| Issue |
A&A
Volume 588, April 2016
|
|
|---|---|---|
| Article Number | L8 | |
| Number of page(s) | 4 | |
| Section | Letters | |
| DOI | https://doi.org/10.1051/0004-6361/201628280 | |
| Published online | 21 March 2016 | |
Detection of protonated formaldehyde in the prestellar core L1689B⋆,⋆⋆
1
Univ. Grenoble Alpes, IPAG,
38000
Grenoble,
France
e-mail:
aurore.bacmann@univ-grenoble-alpes.fr
2
CNRS, IPAG, 38000
Grenoble,
France
Received: 9 February 2016
Accepted: 29 February 2016
Complex organic molecules (COMs) are detected in many regions of the interstellar medium, including prestellar cores. However, their formation mechanisms in cold (~10 K) cores remain to this date poorly understood. The formyl radical HCO is an important candidate precursor for several O-bearing terrestrial COMs in cores, as an abundant building block of many of these molecules. Several chemical routes have been proposed to account for its formation: on grain surfaces, as an incompletely hydrogenated product of H addition to frozen-out CO molecules; and in the gas phase, either as the product of the reaction between H2CO and a radical or as a product of dissociative recombination of protonated formaldehyde H2COH+. The detection and abundance determination of H2COH+, if present, could provide clues as to whether this latter scenario might apply. We searched for protonated formaldehyde H2COH+ in the prestellar core L1689B using the IRAM 30 m telescope. The H2COH+ ion is unambiguously detected, for the first time, in a cold (~10 K) source. The derived abundance agrees with a scenario in which the formation of H2COH+ results from the protonation of formaldehyde. We use this abundance value to constrain the branching ratio of the dissociative recombination of H2COH+ towards the HCO channel to ~10−30%. This value could however be lower if HCO were efficiently formed from neutral-neutral reactions in the gas phase, and we stress the need for laboratory measurements of the rate constants of these reactions at 10 K. Given the experimental difficulties in measuring branching ratios experimentally, observations can place valuable constraints on these values and provide useful input for chemical networks.
Key words: ISM: molecules / line: identification / ISM: abundances
Based on observations carried out with the IRAM 30 m telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain).
Final IRAM data used in the paper (FITS cubes) are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/588/L8
© ESO, 2016
1. Introduction
Despite their low temperatures, prestellar cores harbour a wealth of chemical species. In recent years, complex organic molecules (COMs), which were previously thought to trace mainly warm gas in star-forming regions, have been detected in prestellar sources (e.g. Bacmann et al. 2012; Vastel et al. 2014) where the temperatures are around 10 K. The formation mechanisms of the terrestrial COMs, (i.e. which are stable under Earth-like conditions) currently detected in the cold gas remain poorly understood, and the respective roles of gas-phase reactions or grain surface chemistry are still being debated.
Radicals like the formyl radical HCO or the methoxy radical CH3O have drawn attention as the possible precursors of COMs, either in the gas phase (Vasyunin & Herbst 2013; Balucani et al. 2015) or on grain surfaces (Garrod & Herbst 2006). They are also important intermediates in the grain-surface synthesis of methanol, as products of H-atom additions to CO (e.g. Brown et al. 1988; Pirim & Krim 2011). These radicals are also widely detected in the gas phase of prestellar cores and cold clouds (Bacmann & Faure 2016; Agúndez et al. 2015b; Gerin et al. 2009; Cernicharo et al. 2012).
In a previous survey of these radicals in a sample of prestellar cores, Bacmann & Faure (2016) proposed that the HCO abundances measured in the gas phase could be accounted for by a pure gas-phase scenario, in which HCO results from the dissociative recombination of protonated formaldehyde H2COH+. In dark clouds, H2COH+ is likely the product of the protonation of H2CO, which is an abundant organic species in prestellar cores, with abundances generally around 10-10 −10-9 (Bacmann et al. 2003; Guzmán et al. 2011). Proton donors, such as H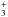 , or HCO+, are the most abundant ions, with abundances close to 10-9 −10-8 (Aikawa et al. 2005; Flower et al. 2005, 2006). It is therefore expected that protonated formaldehyde H2COH+ would easily be formed. Previous searches by Minh et al. (1993) towards Orion A and the two cold clouds L183 and TMC-1 yielded no detection, and Ohishi et al. (1996) detected H2COH+ only towards Sgr B2 and several hot cores and not towards the cold sources of their sample.
, or HCO+, are the most abundant ions, with abundances close to 10-9 −10-8 (Aikawa et al. 2005; Flower et al. 2005, 2006). It is therefore expected that protonated formaldehyde H2COH+ would easily be formed. Previous searches by Minh et al. (1993) towards Orion A and the two cold clouds L183 and TMC-1 yielded no detection, and Ohishi et al. (1996) detected H2COH+ only towards Sgr B2 and several hot cores and not towards the cold sources of their sample.
In this Letter, we present the first detection of protonated formaldehyde in a cold core, and discuss its abundance in terms of the formation of the HCO radical by ion-molecule chemistry, and the possibility to constrain the branching ratio towards HCO of its dissociative recombination with electrons.
2. Observations and data analysis
The frequencies of the rotational transitions of H2COH+ were determined by Chomiak et al. (1994) and Dore et al. (1995) and were retrieved from the CDMS spectroscopy catalogue (Müller et al. 2001, 2005). Line excitation is always an issue in cold (~10 K) gas and only levels with a low energy can be populated. To search for H2COH+, we therefore selected transitions with upper level energies lower than ~20 K, which could be observed in a minimum of frequency setups, and for which good atmospheric transmission did not require very dry weather conditions. The chosen three lines at 2 mm and their spectroscopic parameters are shown in Table 1, to which we added a line at 3 mm from a previous project.
The observations were carried out towards the prestellar core L1689B in January and March 2012 for the 3 mm transition and in March 2015 for the 2 mm transitions with the IRAM 30 m telescope located at Pico Veleta, Spain. The source was selected on the grounds that its molecular lines are usually stronger than in other similar sources (Bacmann & Faure 2016). The integration coordinates were α2000 = 16h34m48.30s and δ2000 = −24°38′04.0″, corresponding to the peak of the millimetre dust continuum emission. We used the receivers E090 and E150 operating at 3 mm and 2 mm, respectively, which were connected to the Fourier transform spectrometer (FTS) at a frequency resolution of ~50 kHz; this corresponded to velocity resolutions of 0.15 km s-1 at 102 GHz, 0.11 km s-1 at 130 GHz, and 0.09 km s-1 at 170 GHz. The weather conditions were good during the 2012 observing runs, and excellent during the March 2015 run with precipitable water vapour of 1 mm on average and system temperatures of ~80 K at 126−132 GHz and ~130 K at 170 GHz. Pointing was checked every 1.5 h on a nearby quasar and found to be within 2−3″ at 2 mm and 3−4″ at 3 mm. Focus was performed on a strong quasar at the beginning of each observing session and on Mercury after sunrise. The data were taken with the frequency switching mode with a frequency throw of 7.5 MHz. The antenna forward efficiency Feff is 0.95 and 0.93 at 3 mm and 2 mm, respectively, and the main beam efficiencies Beff were taken to be 0.79, 0.77, 0.76, and 0.70 at 106.1 GHz, 126.9 GHz, 132.2 GHz, and 168.4 GHz, respectively. From integrations carried out at different offsets (which is discussed elsewhere), we find that the emission fills the main beam but is not very extended, and therefore we used the main beam temperature scale in our analysis, applying 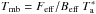 . The main beam size is 24″ at 106.1 GHz, 19″ at 126.9 GHz and at 132.2 GHz, and 15″ at 168.4 GHz.
. The main beam size is 24″ at 106.1 GHz, 19″ at 126.9 GHz and at 132.2 GHz, and 15″ at 168.4 GHz.
Observed H2COH+ transitions.
The data were reduced using the IRAM GILDAS/CLASS1 software: the individual scans were averaged together and a low-order (typically 3) polynomial was fitted to line-free regions of the spectra to subtract a baseline. The resulting spectra were then folded to recover from the effect of the frequency switching procedure.
Line parameters for the observed H2COH+ transitions.
 |
Fig. 1 H2COH+ spectra in L1689B. The vertical dashed lines indicate the positions of the H2COH+ transitions. The feature at ~13 2218 MHz does not correspond to any line from the CDMS or the JPL catalogue. |
3. Column density determination
Protonated formaldehyde is clearly detected, as can be seen from the spectra shown in Fig. 1. The transition at 102 GHz is however not detected, which can be explained by its lower Einstein spontaneous emission coefficient and higher upper level energy. The line parameters, velocity integrated intensities, line widths, and peak line intensities, were determined by fitting a Gaussian to each line. The obtained values are presented in Table 2.
There are no available collisional coefficients for H2COH+, so we perform a simple derivation of the column density assuming local thermodynamic equilibrium (LTE). The method we use has been described in Bacmann & Faure (2016). Briefly, we calculate the integrated intensities of the transitions under the LTE assumption for a range of column density and excitation temperature values, and perform a least-squares fit, defining a χ2 between the calculated integrated intensities and observed integrated intensities for the detected lines.
The best model yields a column density of 6.7 × 1011 cm-2 and a temperature of 4.2 K. Reasonably good fits (i.e. for which χ2 is within 1σ of the minimum χ2 value) can be found for high values of the column density (≳ 1.5 × 1012 cm-2), but these fits are obtained for excitation temperatures that are below 3.5 K. We limit ourselves to excitation temperatures above 3.7 K because lower values become unrealistic. With this additional condition, we find that the beam averaged H2COH+ column density is between 3.3 × 1011 cm-2 and 1.1 × 1012 cm-2. The non-detection of the 102 GHz line does not bring supplementary constraints despite the high sensitivity of the observation because the low excitation temperatures considered (< 5 K) are compatible with a non-detection of this line even for unrealistically high column densities, for which the other lines would be stronger and optically thick. Despite the low number of lines in our analysis, the best fit is only moderately good, as shown in Fig. 2. This probably means that the excitation deviates from LTE.
 |
Fig. 2 Comparison of modelled and observed integrated intensities for the four considered H2COH+ transitions. |
4. Discussion and conclusions
This detection represents the first detection of protonated formaldehyde in a cold prestellar core. Former searches for this ion in similar sources did not yield any detection, most probably because of lack of sensitivity in the observations. The spectral resolution of the H2COH+ non-detections in the cold sources of Ohishi et al. (1996) is ambiguous. If we assume that it was 250 kHz, the rms noise of 20 mK reached by Ohishi et al. (1996) was not low enough to detect the 168.4 GHz line if it was as strong as in L1689B.
Most likely, the dominant reaction route to form H2COH+ in dark clouds is the protonation of formaldehyde 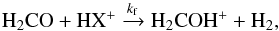 (1)where HX+ stands for a proton donor. Indeed, both H2CO and proton donors (e.g. H
(1)where HX+ stands for a proton donor. Indeed, both H2CO and proton donors (e.g. H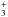 , its deuterated isotopologues, or HCO+) are abundant in prestellar cores, and exothermic ion-molecule processes are generally fast. Bacmann & Faure (2016) estimated the rate for reaction (1)with HX+ = H
, its deuterated isotopologues, or HCO+) are abundant in prestellar cores, and exothermic ion-molecule processes are generally fast. Bacmann & Faure (2016) estimated the rate for reaction (1)with HX+ = H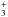 to be kf = 7 × 10-8 cm3 s-1 at 10 K, using the locked dipole approximation. Because of the difference in reduced mass, this rate constant becomes kf = 3.1 × 10-8 cm3 s-1 at 10 K if HX+ = HCO+. The most efficient destruction route for H2COH+ is the dissociative recombination (DR) with electrons
to be kf = 7 × 10-8 cm3 s-1 at 10 K, using the locked dipole approximation. Because of the difference in reduced mass, this rate constant becomes kf = 3.1 × 10-8 cm3 s-1 at 10 K if HX+ = HCO+. The most efficient destruction route for H2COH+ is the dissociative recombination (DR) with electrons 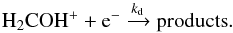 (2)Reaction (2)was studied experimentally by Hamberg et al. (2007, and recently by Osborne et al. 2015 who determined kd = 9.9 × 10-6 cm3s-1 at 10 K. At steady state, if H2COH+ is indeed formed mostly by reaction (1)and destroyed by reaction (2), its abundance is simply given by
(2)Reaction (2)was studied experimentally by Hamberg et al. (2007, and recently by Osborne et al. 2015 who determined kd = 9.9 × 10-6 cm3s-1 at 10 K. At steady state, if H2COH+ is indeed formed mostly by reaction (1)and destroyed by reaction (2), its abundance is simply given by ![\begin{equation} \mathrm{[H_2COH^+]} = \frac{k_{\rm f}}{k_{\rm d}}\mathrm{ \frac{[HX^+]}{[e^-]}[H_2CO]} \label{model} . \end{equation}](/articles/aa/full_html/2016/04/aa28280-16/aa28280-16-eq48.png) (3)Substituting the reaction rates with their values for H
(3)Substituting the reaction rates with their values for H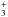 and assuming the proton donor abundance is equal to the electron abundance, we predict an abundance of [H2COH+] ≈ 0.007 [H2CO] as mentioned in Bacmann & Faure (2016), or [H2COH+ ] ≈ 0.003 [H2CO] if HCO+ is the main proton donor. Using an H2CO column density of 1.3 × 1014 cm-2 (Bacmann et al. 2003), we find that the predicted H2COH+ column density Nmod derived from Eq. (3)is 4.1 × 1011−9.1 × 1011 cm-2 (depending on the main proton donor), which is consistent with the observed value we determine in L1689B, Nobs = 6.7 × 1011 cm-2 within the uncertainties. This also nicely confirms the value of the destruction rate of H2COH+ measured by Hamberg et al. (2007) at 10 K.
and assuming the proton donor abundance is equal to the electron abundance, we predict an abundance of [H2COH+] ≈ 0.007 [H2CO] as mentioned in Bacmann & Faure (2016), or [H2COH+ ] ≈ 0.003 [H2CO] if HCO+ is the main proton donor. Using an H2CO column density of 1.3 × 1014 cm-2 (Bacmann et al. 2003), we find that the predicted H2COH+ column density Nmod derived from Eq. (3)is 4.1 × 1011−9.1 × 1011 cm-2 (depending on the main proton donor), which is consistent with the observed value we determine in L1689B, Nobs = 6.7 × 1011 cm-2 within the uncertainties. This also nicely confirms the value of the destruction rate of H2COH+ measured by Hamberg et al. (2007) at 10 K.
Agúndez et al. (2015a) have run a time-dependent chemical model using the UMIST12 network (McElroy et al. 2013) and derive [H2COH+ ] ~ 8 × 10-4−10-3 [H2CO], about six times smaller than our observed value. Though our model better reproduces the observations, it considers only one formation and one destruction route for H2COH+. The H2CO protonation reactions in the UMIST12 network producing H2COH+ have smaller reaction rates than the rate we use (a factor of 2). Our model might also overestimate the amount of proton donors reacting with H2CO, since we assume it equal to the electron abundance. We also neglect other destruction routes than electronic DR for H2COH+, such as proton transfer between H2COH+ and CH3OH or NH3, but these are about 1000 times slower than DR at 10 K and unlikely to be a cause for the discrepancy between both models.
The dissociative recombination of H2COH+ with electrons has several output channels. According to the experiments by Hamberg et al. (2007), the products of the reaction are 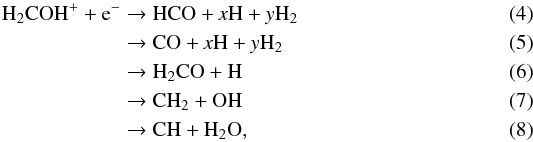 where x and y are integers that account for the different possible combinations of H and H2 in the products. The experimental branching ratios are 6% for CH2, 2% for CH, and 92% for the channels where the C−O bond is conserved (i.e. CO, HCO, and H2CO). This is in contrast to the dissociative recombination of CH3OH for which the C−O bond is preserved in a minority of channels (Geppert et al. 2006). In the case of H2COH+, the experiment by Hamberg et al. (2007) did not distinguish between HCO, CO, and H2CO.
where x and y are integers that account for the different possible combinations of H and H2 in the products. The experimental branching ratios are 6% for CH2, 2% for CH, and 92% for the channels where the C−O bond is conserved (i.e. CO, HCO, and H2CO). This is in contrast to the dissociative recombination of CH3OH for which the C−O bond is preserved in a minority of channels (Geppert et al. 2006). In the case of H2COH+, the experiment by Hamberg et al. (2007) did not distinguish between HCO, CO, and H2CO.
In order to account for the gas-phase abundance of the HCO radical in a sample of prestellar cores, and in particular the constant abundance ratio of ~10 between H2CO and HCO, Bacmann & Faure (2016) suggested that the observed HCO/H2CO abundance ratio can be reproduced if HCO originates from the dissociative recombination of H2COH+, assuming that the branching ratio of the DR is ~10% for the HCO channel (reaction (4)above). Although the detection of H2COH+ in a prestellar core does not provide unambiguous evidence that HCO forms from the protonation of formaldehyde followed by dissociative recombination, it is still in agreement with this scenario, and allows us to directly constrain the branching ratio of the dissociative recombination for the HCO channel. In this framework, and assuming the main destruction route for HCO is with a proton donor like H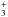 , the branching ratio f for HCO is given by
, the branching ratio f for HCO is given by ![\begin{eqnarray*} k_{\rm dd}\,\mathrm{[HCO][H_3^+]} = f\,k_{\rm d}\,\mathrm{[H_2COH^+][e^-]}, \end{eqnarray*}](/articles/aa/full_html/2016/04/aa28280-16/aa28280-16-eq60.png) where kdd = 5 × 10-8 cm3s-1 is the destruction rate of HCO with H
where kdd = 5 × 10-8 cm3s-1 is the destruction rate of HCO with H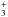 at 10 K (Bacmann & Faure 2016). Assuming as before [H
at 10 K (Bacmann & Faure 2016). Assuming as before [H![\hbox{$_3^+]\approx\,[{\rm e}^-]$}](/articles/aa/full_html/2016/04/aa28280-16/aa28280-16-eq63.png) , the H2COH+ column density determined in this study (
, the H2COH+ column density determined in this study (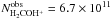 cm-2) and the HCO column density in L1689B given in Bacmann & Faure (2016) (NHCO = 1.3 × 1013 cm-2), we find again f ~ 10%, without invoking the H2CO abundance.
cm-2) and the HCO column density in L1689B given in Bacmann & Faure (2016) (NHCO = 1.3 × 1013 cm-2), we find again f ~ 10%, without invoking the H2CO abundance.
As discussed in Bacmann & Faure (2016), another potential destruction route for HCO is with abundant atoms such as H. In this case, assuming an atomic H abundance of ~10-5 with respect to H2, an electronic abundance of 10-8, and kH = 1.5 × 10-10 cm3 s-1 for the rate constant of the reaction HCO + H (the value at 300 K from Baulch et al. 2005), we find a value for the branching ratio f of 30%. It is however unclear whether the value of kH taken here also applies at 10 K, since no measurements of this rate constant are available at low temperatures.
One major uncertainty in this determination of the branching ratio results from possible alternative formation scenarios for HCO, which could be non-negligible. One such route is the neutral-neutral reaction between H2CO and an abundant radical such as OH or CN. Recent experimental studies have confirmed that such reactions could proceed efficiently via tunnelling at low temperatures. Indeed, Shannon et al. (2013) and Gómez Martín et al. (2014) have shown that the reaction CH3OH + OH gets faster at low temperatures down to ~50 K. A spectacular increase in the reaction constant is also reported by Jiménez et al. (2016) for CH3OCHO + OH; the reaction rate increases by three orders of magnitude between 300 K and 22 K and by an order of magnitude between 60 K and 22 K, reaching as high a value as 1.2 × 10-10 cm3 s-1. In order to be as efficient as the ion-molecule route to form HCO, the constant of the reaction between H2CO and OH would have to be 4 × 10-10 cm3 s-1 at 10 K (Bacmann & Faure 2016). No measurements at 10 K of this reaction, or of reactions similar to this, are available, but they are needed because the behaviour of the reaction constant is not known below 230 K (at which temperature it is 10-11 cm3 s-1) and extrapolation is hazardous. In the case that H2CO + OH would be a major formation channel for HCO, the observed HCO abundance could be accounted for without the need for the DR of H2COH+ to yield a significant amount of HCO. In this respect, the branching ratio we determined above can be considered an upper limit2.
This result can have implications for chemical networks because they assume statistical weights for the three products CO, HCO, and H2CO (1/3, 1/3, 1/3), as in the UMIST12 network or the KIDA3 database (Wakelam et al. 2012), following Prasad & Huntress (1980). As already noted in Hamberg et al. (2007), these branching ratios are still vastly in agreement with their experimental results, which state that CO, HCO and H2CO represent over 90% of the products. However, the branching ratio that is needed here to account for the observed HCO and H2COH+ abundances could be significantly different from the statistical value, as it could be 10%, or lower.
This value should however be taken with some caution because the excitation of H2COH+ is not constrained well in the absence of collisional coefficients. Our estimation of the branching ratio should therefore be repeated once collisional coefficients for H2COH+ become available. Observations of H2COH+ in other prestellar sources are also needed to confirm the current findings.
To conclude, we report the detection of protonated formaldehyde H2COH+ in the prestellar source L1689B. The derived beam-averaged column density is 6.7 × 1011 cm-2, corresponding to an abundance of ~1.9 × 10-11 with respect to H2, if we assume an H2 column density of 3.6 × 1022 cm-2 (Roy et al. 2014). It is likely however that this abundance is overestimated, as the H2 column density in Roy et al. (2014) is averaged over a 36″ beam and is probably higher in the 15−19″ beam of our H2COH+ observations. The H2COH+ column density agrees with the destruction rate of H2COH+ by dissociative recombination as measured by Hamberg et al. (2007), supposing that H2COH+ is mostly formed by protonation of H2CO in prestellar cores and destroyed by electronic dissociative recombination. Using previous observations of the radical HCO in the same source, we constrain the branching ratio of H2COH+ to HCO to be around 10−30% or lower if HCO is significantly formed by gas-phase reactions between H2CO and a radical. The experimental determination of branching ratios is a fundamental piece of data in astrochemistry, but in case these measurements are not available, observations can place valuable constraints on the branching ratios, as demonstrated here.
We also note that the neutral-neutral reaction CH2 + O should yield negligible amounts of HCO in the conditions prevailing in cold cores (see KIDA datasheet on this reaction). Using the rate constant in the KIDA database (2 × 10-12 cm3s-1) and the steady-state abundances of CH2 and O from the model of Le Gal et al. (2014), we find that this reaction is 100 times less efficient than the DR of H2COH+ at forming HCO.
Acknowledgments
This work has benefitted from the support of the CNRS programme “Physique et Chimie du Milieu Interstellaire” (PCMI). E. García-García acknowledges support from an Alpes Grenoble Innovation Recherche (AGIR) grant.
References
- Agúndez, M., Cernicharo, J., de Vicente, P., et al. 2015a, A&A, 579, L10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Cernicharo, J., & Guélin, M. 2015b, A&A, 577, L5 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Aikawa, Y., Herbst, E., Roberts, H., & Caselli, P. 2005, ApJ, 620, 330 [NASA ADS] [CrossRef] [Google Scholar]
- Bacmann, A., & Faure, A. 2016, A&A, 587, A130 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bacmann, A., Lefloch, B., Ceccarelli, C., et al. 2003, ApJ, 585, L55 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bacmann, A., Taquet, V., Faure, A., Kahane, C., & Ceccarelli, C. 2012, A&A, 541, L12 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Balucani, N., Ceccarelli, C., & Taquet, V. 2015, MNRAS, 449, L16 [Google Scholar]
- Baulch, D. L., Bowman, C. T., Cobos, C. J., et al. 2005, J. Phys. Chem. Ref. Data, 34, 757 [NASA ADS] [CrossRef] [Google Scholar]
- Brown, P. D., Charnley, S. B., & Millar, T. J. 1988, MNRAS, 231, 409 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Marcelino, N., Roueff, E., et al. 2012, ApJ, 759, L43 [NASA ADS] [CrossRef] [Google Scholar]
- Chomiak, D., Taleb-Bendiab, A., Civiš, S., & Amano, T. 1994, Can. J. Phys., 72, 1078 [NASA ADS] [CrossRef] [Google Scholar]
- Dore, L., Cazzoli, G., Civiš, S., & Scappini, F. 1995, Chem. Phys. Lett., 244, 145 [NASA ADS] [CrossRef] [Google Scholar]
- Flower, D. R., Pineau Des Forêts, G., & Walmsley, C. M. 2005, A&A, 436, 933 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Flower, D. R., Pineau Des Forêts, G., & Walmsley, C. M. 2006, A&A, 449, 621 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., & Herbst, E. 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Geppert, W. D., Hamberg, M., Thomas, R. D., et al. 2006, Faraday Discuss., 133, 177 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Gerin, M., Goicoechea, J. R., Pety, J., & Hily-Blant, P. 2009, A&A, 494, 977 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Gómez Martín, J., Caravan, R., Blitz, M., Heard, D., & Plane, J. 2014, J. Phys. Chem. A, 118, 2693 [CrossRef] [Google Scholar]
- Guzmán, V., Pety, J., Goicoechea, J. R., Gerin, M., & Roueff, E. 2011, A&A, 534, A49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hamberg, M., Geppert, W. D., Thomas, R. D., et al. 2007, Mol. Phys., 105, 899 [NASA ADS] [CrossRef] [Google Scholar]
- Jiménez, E., Antiñolo, M., Ballesteros, B., Canosa, A., & Albaladejo, J. 2016, Phys. Chem. Chem. Phys., 18, 2183 [CrossRef] [Google Scholar]
- LeGal, R., Hily-Blant, P., Faure, A., et al. 2014, A&A, 562, A83 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- McElroy, D., Walsh, C., Markwick, A. J., et al. 2013, A&A, 550, A36 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Minh, Y. C., Irvine, W. M., & McGonagle, D. 1993, J. Kor. Astron. Soc., 26, 99 [Google Scholar]
- Müller, H. S. P., Thorwirth, S., Roth, D. A., & Winnewisser, G. 2001, A&A, 370, L49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct., 742, 215 [NASA ADS] [CrossRef] [Google Scholar]
- Ohishi, M., Ishikawa, S.-I., Amano, T., et al. 1996, ApJ, 471, L61 [NASA ADS] [CrossRef] [Google Scholar]
- Osborne, Jr., D. S., Lawson, P. A., & Adams, N. G. 2015, Int. J. Mass Spectr., 378, 193 [CrossRef] [Google Scholar]
- Pirim, C., & Krim, L. 2011, Chem. Phys., 380, 67 [NASA ADS] [CrossRef] [Google Scholar]
- Prasad, S. S., & Huntress, Jr., W. T. 1980, ApJS, 43, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Roy, A., André, P., Palmeirim, P., et al. 2014, A&A, 562, A138 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Shannon, R. J., Blitz, M. A., Goddard, A., & Heard, D. E. 2013, Nature Chem., 5, 745 [Google Scholar]
- Vastel, C., Ceccarelli, C., Lefloch, B., & Bachiller, R. 2014, ApJ, 795, L2 [Google Scholar]
- Vasyunin, A. I., & Herbst, E. 2013, ApJ, 769, 34 [Google Scholar]
- Wakelam, V., Herbst, E., Loison, J.-C., et al. 2012, ApJS, 199, 21 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 H2COH+ spectra in L1689B. The vertical dashed lines indicate the positions of the H2COH+ transitions. The feature at ~13 2218 MHz does not correspond to any line from the CDMS or the JPL catalogue. |
| In the text | |
 |
Fig. 2 Comparison of modelled and observed integrated intensities for the four considered H2COH+ transitions. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


