné & Tielens 2012). The top-down processes behind PAH breakdown initiated by UV irradiation or energetic particle bombardment are balanced by bottom-up molecular growth processes (Tielens 2013; Zhen et al. 2018a), where inside the benign environments of molecular clouds, small PAHs can collide and cluster into larger structures (Tielens 2008; Candian et al. 2018). So far, experimental evidence of this process has been lacking. Specifically, laboratory studies of processing of van der Waals clusters of PAHs and fullerenes by energetic ions (e.g., 24 keV O2+ or 12 keV Ar2+) have revealed the formation of chemically bonded large species through direct knock-out of carbon atoms (Zettergren et al. 2010, 2013; Chen et al. 2015; Delaunay et al. 2015).
In the present study, we obtained the formation process of large molecules (~100 atoms, e.g., PAH clusters or graphene) under laser irradiation (355 nm, 3.49 eV), the main aim is to investigate the inherent of large PAH cluster stabilities, the related fragmentation processes, and their collision reaction process (gas-phase condensation) at same time. We explored dicoronylene (DC, C48H20)/anthracene (C14H10) cluster cations as the initial precursor, and experiments were performed by using of newly constructed set up: a quadrupole mass filter-quadrupole ion trap source, analyzed by TOF mass spectrometry. We selected DC as it is an all-benzenoid PAH with size (48 C-atoms) in the astrophysically relevant range (Tielens 2008; Croiset et al. 2016) that possibly serves as a prototypical example for large(r) PAHs. The photo-fragmentation behavior and the IR spectra of DC+ have been obtained (Zhen et al. 2018b; Castellanos et al. 2018), its IR spectra provides a better agreement with astronomical observations (Ricca et al. 2012).
2. Experimental methods
The experiments and the information on experimental procedures are available in Zhen et al. (2018c). We provide only the essential relevant details here. DC is evaporated by heating the powder (Kentax, with a purity better than 99.5%) in the oven at a temperature of ~633 K. Subsequently, evaporated molecules are ionized using electron impact ionization and transported into the ion trapvia ion gate and quadrupole mass filter into the ion trap. Another oven (neutral molecules source, ~300 K) is located under the trap that the vapor phase molecules (anthracene power, J&K, with a purity better than 99%) effuse continuously toward the center of the trap. In the trap, DC/anthracene cluster cations are formed by a collision reaction between cations and neutral anthracene molecule. Helium gas is introduced continuously into the trap to thermalize the ion cloud through collisions via a leak valve.
The third harmonic of Nd:YAG laser (INDI, Spectra-Physics), 355 nm, ~6 ns, operatedat 10 Hz, is used to irradiate the trapped, newly formed ion clusters. A beam shutter (Uniblitz, XRS−4) acts as a physical shield inside in the chamber and determines the interaction time of the light with the trapped ionic clusters. The shutter is externally triggered to guarantee that the ion cloud is irradiated only for a specified amount of time during each cycle. A high precision delay generator (SRS DG535) controls the full timing sequence.
Our setup operates at a typical frequency of 0.2 Hz, meaning that one full measuring cycle lasts 5 s. We used two time strategies here:
(1) At the leading edge of the master trigger the ion gate opens (0–4.0 s), allowing the ion trap to fill for a certain amount of ions. In this period the trapped ion collision reaction with anthracene molecules to form new cluster cations. Then the beam shutter opens and the ion cloud is irradiated (4.0–4.8 s). At the end of the irradiation, a negative square pulse (4.88 s) is applied to the end cap of the ion trap, accelerating the ions out of the trap and into the field-free TOF region where the resulting mass fragments are measured. The resulting mass spectrum is shown in Figs. 1 and 2.
(2) At the leading edge of the master trigger the ion gate and beam shutter opens (0–4.0 s), allowing the ion trap to fill for a certain amount of ions and irradiated by the laser at the same time. Then with a time delay period (4.0–4.8 s), the trapped new photo-formed ions collision reaction with anthracene molecules to form new cluster cations, then the resulting mass is measured (4.88 s). The resulting mass spectrum is shown in Fig. 3.
 |
Fig. 1 Mass spectrum of DC/anthracene cluster cations trapped in QIT without laser irradiation. The inset is a zoom-in mass spectrum, revealing the presence of newly formed clusters: C14 H10DC+ and (C14 H10)2DC+. |
3. Experimental results and discussion
A typical mass spectrum of DC/anthracene cluster cations without laser irradiation is shown in Fig. 1. A series of DC/ anthracene cluster cations are produced, similarly to what was observed for HBC/anthracene (hexa-peri-hexabenzocoronene, (C42 H18, HBC) in Zhen et al. (2018c). The DC/anthracene cluster cations are labeled in detail in a zoom-in mass spectrum in Fig. 1. The inset spectrum provides more details on the new formed DC/anthracene cluster cations: all of them are dehydrogenated, in other words, partially H-stripped ion species are the most abundant species, that as C14 H10DC+ (e.g., C14 H10C48H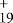 , m/z = 773) and (C14 H10)2DC+ (e.g., (C14 H10)2C48H
, m/z = 773) and (C14 H10)2DC+ (e.g., (C14 H10)2C48H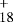 , m/z = 950).
, m/z = 950).
Based on the results in Fig. 1 and our previous work in Zhen et al. (2018c), we propose DC/anthracene cluster cations are formed through collision formation reaction between DC fragmented cations and neutral anthracene molecular, and occurs step by step. As for C14 H10DC+ and (C14 H10)2DC+, the formation reaction pathways are shown below.
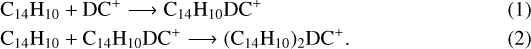
Figure 2 shows the resulting mass spectrum of trapped DC/anthracene cluster cations upon 355 nm irradiation at 4.0 mJ laser energy (irradiation times amounting to 0.8 s; i.e., typically approximately eight pulses, from 4.0 to 4.8 s). Upon laser irradiation, many new peaks are observed in the mass spectrum, in general, two similar fragmentation sequences can be distinguished in the range of m/z = 384–620 and m/z = 620–800, respectively. The fragmentation sequence similar to that observed of large PAHs (Zhen et al. 2014).
In the range of m/z = 384–620, the photo-dissociation of DC+ mainly follows dehydrogenation, leading to the species of C48 H with n = [0–19]. Masses corresponding to C
with n = [0–19]. Masses corresponding to C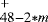 with m = [0–7] appear as well, all ions are almost completely dehydrogenated, resulting in mass spectrum dominated by species (graphene) with masses by C2 difference (separated by 24Da mass differences). In the m/z = 620–800 range, similar to DC+, the photo-dissociation of C14 H10DC+ mainly follows dehydrogenation, leading to the species of C60 H
with m = [0–7] appear as well, all ions are almost completely dehydrogenated, resulting in mass spectrum dominated by species (graphene) with masses by C2 difference (separated by 24Da mass differences). In the m/z = 620–800 range, similar to DC+, the photo-dissociation of C14 H10DC+ mainly follows dehydrogenation, leading to the species of C60 H with n = [0–23] and C62 H
with n = [0–23] and C62 H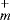 with m = [0–25], as shown in a zoom-in mass spectrum in Fig. 2 in the range of m/z = 704–784. Masses corresponding to C
with m = [0–25], as shown in a zoom-in mass spectrum in Fig. 2 in the range of m/z = 704–784. Masses corresponding to C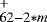 with m = [0–5] also appear, resulting in mass spectrum dominated by species (graphene) with masses following a C2 difference. The similar photo-behavior of C14 H10DC+ to DC+ means that the photo-evolution of C14 H10DC+ mainly converts to more aromatic species by dehydrogenation process (e.g., PAHs, such as C62 H26) and then to other aromatic species (e.g., graphene, such as C62 and C60) (Kroto et al. 1985; Ekern et al. 1998; Castellanos et al. 2018; West et al. 2018; Zhen et al. 2018a).
with m = [0–5] also appear, resulting in mass spectrum dominated by species (graphene) with masses following a C2 difference. The similar photo-behavior of C14 H10DC+ to DC+ means that the photo-evolution of C14 H10DC+ mainly converts to more aromatic species by dehydrogenation process (e.g., PAHs, such as C62 H26) and then to other aromatic species (e.g., graphene, such as C62 and C60) (Kroto et al. 1985; Ekern et al. 1998; Castellanos et al. 2018; West et al. 2018; Zhen et al. 2018a).
Based on the above discussion, we propose the photo-dissociation channel upon laser irradiation for DC cations and C14 H10DC cluster cations are shown as below:
![\begin{eqnarray}&&\hspace*{-6pt}\textrm{C}_{48}\textrm{H}_{20}^{+} + \textrm{h}\nu \longrightarrow {\textrm{C}}_{48}\textrm{C}_{n}^+, n=[0{-}19]\\ &&\hspace*{-6pt}{\textrm{C}_{14}\textrm{H}_{10}\textrm{DC}}^+ + \textrm{h}\nu \longrightarrow {\textrm{C}}_{62}\textrm{H}_{26}^+ \\ &&\hspace*{-6pt}\textrm{C}_{62}\textrm{H}_{26}^+ + \textrm{h}\nu \longrightarrow {\textrm{C}}_{62}\textrm{H}_{m}^+, m=[0{-}25]. \end{eqnarray}](/articles/aa/full_html/2019/03/aa34847-18/aa34847-18-eq13.png)
Figure 3 shows the resulting mass spectrum of trapped DC/anthracene cluster cations upon 355 nm irradiation at 4.0 mJ laser energy (irradiation times amounting to 4.0 s; i.e., typically ~40 pulses, from 0 to 4.0 s) and then collision reaction with neutral anthracene molecular (collision reaction times amounting to 0.8 s, from 4.0 to 4.8 s). Upon laser irradiation and then with the collision reaction, a series of cationic clusters are generated and many more new peaks (three groups) are observed in the mass spectrum in the range of m/z = 620–1344 (Fig. 3, upper panel A).
Further detail on the three groups of new formation species are labeled and illustrated in three zoom-in mass spectra in Figs. 3B–D, respectively. In Fig. 3B, C14 H10C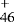 (m/z = 730) and C14 H10C48H
(m/z = 730) and C14 H10C48H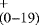 (m/z = 754–773) are produced, and formed through the fragmented cations (C
(m/z = 754–773) are produced, and formed through the fragmented cations (C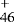 and C48 H
and C48 H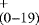 ), that are generated from the fragmentation process of DC+, collision reaction with neutral anthracene molecular. C
), that are generated from the fragmentation process of DC+, collision reaction with neutral anthracene molecular. C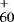 (m/z = 720) and C
(m/z = 720) and C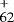 (m/z = 744) are produced, and formed through the photo-dissociation of C14 H10DC+. In Fig. 3C, (C14H10)2C
(m/z = 744) are produced, and formed through the photo-dissociation of C14 H10DC+. In Fig. 3C, (C14H10)2C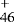 (m/z = 908), C14 H10C
(m/z = 908), C14 H10C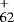 (m/z = 922) and (C14H10)2C
(m/z = 922) and (C14H10)2C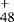 (m/z = 932) are produced, and formed by C14 H10C
(m/z = 932) are produced, and formed by C14 H10C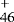 , C
, C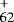 and C14 H10C
and C14 H10C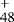 collision reaction with neutral anthracene molecular. C
collision reaction with neutral anthracene molecular. C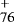 (m/z = 912)is produced, and formed by the photo-dissociation of C14 H10C
(m/z = 912)is produced, and formed by the photo-dissociation of C14 H10C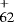 . In Fig. 3D, (C14H10)3C
. In Fig. 3D, (C14H10)3C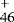 (m/z = 1086), C14 H10C
(m/z = 1086), C14 H10C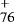 (m/z = 1090), (C14H10)2C
(m/z = 1090), (C14H10)2C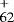 (m/z = 1100) and (C14H10)3C
(m/z = 1100) and (C14H10)3C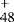 (m/z = 1110) are produced, and formed by (C14H10)2C
(m/z = 1110) are produced, and formed by (C14H10)2C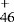 , C
, C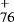 , C14 H10C
, C14 H10C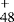 and (C14H10)2C
and (C14H10)2C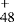 collision reaction with neutral anthracene molecular.
collision reaction with neutral anthracene molecular.
Based on the discussion above, we propose the formation and photo-dissociation under laser irradiation for these new species in different time range are shown as below:
In the time range from 0 to 4.0 s, the photo-dissociation and formation behavior is similar to what we see in Fig. 2 (we may have a lower intensify of formed DC/anthracene cluster cations), we summarize the formation reaction pathway for some new species are shown as below:
![\begin{eqnarray}&&\hspace*{-6pt}\textrm{C}_{48}\textrm{H}_{20}^+ + \textrm{h}\nu \longrightarrow {\textrm{C}}_{48}\textrm{H}_{n}^+, n=[0-19]\\[2pt] &&\hspace*{-6pt}\textrm{C}_{14}\textrm{H}_{10}\textrm{DC}^+ + \textrm{h}\nu \longrightarrow {\textrm{C}}_{62}\textrm{H}_{26}^+ \\[2pt] &&\hspace*{-6pt}\textrm{C}_{62}\textrm{H}_{26}^+ + \textrm{h}\nu \longrightarrow {\textrm{C}}_{62}\textrm{H}_{m}^+, m=[0-25]. \end{eqnarray}](/articles/aa/full_html/2019/03/aa34847-18/aa34847-18-eq36.png)
In the time range from 4.0 to 4.8 s, we propose that new cluster cations are formed through a collision formation reaction between fragmented cations (shown as above) and neutral anthracene molecular, we summarize the formation reaction pathway for some new species below.
![\begin{eqnarray}&&\hspace*{-6pt}\textrm{C}_{14}\textrm{H}_{10} + \textrm{C}_{48}\textrm{H}_{n}^+ \longrightarrow (\textrm{C}_{14}\textrm{H}_{10})C_{48}\textrm{H}_{n}^+, n=[0{-}19]\\ &&\hspace*{-6pt}\textrm{C}_{14}\textrm{H}_{10} + \textrm{C}_{62}\textrm{H}_{m}^+ \longrightarrow (\textrm{C}_{14}\textrm{H}_{10})\textrm{C}_{62}\textrm{H}_{m}^+, m=[0{-}25]. \end{eqnarray}](/articles/aa/full_html/2019/03/aa34847-18/aa34847-18-eq37.png)
 |
Fig. 2 Mass spectrum of DC/anthracene cluster cations trapped in QIT with laser irradiation upon 355 nm irradiation at 4.0 mJ laser energy (irradiation times amounting to 0.8 s, from 4.0 to 4.8 s). The inset is a zoom-in mass spectrum is in the range of m/z = 704–784, revealing the presence of newly formed clusters: C |
 |
Fig. 3 Mass spectrum of DC/anthracene cluster cations trapped in QIT with laser irradiation upon 355 nm irradiation at 4.0 mJ laser energy (irradiation times amounting to 4.0 s, from 0 to 4.0 s), and then collision reaction in the period of 4.0–4.8 s. Panel A: whole mass spectrum in the range of m/z = 384–1344. Panels B, C, and D: zoom-ins of the mass spectrum, revealing the details of the presence newly formed clusters. |
4. Astronomical implications and conclusions
The origin and evolution of large molecular (e.g., PAH clusters or graphene) in space, especially in the range of ~100 atoms or nanometer-size, is a very interesting topic. It has been suggested that the presence of large molecular may help explain certain characteristics of the astronomical observations of the IR spectra (Tielens 2013). While the formation process of such clusters in the experiment is not entirely clear and hence it is difficult to assess their formation in the ISM (Candian et al. 2018). Different from the energetic ions induce intra-cluster reactions to form large molecules, in other words, ion-driven (Zettergren et al. 2013; Delaunay et al. 2015). Our experimental results presented here provide further insight on the formation process of large molecular in the ISM. New larger molecules can be built up under the promotion of irradiation by laser, in other words, photo-driven, the conversion is suggested to take place in the areas of high luminosity where PAH clusters absorb multiple photons. The multiphoton absorption leads to the dehydrogenation of PAHs with the following polymerization with smaller PAHs. The DC/anthracene clusters, as an example of initial precursor molecular, the formation mechanism (gas-phase condensation) and photo-dissociation behavior obtained in here can be generalized and provides a reasonable explanation for the formation and evolution of large molecular in space.
We stress that given the new species produced in this work, PAH cluster cations (e.g., (C14H10)C48H , n = [1–19] and (C14H10)C62H
, n = [1–19] and (C14H10)C62H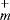 , m = [1–25]) and PAH-graphene cluster cations (e.g., (C14H10)nC
, m = [1–25]) and PAH-graphene cluster cations (e.g., (C14H10)nC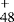 , n = 0, 1, 2, 3 and (C14H10)mC
, n = 0, 1, 2, 3 and (C14H10)mC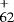 , m = 0, 1, 2), range from 48 to 120 atoms or ~2 nm in size, that can be a nice example of carbonaceous dust grains in the ISM (Kroto et al. 1985; Henning & Salama 1998; Jäger et al. 2009, 2011). The newly formed species produced here offer an approach of carbonaceous dust grains that provide a basic understanding (e.g., composition and structural properties) of the grain formation processes in the gas-phase.
, m = 0, 1, 2), range from 48 to 120 atoms or ~2 nm in size, that can be a nice example of carbonaceous dust grains in the ISM (Kroto et al. 1985; Henning & Salama 1998; Jäger et al. 2009, 2011). The newly formed species produced here offer an approach of carbonaceous dust grains that provide a basic understanding (e.g., composition and structural properties) of the grain formation processes in the gas-phase.
Finally, in conclusion, we have presented the experimental results on the large interstellar molecules (e.g., a family group of molecular clusters and graphene). With the photo-dissociation by the laser and with the formation pathway by gas-phase condensation (collision reaction), PAH clusters are evolving to become more aromatic, structurally complicated, and larger. Theoretical studies regarding the dynamic pathway will be carried out in a subsequent paper (Zhang & Zhen, in prep.).
Acknowledgements
This work is supported by the Fundamental Research Funds for the Central Universities and from the National Science Foundation of China (NSFC, Grant No. 11421303 and Grant No. 11590782).
References
- Allamandola, L. J., Tielens, A. G. G. M., & Barker, J. R. 1989, ApJS, 71, 733 [NASA ADS] [CrossRef] [Google Scholar]
- Berné, O., & Tielens, A. G. G. M. 2012, Proc. Natl. Acad. Sci., 109, 401 [NASA ADS] [CrossRef] [Google Scholar]
- Berné, O., Joblin, C., Deville, Y., et al. 2007, A&A, 469, 575 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Candian, A., Zhen, J., & Tielens, A. G. G. M. 2018, Phys. Today, 71, 38 [CrossRef] [Google Scholar]
- Castellanos, P., Candian, A., Zhen, J., Linnartz, H., & Tielens, A. G. G. M. 2018, A&A, 616, A166 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Chen, T., Gatchell, M., Stockett, M. H., et al. 2015, J. Chem. Phys., 142, 144305 [NASA ADS] [CrossRef] [Google Scholar]
- Croiset, B. A., Candian, A., Berné, O., & Tielens, A. G. G. M. 2016, A&A, 590, A26 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Delaunay, R., Gatchell, M., Rousseau, P., et al. 2015, J. Phys. Chem. Lett., 6, 1536 [CrossRef] [PubMed] [Google Scholar]
- Ekern, S. P., Marshall, A. G., Szczepanski, J., & Vala, M. 1998, J. Phys. Chem. A, 102, 3498 [NASA ADS] [CrossRef] [Google Scholar]
- Henning, T., & Salama, F. 1998, Science, 282, 2204 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Hollenbach, D. J., & Tielens, A.G.G.M. 1999, Rev. Mod. Phys., 71, 173 [Google Scholar]
- Jäger, C., Huisken, F., Mutschke, H., Llamas Jansa, I., & Henning, T. 2009, ApJ, 696, 706 [NASA ADS] [CrossRef] [Google Scholar]
- Jäger, C., Mutschke, H., Henning, T., & Huisken, F. 2011, EAS Pub. Ser., 46, 293 [CrossRef] [Google Scholar]
- Kroto, H., Heath, J., O’Brien, S., Curl, R., & Smalley, R. 1985, Nature, 318, 162 [NASA ADS] [CrossRef] [Google Scholar]
- Malloci, G., Mulas, G., Cecchi-Pestellini, C., & Joblin, C. 2008, A&A, 489, 1183 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Micelotta, E. R., Jones, A. P., & Tielens, A. G. G. M. 2010, A&A, 510, A37 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pety, J., Teyssier, D., Fossé, D., et al. 2005, A&A, 435, 885 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pilleri, P., Joblin, C., Boulanger, F., & Onaka, T. 2015, A&A, 577, A16 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Puget, J. L., & Leger, A. 1989, ARA&A, 27, 161 [NASA ADS] [CrossRef] [Google Scholar]
- Rapacioli, M., Joblin, C., & Boissel, P. 2005, A&A, 429, 193 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Rapacioli, M., Calvo, F., Joblin, C., et al. 2006, A&A, 460, 519 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ricca, A., Bauschlicher, C. W., Jr., Boersma, C., Tielens, A. G. G. M., & Allamandola, L. J. 2012, ApJ, 754, 75 [NASA ADS] [CrossRef] [Google Scholar]
- Richter, H., Granata, S., Green, W.H., & Howard, J.B. 2004, PCI, 30, 1397 [Google Scholar]
- Rhee, Y. M., Lee, T. J., Gudipati, M. S., Allamandola, L. J. & Head-Gordon, M. 2007, Proc. Natl. Acad. Sci., 104, 5274 [NASA ADS] [CrossRef] [Google Scholar]
- Sellgren, K. 1984, ApJ, 277, 623 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. G. G. M. 2008, ARA&A, 46, 289 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Tielens, A. G. G. M. 2013, Rev. Mod. Phys., 85, 1021 [NASA ADS] [CrossRef] [Google Scholar]
- van Diedenhoven, B., Peeters, E., Van Kerckhoven, C., et al. 2004, ApJ, 611, 928 [NASA ADS] [CrossRef] [Google Scholar]
- West, B., Castillo, S. R., Sit, A., et al. 2018, Phys. Chem. Chem. Phys., 20, 7195 [CrossRef] [Google Scholar]
- Zettergren, H, Johansson, H. A. B., Schmidt, H. T., et al. 2010, J. Chem. Phys., 133, 104301 [NASA ADS] [CrossRef] [Google Scholar]
- Zettergren, H., Rousseau, P., Wang, Y., et al. 2013, Phys. Rev. Lett., 110, 185501 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Zhen, J., Paardekooper, D. M., Candian, A., Linnartz, H., & Tielens, A. G. G. M. 2014, Chem. Phys. Lett., 592, 211 [NASA ADS] [CrossRef] [Google Scholar]
- Zhen, J., Chen, T., & Tielens, A. G. G. M. 2018a, ApJ, 863, 128 [NASA ADS] [CrossRef] [Google Scholar]
- Zhen, J., Candian, A., Castellanos, P., et al. 2018b, ApJ, 854, 27 [NASA ADS] [CrossRef] [Google Scholar]
- Zhen, J., Zhang, W. W., & Yang, Y. Y. 2018c, ApJ, submitted [Google Scholar]
All Figures
 |
Fig. 1 Mass spectrum of DC/anthracene cluster cations trapped in QIT without laser irradiation. The inset is a zoom-in mass spectrum, revealing the presence of newly formed clusters: C14 H10DC+ and (C14 H10)2DC+. |
| In the text | |
 |
Fig. 2 Mass spectrum of DC/anthracene cluster cations trapped in QIT with laser irradiation upon 355 nm irradiation at 4.0 mJ laser energy (irradiation times amounting to 0.8 s, from 4.0 to 4.8 s). The inset is a zoom-in mass spectrum is in the range of m/z = 704–784, revealing the presence of newly formed clusters: C |
| In the text | |
 |
Fig. 3 Mass spectrum of DC/anthracene cluster cations trapped in QIT with laser irradiation upon 355 nm irradiation at 4.0 mJ laser energy (irradiation times amounting to 4.0 s, from 0 to 4.0 s), and then collision reaction in the period of 4.0–4.8 s. Panel A: whole mass spectrum in the range of m/z = 384–1344. Panels B, C, and D: zoom-ins of the mass spectrum, revealing the details of the presence newly formed clusters. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.