| Issue |
A&A
Volume 591, July 2016
|
|
|---|---|---|
| Article Number | A110 | |
| Number of page(s) | 6 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201628201 | |
| Published online | 22 June 2016 | |
Terahertz spectroscopy of the 15NH2 amidogen radical⋆
1
Univ. Lille, CNRS, UMR 8523 – PhLAM – Physique des Lasers,
Atomes et Molécules,
59000
Lille,
France
e-mail:
laurent.margules@univ-lille1.fr
2
SOLEIL Synchrotron, AILES beamline, l’Orme des Merisiers,
Saint-Aubin, 91192
Gif-sur-Yvette Cedex,
France
3
Institut des Sciences Moléculaires d’Orsay (ISMO), CNRS, Univ.
Paris-Sud, Université Paris-Saclay, 91405
Orsay,
France
4
LERMA, Observatoire de Paris, PSL Research University, CNRS,
Sorbonne Universités, UPMC Univ.
Paris 06, 92190
Meudon,
France
5
LERMA, UMR 8112 CNRS, 24 Rue Lhomond, 75231
Paris Cedex 05,
France
Received: 27 January 2016
Accepted: 31 March 2016
Context. The determination of isotopic ratios in interstellar molecules is a powerful probe of chemical routes leading to their formation. In particular, the 14N/15N abundance ratio of nitrogen-bearing species provides information on possible fractionation mechanisms. Up to now there is no accurate determination of this ratio in the interstellar medium (ISM) for the amidogen radical, NH2.
Aims. This work is aimed at determining rotational frequencies of 15NH2 to enable its astronomical detection, which will help to understand the formation mechanisms of nitrogen hydrides in the ISM.
Methods. We performed complementary measurements using both synchrotron-based, broadband far-infrared and high-resolution, submillimeter-wave frequencies to investigate the pure rotational spectrum of the 15NH2 species.
Results. The first spectroscopic study of the 15N-isotopologue of the amidogen radical yielded an accurate set of molecular parameters.
Conclusions. Accurate frequencies are now available for 15NH2 up to 7 THz (with N′′ ≤ 13) allowing dedicated astronomical searches to be undertaken.
Key words: submillimeter: ISM / line: identification / astronomical databases: miscellaneous / ISM: molecules
Full Table 2 (S1) and fitting files (S2-S4) are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/591/A110
© ESO, 2016
1. Introduction
The formation mechanism of most of the complex organic molecules being still unknown, the determination of isotopic ratios in the interstellar medium (ISM) is a great help to improve knowledge about their formation process. The 14N/15N ratio is less characterized than H/D or 12C/13C ratios. Using the N = 1 − 0 pattern for CN, HCN, and HNC at 3 mm, Adande & Ziurys (2012) retrieved the 14N/15N ratio and its gradient towards 11 molecular clouds spanning a wide range of distances from the Galactic center that are representative of star-forming regions. Isotopic ratios are also used to test the link between cometary and interstellar molecules as reviewed recently by Mumma & Charnley (2011). Hily-Blant et al. (2013b) used CN transitions to study the 14N/15N ratio isotopic ratio in two dark clouds, whereas Hily-Blant et al. (2013a) discussed 15N enrichment in the dark clouds L1544 and L1498 and solar system objects and raised a possible chemical origin for the observational differences between nitriles and nitrogen hydrides (misleadingly called amines by these authors). We also mention the detection of HC15N in the extragalactic Arp220 environment. Recently, Füri & Marty (2015) suggested that the variation of nitrogen isotopic ratio offers a powerful tracer for investigating the origins of planetary atmospheres.
The present study offers an additional possibility to measure the 14N/15N abundance ratio in the NH2 amidogen molecule. NH2 has been detected in its ortho and para forms as a result of its absorption transitions in the submillimeter region towards high-mass star-forming regions W31C, W49N, W51, and G34.3 (Persson et al. 2012, 2016) with a relative abundance compared to molecular hydrogen of several 10-9 in the molecular envelopes. This nitrogen hydride witnesses the chain of formation reactions involving molecular hydrogen, starting with N+ + H2 and terminating in NH from which NH2 and NH3 are produced through dissociative recombination. The 14N/15N ratio in NH2 and NH3 depends on the ortho/para ratio of molecular hydrogen, which triggers the initial step as discussed in Dislaire et al. (2012) and Roueff et al. (2015). It should be stressed that the 14N/15N isotopic ratio of NH3 and NH2D were determined in the B1 dark molecular cloud (Lis et al. 2010; Gerin et al. 2009; Daniel et al. 2013) and are satisfactorily explained through gas-phase chemistry (Roueff et al. 2015). However, present models fail to explain the large depletions of 15N found in the two isotopologues of N2H+ (Bizzocchi et al. 2010, 2013; Fontani et al. 2015). It is finally worth mentioning the recent detection of 15NH2 in two comets (Rousselot et al. 2014; Shinnaka et al. 2014) thanks to visible spectroscopy that allowed the authors to derive a 14N/15N ratio of 127 for NH2, which is similar to the ratio observed for HCN/CN molecules in these objects. This first detection in comets is indeed very encouraging for interstellar studies as well. The present measurement of the first rotational lines of 15NH2, around 640 GHz, is mandatory for its detection in the ISM using the very sensitive ALMA facility.
from which NH2 and NH3 are produced through dissociative recombination. The 14N/15N ratio in NH2 and NH3 depends on the ortho/para ratio of molecular hydrogen, which triggers the initial step as discussed in Dislaire et al. (2012) and Roueff et al. (2015). It should be stressed that the 14N/15N isotopic ratio of NH3 and NH2D were determined in the B1 dark molecular cloud (Lis et al. 2010; Gerin et al. 2009; Daniel et al. 2013) and are satisfactorily explained through gas-phase chemistry (Roueff et al. 2015). However, present models fail to explain the large depletions of 15N found in the two isotopologues of N2H+ (Bizzocchi et al. 2010, 2013; Fontani et al. 2015). It is finally worth mentioning the recent detection of 15NH2 in two comets (Rousselot et al. 2014; Shinnaka et al. 2014) thanks to visible spectroscopy that allowed the authors to derive a 14N/15N ratio of 127 for NH2, which is similar to the ratio observed for HCN/CN molecules in these objects. This first detection in comets is indeed very encouraging for interstellar studies as well. The present measurement of the first rotational lines of 15NH2, around 640 GHz, is mandatory for its detection in the ISM using the very sensitive ALMA facility.
A strength of this project lies in the complementary measurements that were performed using synchrotron-based, far-infrared (FIR) and submillimeter spectroscopy to determine accurate frequencies over a wide spectral region (550−6000 GHz/18−200 cm-1); these measurements are suitable for detection in the ISM.
2. Experiments
2.1. Production of the radical
The 15NH2 radical was generated in a positive column discharge using 15NH3 as a precursor (98%; Cambridge Isotope Laboratories, Inc.). Two such discharge cells were used for Fourier-transform FIR (FT-FIR) and submillimeter-wave spectroscopy. A description of the cells can be found in Martin-Drumel et al. (2011) and Ozeki et al. (2011), respectively. Briefly, for FT-FIR spectroscopy the 1-m-long, White-type cell available at SOLEIL allowed a 24 m absorption path length in the positive column of the discharge. For submillimeter-wave measurements (PhLAM laboratory), the single path discharge cell was 2 m long. He (1.26 mbar) and Ar (18 μbar) were used as buffer gas for a total pressure of 1 mbar and 25 μbar, respectively. The discharge current sustained between the water-cooled electrodes in the corresponding experimental set-ups was adjusted to 100 mA and 65 mA, respectively.
 |
Fig. 1 Portion of the FT-FIR spectrum recorded using 15NH3 as precursor: both 15NH2 and 15NH are simultaneously produced in the discharge. |
2.2. Synchrotron-based FT-FIR spectroscopy
The rotational spectrum of 15NH2 has been investigated below 225 cm-1 (~7 THz) at the AILES beamline of SOLEIL synchrotron (Brubach et al. 2010) with a Bruker IFS125HR Fourier transform spectrometer making use of the high sensitivity and wide spectral range of the synchrotron radiation. The high-resolution (0.001 cm-1 or 30 MHz) FT-FIR spectrum of the radical was recorded using a 6 μm-thick Mylar beamsplitter and a 4.2 K liquid helium cooled Si-bolometer equipped with a 8 THz low-pass optical filter. The spectrum is the result of about seven hours of integration time (120 co-averaged interferograms). The spectrum was calibrated using residual H2O lines against accurate frequencies from Matsushima et al. (1995) allowing a 0.0001 cm-1 measurement accuracy of 15NH2 experimental frequencies. In addition, pure rotational transitions of 15NH2, absorption features of 15NH – which was analysed in Bailleux et al. (2012) – and residual 15NH3, 14NH3, 14NH2, 14NH, H2O, and OH are also visible (see Martin-Drumel et al. 2014, Fig. 1).
2.3. Submillimeter-wave spectroscopy
In the 580−660 GHz frequency range, a backwards wave oscillator (BWO; Istok company) was used as a source of radiation. It was phase-locked to a harmonic of an Agilent E8257D synthesizer (2−20 GHz) (Ozeki et al. 2011). Additional measurements between 660 and 990 GHz were performed using a solid state source (Motiyenko et al. 2010). In this case, the frequency of the Agilent synthesizer feeded a Spacek active sextupler providing an output power of +15 dBm in the W-band range (75−110 GHz). This power is high enough to use passive Schottky multipliers (×6 and ×9 from Virginia Diodes, Inc.) in the next stage of the frequency multiplication chain. Both types of sources were frequency modulated at 10 kHz. A very sensitive InSb liquid, He-cooled bolometer (QMC Instruments Ltd) was used for detection.
 |
Fig. 2 Example of hyperfine splittings due to 15N and H resolved in the submillimeter-wave spectrum. The lineshape appears as second derivative due to frequency modulation at f = 10 kHz and detection at 2f. |
With a total pressure of 25 μbar the linewidths were limited by Doppler broadening. As a result, isolated lines were measured with an accuracy better than 30 kHz, while blended lines and those observed with a poor signal-to-noise ratio were given an accuracy of 100 or 200 kHz.
3. Analysis of the rotational spectrum
3.1. Theory
The NH2 radical in its ground electronic state (
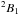 ) is a very asymmetric prolate rotor with an asymmetry parameter κ = − 0.374. The 1.82 D dipole moment lies along the b-axis (Brown et al. 1979). There are many high-resolution spectroscopic studies concerning the amidogen radical in different wavelengths domains. A summary of the spectroscopic history can be found in the latest rotational spectroscopy study (Martin-Drumel et al. 2014). Although the deuterated species were studied (Morino & Kawaguchi 1997; Kobayashi et al. 1997), there has been no investigation concerning the 15N isotopologue until now.
) is a very asymmetric prolate rotor with an asymmetry parameter κ = − 0.374. The 1.82 D dipole moment lies along the b-axis (Brown et al. 1979). There are many high-resolution spectroscopic studies concerning the amidogen radical in different wavelengths domains. A summary of the spectroscopic history can be found in the latest rotational spectroscopy study (Martin-Drumel et al. 2014). Although the deuterated species were studied (Morino & Kawaguchi 1997; Kobayashi et al. 1997), there has been no investigation concerning the 15N isotopologue until now.
Each rotational level with N> 0 is split into two sublevels because of the spin S of the unpaired electron. There is only one spin sublevel if N = 0 (corresponding to 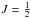 ). These fine structure levels are further split because of the nuclear spin of 15N (
). These fine structure levels are further split because of the nuclear spin of 15N (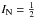 ). Finally, the two equivalent hydrogen nuclei (I
H1
= I
H2
=
). Finally, the two equivalent hydrogen nuclei (I
H1
= I
H2
= 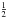 ) give additional splitting of each of these sublevels for rotational levels with even Ka + Kc (ortho levels), whereas no further splitting is observed with odd Ka + Kc (para levels). These properties mean that the energy level diagram of 15NH2 is similar to that of PH2. The representation of the resulting energy level diagram for the ortho levels can be found in Fig. 2 of Margulès et al. (2002). The rotational and spin angular momenta of 15NH2 are coupled in the following way:
) give additional splitting of each of these sublevels for rotational levels with even Ka + Kc (ortho levels), whereas no further splitting is observed with odd Ka + Kc (para levels). These properties mean that the energy level diagram of 15NH2 is similar to that of PH2. The representation of the resulting energy level diagram for the ortho levels can be found in Fig. 2 of Margulès et al. (2002). The rotational and spin angular momenta of 15NH2 are coupled in the following way:  (1)Following the coupling scheme above, each level is characterized with the following set of quantum numbers: N,Ka,Kc,J,F1,F. Pickett’s programs SPFIT and SPCAT were employed for fitting and predicting the spectra (Pickett 1991). The decomposition of the Hamiltonian is the same as that used in previous studies of NH2 (Müller et al. 1999; Gendriesch et al. 2001; Martin-Drumel et al. 2014) and PH2 (Margulès et al. 2002), i.e.
(1)Following the coupling scheme above, each level is characterized with the following set of quantum numbers: N,Ka,Kc,J,F1,F. Pickett’s programs SPFIT and SPCAT were employed for fitting and predicting the spectra (Pickett 1991). The decomposition of the Hamiltonian is the same as that used in previous studies of NH2 (Müller et al. 1999; Gendriesch et al. 2001; Martin-Drumel et al. 2014) and PH2 (Margulès et al. 2002), i.e. 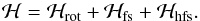 (2)The value ℋrot is the Watson A-reduced rotational Hamiltonian in the Ir representation. In the present investigation, it contains centrifugal distortion constants up to the eighth order. The octic terms, which could not be determined, were kept fixed at the 14NH2 value (Martin-Drumel et al. 2014). Quartic, sextic, and octic centrifugal distortion terms have also been included in the fine structure Hamiltonian ℋfs.
(2)The value ℋrot is the Watson A-reduced rotational Hamiltonian in the Ir representation. In the present investigation, it contains centrifugal distortion constants up to the eighth order. The octic terms, which could not be determined, were kept fixed at the 14NH2 value (Martin-Drumel et al. 2014). Quartic, sextic, and octic centrifugal distortion terms have also been included in the fine structure Hamiltonian ℋfs.
The hyperfine structure Hamiltonian ℋhfs can be separated into three parts: 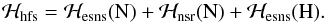 (3)The first and third terms describe the electron spin-nuclear spin couplings between the electronic spin (es) and the nuclear spins (ns). The second term represents the nuclear spin-rotation (nsr) interactions caused by the nitrogen and hydrogen nuclei. As previously found in 14NH2, other terms concerning the nuclear spin-rotation interactions and the electron spin-nuclear spin couplings were found to be insignificant.
(3)The first and third terms describe the electron spin-nuclear spin couplings between the electronic spin (es) and the nuclear spins (ns). The second term represents the nuclear spin-rotation (nsr) interactions caused by the nitrogen and hydrogen nuclei. As previously found in 14NH2, other terms concerning the nuclear spin-rotation interactions and the electron spin-nuclear spin couplings were found to be insignificant.
3.2. Assignment
The initial prediction was made with a somewhat rough method. Rotational constants for both 14N and 15N amidogen radicals were first inferred from the r0 structure deduced from the FT-FIR study (0.0075 cm-1 resolution) of NH2, NHD, and ND2 by Morino & Kawaguchi (1997). These calculated constants are referred to as 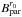 and
and 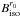 , respectively, where B stands for the A, B, or C rotational constant of the parent (par) and isotologue (iso) species. The 15NH2 rotational constants, noted as
, respectively, where B stands for the A, B, or C rotational constant of the parent (par) and isotologue (iso) species. The 15NH2 rotational constants, noted as 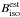 , were precisely estimated by correcting the three
, were precisely estimated by correcting the three 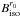 with the (
with the (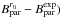 differences, where
differences, where 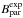 are the experimentally determined rotational constants of 14NH2 from Müller et al. (1999):
are the experimentally determined rotational constants of 14NH2 from Müller et al. (1999): 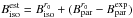 . This method yields 15NH2 estimated rotational constants with an accuracy better than 30 MHz (0.01% on C). The centrifugal distortion and fine structure terms were fixed at their corresponding 14NH2 values. The hyperfine parameters related to the 15N nucleus were obtained by applying the gyromagnetic corrections to the related 14NH2 parameters, as in our 15NH previous study (Bailleux et al. 2012).
. This method yields 15NH2 estimated rotational constants with an accuracy better than 30 MHz (0.01% on C). The centrifugal distortion and fine structure terms were fixed at their corresponding 14NH2 values. The hyperfine parameters related to the 15N nucleus were obtained by applying the gyromagnetic corrections to the related 14NH2 parameters, as in our 15NH previous study (Bailleux et al. 2012).
This prediction was accurate enough to readily assign and fit 142 lines in the FT-FIR spectrum (N′′ ≤ 13 and 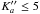 ). A zoom of the region around 129 cm-1 gives an example of the fine structures of 15NH2 and 15NH rotational transitions observed, 12 of which exhibit a resolved-nitrogen, hyperfine structure. A preliminary fit of these data enabled a prediction that was better than a few MHz in the submillimeter-wave region, resulting in a straightforward analysis of the corresponding transition frequencies. The hyperfine structure due to the hydrogen spins was resolved owing to the higher resolution achieved in this spectral region, as shown in Fig. 2. Seventy-seven additional lines, with N′′ ≤ 5 and
). A zoom of the region around 129 cm-1 gives an example of the fine structures of 15NH2 and 15NH rotational transitions observed, 12 of which exhibit a resolved-nitrogen, hyperfine structure. A preliminary fit of these data enabled a prediction that was better than a few MHz in the submillimeter-wave region, resulting in a straightforward analysis of the corresponding transition frequencies. The hyperfine structure due to the hydrogen spins was resolved owing to the higher resolution achieved in this spectral region, as shown in Fig. 2. Seventy-seven additional lines, with N′′ ≤ 5 and 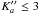 , were assigned and fitted.
, were assigned and fitted.
Spectroscopic parameters of 15NH2 were derived from a global fit of a total of 219 lines. A root mean square (rms) deviation of 1.0 × 10-4 cm-1 for the FT-FIR lines and 115 kHz for the submillimeter-wave lines was achieved (see Table 1). The list of measured rotational lines that are potential candidates for detection with the ALMA facility is provided in Table 2. Owing to its large size, the complete version of the global fit Table (S1) is supplied at the CDS. The fitting files .lin (S2), .par (S3), and the prediction .cat (S4) are also available at CDS.
Spectroscopic parameters of the 15NH2 and 14NH2 radicals in their ground vibronic state.
Measured frequencies of the 21,1 − 20,2 transition, and residuals from the global fit of the Infrared, and submillimeter-wave data for 15NH2.
4. Conclusion
The first high-resolution rotational spectroscopic study of 15NH2 was performed using synchrotron-based broad range FIR measurements and submillimeter spectroscopy. A large number of lines were fitted within the experimental accuracy. Accurate frequencies of 15NH2 can now be predicted up to at least 7 THz. The rotational transition 21,1 − 20,2 lies near 645 GHz and is therefore a good candidate for a possible detection with ALMA.
Acknowledgments
This work was supported by the Programme National “Physique et Chimie du Milieu Interstellaire” and the Centre National d’Études Spatiales (CNES). This work was also done under ANR-13-BS05-0008-02 IMOLABS. The authors are grateful to SOLEIL for providing beam time on the AILES beamline under the proposal 20110017.
References
- Adande, G., & Ziurys, L. 2012, ApJ, 744, 194 [NASA ADS] [CrossRef] [Google Scholar]
- Bailleux, S., Martin-Drumel, M., Margulès, L., et al. 2012, A&A, 538, A135 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bizzocchi, L., Caselli, P., & Dore, L. 2010, A&A, 510, L5 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bizzocchi, L., Caselli, P., Leonardo, E., & Dore, L. 2013, A&A, 555, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Brown, J., Chalkley, S., & Wayne, F. 1979, Mol. Phys., 38, 1521 [NASA ADS] [CrossRef] [Google Scholar]
- Brubach, J.-B., Manceron, L., Rouzières, M., et al. 2010, in WIRMS 2009 5th international workshop on infrared microscopy and spectroscopy with accelerator based sources, AIP Conf. Proc., 1214, 81 [NASA ADS] [CrossRef] [Google Scholar]
- Daniel, F., Gérin, M., Roueff, E., et al. 2013, A&A, 560, A3 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Dislaire, V., Hily-Blant, P., Faure, A., et al. 2012, A&A, 537, A20 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fontani, F., Caselli, P., Palau, A., Bizzocchi, L., & Ceccarelli, C. 2015, ApJ, 808, L46 [NASA ADS] [CrossRef] [Google Scholar]
- Füri, E., & Marty, B. 2015, Nature Geoscience, 8, 515 [CrossRef] [Google Scholar]
- Gendriesch, R., Lewen, F., Winnewisser, G., & Müller, H. S. 2001, J. Mol. Struct., 599, 293 [NASA ADS] [CrossRef] [Google Scholar]
- Gerin, M., Marcelino, N., Biver, N., et al. 2009, A&A, 498, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hily-Blant, P., Bonal, L., Faure, A., & Quirico, E. 2013a, Icarus, 223, 582 [Google Scholar]
- Hily-Blant, P., Des Forêts, G. P., Faure, A., Le Gal, R., & Padovani, M. 2013b, A&A, 557, A65 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Kobayashi, K., Ozeki, H., Saito, S., Tonooka, M., & Yamamoto, S. 1997, J. Chem. Phys., 107, 9289 [NASA ADS] [CrossRef] [Google Scholar]
- Lis, D., Wootten, A., Gerin, M., & Roueff, E. 2010, ApJ, 710, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Margulès, L., Herbst, E., Ahrens, V., et al. 2002, J. Mol. Spectr., 211, 211 [NASA ADS] [CrossRef] [Google Scholar]
- Martin-Drumel, M., Pirali, O., Balcon, D., et al. 2011, Rev. Sci. Inst., 82, 113106 [Google Scholar]
- Martin-Drumel, M., Pirali, O., & Vervloet, M. 2014, J. Phys. Chem. A, 118, 1331 [CrossRef] [Google Scholar]
- Matsushima, F., Odashima, H., Iwasaki, T., Tsunekawa, S., & Takagi, K. 1995, J. Mol. Struct., 352, 371 [NASA ADS] [CrossRef] [Google Scholar]
- Morino, I., & Kawaguchi, K. 1997, J. Mol. Spectr., 182, 428 [NASA ADS] [CrossRef] [Google Scholar]
- Motiyenko, R., Margulès, L., Alekseev, E., Guillemin, J.-C., & Demaison, J. 2010, J. Mol. Spectr., 264, 94 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S., Klein, H., Belov, S. P., et al. 1999, J. Mol. Spectr., 195, 177 [NASA ADS] [CrossRef] [Google Scholar]
- Mumma, M. J., & Charnley, S. B. 2011, ARA&A, 49, 471 [NASA ADS] [CrossRef] [Google Scholar]
- Ozeki, H., Bailleux, S., & Wlodarczak, G. 2011, A&A, 527, A64 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Persson, C. M., De Luca, M., Mookerjea, B., et al. 2012, A&A, 543, A145 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Persson, C. M., Olofsson, A. O. H., Le Gal, R., et al. 2016, A&A, 586, A128 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pickett, H. M. 1991, J. Mol. Spectr., 148, 371 [NASA ADS] [CrossRef] [Google Scholar]
- Roueff, E., Loison, J. C., & Hickson, K. M. 2015, A&A, 576, A99 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Rousselot, P., Pirali, O., Jehin, E., et al. 2014, ApJ, 780, L17 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Shinnaka, Y., Kawakita, H., Kobayashi, H., Nagashima, M., & Boice, D. C. 2014, ApJ, 782, L16 [NASA ADS] [CrossRef] [Google Scholar]
- Steimle, T., Brown, J., & Curl Jr, R. 1980, J. Chem. Phys., 73, 2552 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Spectroscopic parameters of the 15NH2 and 14NH2 radicals in their ground vibronic state.
Measured frequencies of the 21,1 − 20,2 transition, and residuals from the global fit of the Infrared, and submillimeter-wave data for 15NH2.
All Figures
 |
Fig. 1 Portion of the FT-FIR spectrum recorded using 15NH3 as precursor: both 15NH2 and 15NH are simultaneously produced in the discharge. |
| In the text | |
 |
Fig. 2 Example of hyperfine splittings due to 15N and H resolved in the submillimeter-wave spectrum. The lineshape appears as second derivative due to frequency modulation at f = 10 kHz and detection at 2f. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


