| Issue |
A&A
Volume 555, July 2013
|
|
|---|---|---|
| Article Number | A85 | |
| Number of page(s) | 6 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201321517 | |
| Published online | 04 July 2013 | |
Highly resolved infrared spectra of pure CO2 ice (15–75 K)⋆
1
Raymond and Beverly Sackler Laboratory for Astrophysics, Leiden
Observatory, Leiden University, PO
Box 9513
2300 RA, Leiden, The Netherlands
e-mail: isokoski@strw.leidenuniv.nl
2
Department of Physics and Astronomy, The University of
Toledo, 2801 West Bancroft
Street, Toledo,
OH
43606,
USA
3
Current address: New York Center for Astrobiology, Rensselaer
Polytechnic Institute, 110 Eighth
Street, Troy,
NY
12180,
USA
Received: 20 March 2013
Accepted: 14 May 2013
Context. The ν2 bending mode of pure CO2 ice around 15.2 μm exhibits a fine double-peak structure that offers a sensitive probe to study the physical and chemical properties of solid CO2 in space. Current laboratory spectra do not fully resolve the CO2 ice features.
Aims. To improve the fitting of the observed CO2 features, high-resolution solid-state infrared spectra of pure CO2 ice are recorded in the laboratory for a series of astronomically relevant temperatures and at an unprecedented level of detail.
Methods. The infrared spectra of pure CO2 ice were recorded in the 4000 to 400 cm-1 (2.5–25 μm) region at a resolution of 0.1 cm-1 using Fourier transform infrared spectroscopy.
Results. Accurate band positions and band widths (FWHM) of pure CO2 ice are presented for temperatures of 15, 30, 45, 60, and 75 K. The focus of this spectroscopic work is on the CO2 (ν2) bending mode, but more accurate data are also reported for the 12CO2 and 13CO2 (ν3) stretching mode, and CO2 (ν1+ν3) and (2ν2+ν3) combination bands.
Key words: astrochemistry / line: profiles / methods: laboratory / techniques: spectroscopic / ISM: lines and bands / ISM: molecules
FITS files of the spectra are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/555/A85
© ESO, 2013
1. Introduction
Solid carbon dioxide, CO2, is an important tracer of the chemical and physical history of protostars and their surroundings (Whittet et al. 1998; Ehrenfreund et al. 1998; Gerakines et al. 1999; Boogert et al. 2000; Nummelin et al. 2001; Pontoppidan et al. 2008). It constitutes a significant part of interstellar ice with abundances relative to solid H2O varying from ~15 to 40% in quiescent dark clouds (d’Hendecourt & Jourdain de Muizon 1989; Whittet et al. 1998, 2007, 2009; Bergin et al. 2005; Knez et al. 2005) and circumstellar envelopes of low- and high-mass protostars (Gerakines et al. 1999; Nummelin et al. 2001; Boogert et al. 2004; Pontoppidan et al. 2008; Zasowski et al. 2009; Cook et al. 2011). Also in planetary environments CO2 ice has been detected (see e.g., Cruikshank et al. 2010). The observed abundances of solid CO2 in the interstellar medium (ISM) are a factor of ~100 higher than in the gas phase (van Dishoeck et al. 1996; Boonman et al. 2003) and cannot be reproduced by gas-phase chemical models (Bergin et al. 1995). The formation of CO2 is therefore assumed to proceed through reactions in ices on interstellar dust grains.
CO2 is readily produced in UV photoprocessed CO-H2O laboratory ice, with an efficiency high enough to be driven by the cosmic-ray induced UV field in dense interstellar regions (Watanabe & Kouchi 2002). Comparable CO2 ice abundances are however observed in regions with and without additional UV photons from a nearby protostar, suggesting a formation route which does not depend critically on the available UV field. Cosmic-ray processing of pure CO laboratory ice has been also shown to be a viable mechanism for CO2 production in the ISM (Jamieson et al. 2006), and seems plausible given the large abundances of pure CO ice (Pontoppidan et al. 2003). A number of other reaction schemes have been proposed and/or studied including nonenergetic surface-catalyzed CO oxidation (Tielens & Hagen 1982; Roser et al. 2001; Madzunkov et al. 2006; Raut & Baragiola 2011; Talbi et al. 2006; Slanger et al. 1972; Goumans & Andersson 2010); hydrogenation of CO-O2 binary ice, providing a formation route through the CO-OH intermediate (Ioppolo et al. 2011); and energetic processing (photon and ion irradiation) of ices containing C- and O-bearing molecules as well as carbon grains covered by water ice (Moore et al. 1991; Gerakines et al. 1996; Palumbo et al. 1998; Satorre et al. 2000; Mennella et al. 2004; Loeffler et al. 2005; Gomis & Strazzulla 2005; Mennella et al. 2006; Ioppolo et al. 2009; Fulvio et al. 2012). Recent observational and experimental work suggests that interstellar ices are unlikely to contain significant amounts of CO intimately mixed in H2O (Pontoppidan et al. 2003; Cuppen et al. 2011). This is inconsistent with the CO2 formation mechanisms relying on or resulting from CO in H2O-rich ices. Cuppen et al. (2011) further demonstrated that the astronomically observed CO ice feature is better reproduced by CO mixed in CH3OH ice. Indeed, CH3OH is readily produced through CO hydrogenation (Watanabe et al. 2004, 2006; Fuchs et al. 2009) and CO2 produced in water-poor CO ice should therefore be intimately mixed also with CH3OH.
The molecular environment in which CO2 is observed provides, therefore, valuable information on its formation mechanism; CO2 located in ice dominated by H2O, CO and/or CH3OH is a direct hint for a possible chemical connection. The CO2 (ν2) bending mode at 15.2 μm (660 cm-1) is particularly sensitive to its molecular environment and has been observed toward a number of sources ranging from luminous young stars (Gerakines et al. 1999; Pontoppidan et al. 2008) to cold, quiescent molecular clouds (Knez et al. 2005; Bergin et al. 2005; Whittet et al. 2007). The appearance of absorption features not only depends on ice composition, but also on ice temperature. To characterize different ice environments, the observed CO2 (ν2) feature can be decomposed phenomenologically into a finite set of known laboratory ice compositions (Pontoppidan et al. 2008) including pure CO2 ice. While the profile of the CO2 feature in each component is fixed, the relative contribution varies from source to source, thus characterizing and quantifying the ices along the line of sights.
A large number of laboratory spectra of solid CO2 in different molecular environments and for different temperatures are available (Sandford & Allamandola 1990; Ehrenfreund et al. 1997, 1999; van Broekhuizen et al. 2006; White et al. 2009, 2012). For most sources, the analysis of the CO2 (ν2) feature implies the presence of both hydrogen-rich (H2O:CO2) and hydrogen-poor (CO:CO2) CO2 ice, as well as CH3OH containing CO2 ice (Ehrenfreund et al. 1998), consistent with several of the proposed formation schemes. Most sources also exhibit some contribution from pure CO2 ice. The occurrence of pure CO2 ice is considered to be a result of thermal processing, which causes segregation and/or distillation of mixed components with different volatility (Ehrenfreund et al. 1998; Öberg et al. 2009; Fayolle et al. 2011). Distillation of CO-rich ice happens at 20–30 K (van Broekhuizen et al. 2006; Pontoppidan et al. 2008). In the laboratory, segregation from H2O-rich ice requires strong (>100 K) heating (Gerakines et al. 1999), but is lowered to 30 K in the ISM due to longer time scales (Öberg et al. 2009).
Low-temperature (<20 K) H2O- and CO-rich ices are characterized by a broad single-peaked CO2 (ν2) feature, whereas pure CO2 produces a double-peaked substructure (Ehrenfreund et al. 1997; van Broekhuizen et al. 2006). In a pure CO2 lattice the axial symmetry of the linear molecule is broken, giving rise to the so-called Davydov splitting (Davydov 1962; Tso & Lee 1985). The splitting makes the CO2 bending mode a sensitive probe for the changes in its environment. Prior to this work, the available laboratory spectra of pure CO2 ice (Sandford & Allamandola 1990; Hudgins et al. 1993; Ehrenfreund et al. 1997; Baratta & Palumbo 1998) were recorded at resolutions of 1–2 cm-1, too low to fully resolve the CO2 (ν2) bending mode. van Broekhuizen et al. (2006) recorded CO2 ice spectra at 15–90 K with 0.5 cm-1 resolution but the low signal-to-noise ratio (S/N) prohibited an accurate comparison with astronomical data. Particularly for sources with a prominent double-peak structure, such as HOPS-68 (Poteet et al. 2011, 2013), the spectral quality of the used pure CO2 component is important. Using a pure CO2 component with a properly resolved Davydov splitting can help to avoid overestimation and misinterpretation of the underlying broader features. Ehrenfreund et al. (1997) did not make available the entire temperature series for pure CO2 ice. Here we present a complete, high-resolution temperature series. In addition to the 15.2 μm bending mode, the resolution of the previous data is not sufficient when studying the narrow (ν3) 13CO2 or (ν1 + ν3) CO2 combination ice bands. These bands are studied here as well and will be accessible with the James Webb Space Telescope.
2. Experimental procedure
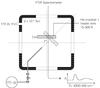 |
Fig. 1 Schematic drawing of the experimental setup for preparation and spectroscopic characterization of the cryogenic CO2 samples. |
The experiments are performed in a high-vacuum (HV) setup first described by Gerakines et al. (1995) (Fig. 1). A stainless steel chamber is evacuated by a turbomolecular pump (170 l s-1; Pfeiffer TPH 170) and a rotary vane pump (6 m3 h-1; Edwards E2M8) separated by an oil mist filter, allowing a base pressure of 2.5 × 10-7 mbar at room temperature. The chamber houses a CsI (Caesium Iodide) sample substrate that is cooled down to 15 K by a closed cycle helium cryostat (ADP DE-202). The substrate temperature is controlled between 15 and 300 K, with a precision of 0.1 K, by a resistive heater element and a silicon diode sensor using an external temperature control unit (LakeShore 330). CO2 (Praxair, 99.998% purity) is introduced into the system from a gas bulb at 10 mbar filled in a separate vacuum manifold (base pressure ~10-5 mbar). CO2 ices are grown onto the substrate at 15 K via effusive dosing of the gaseous sample through a stainless steel capillary along the surface normal. The approximate growth rate is determined by setting the exposure to ~1016 molecules cm-2 s-1. Assuming a monolayer surface coverage of 1015 molecules cm-2 and a sticking probability of 1, this results in a growth rate of 10 L s-11. Ices are deposited for 5 min resulting in approxmately ~3000 ML. The exact thickness of the samples is derived from the IR band strengths (Sect. 4).
The ices are heated from 15 K to 30, 45, 60, 75 and 90 K at a rate of 2 K min-1 and allowed to relax for 5 min before recording the absorption spectra. A Fourier transform infrared (FTIR) spectrometer (Varian 670-IR) is used to record the ice spectra in transmission mode from 4000 to 400 cm-1 (2.5–25 μm) with a spectral resolution of 0.1 cm-1, averaging a total of 256 scans to increase the S/N. Background spectra are acquired at 15 K prior to deposition and subtracted from the recorded ice spectra. The spectra recorded at different temperatures correspond to different ice samples prepared under identical conditions. This procedure is used to minimize sample contamination during the relatively long acquisition time (~2 h).
3. Results
 |
Fig. 2 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the 12CO2 (ν2) bending mode. The displayed spectra are obtained by smoothing through superposition of Gaussians. |
 |
Fig. 3 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the 12CO2 and 13CO2 (ν3) stretching modes. The zoom-in of the latter is shown in Fig. 4. The displayed spectra are baseline corrected. Gaseous CO2 absorption features have been subtracted. |
 |
Fig. 4 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the 13CO2 (ν3) stretching mode. The displayed spectra are baseline corrected. |
 |
Fig. 5 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the (ν1 + ν3) and (2ν2 + ν3) combination bands. The displayed spectra are baseline corrected. |
Figures 2–5 show the high-resolution (0.1 cm-1) FTIR spectra of CO2 ice at 15, 30, 45, 60 and 75 K. The separate graphs are offset for clarity. Figure 2 shows the spectral region around the ν2 bending mode (680–645 cm-1). Figures 3 and 4 show the CO2 (ν3) asymmetric stretching fundamental, for 12C and 13C isotopologues (in natural abundance), respectively. Figure 5 shows the (ν1 + ν3) and (2ν2 + ν3) CO2 combination bands.
The CO2 bending fundamental (ν2) has a double-peaked substructure at ~660/655 cm-1. The high-frequency component is highly asymmetric with a long blue (high wavenumber) wing, while the low-frequency component is relatively symmetric. The 12CO2 asymmetric stretching fundamental (ν3) is located at ~2345 cm-1 (4.26 μm), and is redshifted from the gas-phase value at 2348 cm-1 due to interactions with the surrounding matrix environment. The profile is asymmetric with a prominent blue shoulder and a weaker red (low wavenumber) shoulder. The asymmetric stretching fundamental (ν3) of the 13CO2 isotopologue is found in its natural abundance at ~2283 cm-1 (4.38 μm), similarly redshifted from its gas-phase value. The 13CO2 (ν3) feature also exhibits shoulders on both sides (Fig. 4). The CO2 (ν1) symmetric stretching fundamental at ~1385 cm-1 (7.22 μm) is IR inactive (Falk & Seto 1986). The CO2 (ν1 + ν3) combination band and (2ν2 + ν3) combination/overtone bands appear at ~3708 cm-1 (2.70 μm) and 3599 cm-1 (2.78 μm), respectively. The combination modes have narrow, relatively symmetric profiles. These bands are close to the broad H2O stretching mode around 3300 cm-1 (3.03 μm) which typically dominates the observational ice spectra. However, unlike the strong ν2 and ν3 modes, the CO2 combination and the 13CO2 (ν3) modes are weak and unaffected by grain shape effects. Thus, their band shape profiles depend only on the chemical composition of interstellar CO2 ice (e.g., Keane et al. 2001).
3.1. Corrections
In the laboratory, the CO2 ice feature around 2350 cm-1 is easily contaminated with absorptions of gaseous CO2 along the IR beam path. Variable concentrations relative to the background spectrum may cause under- or over-subtraction of interfering rotational lines. Same is true for the CO2 bending mode around 660 cm-1 where an absorption band is seen around 668 cm-1. The spectra are corrected using a gaseous CO2 spectrum extracted from the background spectrum (Fig. 6). The bending mode region also suffers from spectral artifacts due to being located in the edge of the spectrometer spectral range. The (ν2) bands presented here are therefore smoothed by superposition of several Gaussians. All spectra have been baseline corrected, and are available in reduced as well as in raw format in the Leiden ice database2.
 |
Fig. 6 Spectral corrections performed on the CO2 (ν3) mode. Rotational fine-structure of gaseous CO2 (green trace), overlapping with the CO2 (ν3) ice band, is subtracted from the raw spectrum (black trace). The corrected spectrum is shown in red. |
4. Discussion
High-resolution (0.1 cm-1) band positions
(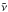 ) and linewidths (FWHM) in pure
CO2 ice.
) and linewidths (FWHM) in pure
CO2 ice.
 |
Fig. 7 Relative peak position (in cm-1), width, peak intensity and integrated area of the CO2 ice features with respect to the 15 K values as a function of temperature. |
Table 1 lists the band positions and bandwidths of pure CO2 ice features for the five different temperatures in the 0.1 cm-1 resolution spectra. The values are obtained by the integration procedure in the Origin 7.5 software package. Figure 7 visualizes the derived values, including relative peak intensity and integrated area, as a function of temperature, together with other values available from the literature. The values are normalized to those in the 0.1 cm-1 spectra at 15 K. The influence of thermal annealing on the peak position depends on the influence of the trapping site on the specific vibrational mode. In general, all CO2 band widths decrease at elevated temperatures. The narrowing is gradual and is observed already between 15 and 30 K. During the thermal annealing process, molecules rearrange themselves by finding energetically more favorable orientations. As the range of environments is reduced, the band profiles become more narrow. Simultaneously, the peak intensities increase. The integrated area of all features peaks around 40 K, after which it declines until the ice desorbs between 80 and 90 K.
Comparison of the peak positions in the high-resolution spectra with those from lower resolution studies (Sandford & Allamandola 1990; Ehrenfreund et al. 1997; van Broekhuizen et al. 2006) generally shows agreement within the limitations due to resolution, assuming that at 1 cm-1 resolution, the error in position is 0.5 cm-1. At 15 K, the CO2 (ν2) bending mode peaks at 659.7 and 654.5 cm-1. The low-wavenumber component shifts by 0.12 cm-1 between 45 and 75 K. The observed shift is below the resolution of previous studies. The high-wavenumber component does not shift with temperature. The CO2 (ν3) stretching mode, peaking at 2344.4 cm-1 in the 15 K spectrum, red-shifts by 0.7 cm-1 for temperatures between 30 and 75 K. The 13CO2 stretching mode undergoes a smaller (0.3 cm-1) red-shift in a similar temperature range. The peak positions derived for the combination modes (ν1 + ν3) and (2ν2 + ν3) at 15 K are 3708.0 and 3599.5 cm-1. The position of the combination modes shifts <0.25 cm-1 between 15 and 75 K. The shift of the (ν1 + ν3) band occurs above 30 K, toward lower wavenumbers.
 |
Fig. 8 Dip-to-peak ratio at different temperatures derived from the 0.1 cm-1 (filled squares) and 1.0 cm-1 (open triangles) resolution spectra of the CO2 bending mode. |
The main objective of this high-resolution study is to fully resolve the Davydov splitting in the CO2 (ν2) bending mode. Figure 8 shows the dip-to-peak ratio, defined by Zasowski et al. (2009) as the local minimum to local maximum ratio of the blue peak, for 0.1 and 1.0 cm-1 resolution spectra as a function of temperature. For the high-resolution CO2 ice feature, the dip-to-peak ratio decreases from 0.3 to <0.10 between 15 and 75 K. The ratio derived from the lower resolution spectra (Ehrenfreund et al. 1997) is ~20 % larger for all studied temperatures. A smaller dip-to-peak ratio confirms that the CO2 bending mode and in particular the Davydov split is better resolved in the 0.1 cm-1 spectra.
The double peaked structure of the CO2 bending mode is a diagnostic for the observed 15.2 μm CO2 ice band. The interpretation of the CO2 ice composition from observational spectra relies on laboratory spectra of CO2 for different ice environments (Gerakines et al. 1999; Keane et al. 2001; Pontoppidan et al. 2008). The quality of the laboratory spectra therefore directly influences the derived ice composition from observations. Particularly for sources with larger contributions from pure CO2 ice, a properly resolved Davydov splitting is a prerequisite for an accurate interpretation. Thus, the incorporation of 0.1 cm-1 resolution laboratory spectra (Table 1) into the fitting procedure is expected to improve the interpretation of the astronomical observations.
5. Summary
In this work we provide highly resolved (0.1 cm-1) IR spectra of pure CO2 ice at a spectral range of 4000–400 cm-1, at temperatures 15, 30, 45, 60, 75 K. The spectral range covers the fundamental bending mode (ν2), antisymmetric 12CO2 and 13CO2 stretching modes (ν3) and the CO2 combination mode (ν1 + ν3) and combination/overtone mode (2ν2 + ν3). The improved spectral parameters are needed for a more accurate interpretation of astronomical ice data. Moreover, the high spectral resolution allows an accurate quantification of thermally induced changes in CO2 band profiles that are below the resolution of previous work. This is particularly true for shifts in line position below 0.5 cm-1. Also, the Davydov splitting in the ν2 band is characterized more accurately and allows a better interpretation of astronomical spectra along lines of sight with thermally processed ices, where segregation and/or distillation causes CO2 to be present in pure form as well. All data are available in the Leiden ice database3.
Acknowledgments
This work has been financially supported through the NOVA instrumentation program and benefits from a NWO-VICI grant. We thank Elisabetta Palumbo for very helpful discussions.
References
- Baratta, G. A., & Palumbo, M. E. 1998, J. Opt. Soc. Am. A, 15, 3076 [Google Scholar]
- Bergin, E. A., Langer, W. D., & Goldsmith, P. F. 1995, ApJ, 441, 222 [NASA ADS] [CrossRef] [Google Scholar]
- Bergin, E. A., Melnick, G. J., Gerakines, P. A., Neufeld, D. A., & Whittet, D. C. B. 2005, ApJ, 627, L33 [NASA ADS] [CrossRef] [Google Scholar]
- Boogert, A. C. A., Ehrenfreund, P., Gerakines, P. A., et al. 2000, A&A, 353, 349 [NASA ADS] [Google Scholar]
- Boogert, A. C. A., Pontoppidan, K. M., Lahuis, F., et al. 2004, ApJS, 154, 359 [NASA ADS] [CrossRef] [Google Scholar]
- Boonman, A. M. S., van Dishoeck, E. F., Lahuis, F., & Doty, S. D. 2003, A&A, 399, 1063 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cook, A. M., Whittet, D. C. B., Shenoy, S. S., et al. 2011, ApJ, 730, 124 [NASA ADS] [CrossRef] [Google Scholar]
- Cruikshank, D. P., Meyer, A. W., Brown, R. H., et al. 2010, Icarus, 206, 561 [NASA ADS] [CrossRef] [Google Scholar]
- Cuppen, H. M., Penteado, E. M., Isokoski, K., van der Marel, N., & Linnartz, H. 2011, MNRAS, 417, 2809 [NASA ADS] [CrossRef] [Google Scholar]
- Davydov, A. 1962, Nucl. Phys. A, 37, 106 [CrossRef] [Google Scholar]
- d’Hendecourt, L. B., & Jourdain de Muizon, M. 1989, A&A, 223, L5 [NASA ADS] [Google Scholar]
- Ehrenfreund, P., Boogert, A. C. A., Gerakines, P. A., Tielens, A. G. G. M., & van Dishoeck, E. F. 1997, A&A, 328, 649 [NASA ADS] [Google Scholar]
- Ehrenfreund, P., Dartois, E., Demyk, K., & D’Hendecourt, L. 1998, A&A, 339, L17 [NASA ADS] [Google Scholar]
- Ehrenfreund, P., Kerkhof, O., Schutte, W. A., et al. 1999, A&A, 350, 240 [NASA ADS] [Google Scholar]
- Falk, M., & Seto, P. F. 1986, Can. J. Spectros., 31, 134 [Google Scholar]
- Fayolle, E. C., Öberg, K. I., Cuppen, H. M., Visser, R., & Linnartz, H. 2011, A&A, 529, A74 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fuchs, G. W., Cuppen, H. M., Ioppolo, S., et al. 2009, A&A, 505, 629 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Fulvio, D., Raut, U., & Baragiola, R. A. 2012, ApJ, 752, L33 [NASA ADS] [CrossRef] [Google Scholar]
- Gerakines, P. A., Schutte, W. A., Greenberg, J. M., & van Dishoeck, E. F. 1995, A&A, 296, 810 [NASA ADS] [Google Scholar]
- Gerakines, P. A., Schutte, W. A., & Ehrenfreund, P. 1996, A&A, 312, 289 [NASA ADS] [Google Scholar]
- Gerakines, P. A., Whittet, D. C. B., Ehrenfreund, P., et al. 1999, ApJ, 522, 357 [NASA ADS] [CrossRef] [Google Scholar]
- Gomis, O., & Strazzulla, G. 2005, Icarus, 177, 570 [NASA ADS] [CrossRef] [Google Scholar]
- Goumans, T. P. M., & Andersson, S. 2010, MNRAS, 406, 2213 [NASA ADS] [CrossRef] [Google Scholar]
- Hudgins, D. M., Sandford, S. A., Allamandola, L. J., & Tielens, A. G. G. M. 1993, ApJS, 86, 713 [NASA ADS] [CrossRef] [Google Scholar]
- Ioppolo, S., Palumbo, M. E., Baratta, G. A., & Mennella, V. 2009, A&A, 493, 1017 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ioppolo, S., van Boheemen, Y., Cuppen, H. M., van Dishoeck, E. F., & Linnartz, H. 2011, MNRAS, 413, 2281 [NASA ADS] [CrossRef] [Google Scholar]
- Jamieson, C. S., Mebel, A. M., & Kaiser, R. I. 2006, ApJS, 163, 184 [NASA ADS] [CrossRef] [Google Scholar]
- Keane, J. V., Boogert, A. C. A., Tielens, A. G. G. M., Ehrenfreund, P., & Schutte, W. A. 2001, A&A, 375, L43 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Knez, C., Boogert, A. C. A., Pontoppidan, K. M., et al. 2005, ApJ, 635, L145 [NASA ADS] [CrossRef] [Google Scholar]
- Loeffler, M. J., Baratta, G. A., Palumbo, M. E., Strazzulla, G., & Baragiola, R. A. 2005, A&A, 435, 587 [NASA ADS] [CrossRef] [EDP Sciences] [MathSciNet] [Google Scholar]
- Madzunkov, S., Shortt, B. J., Macaskill, J. A., Darrach, M. R., & Chutjian, A. 2006, Phys. Rev. A, 73, 020901 [NASA ADS] [CrossRef] [Google Scholar]
- Mennella, V., Palumbo, M. E., & Baratta, G. A. 2004, ApJ, 615, 1073 [NASA ADS] [CrossRef] [Google Scholar]
- Mennella, V., Baratta, G. A., Palumbo, M. E., & Bergin, E. A. 2006, ApJ, 643, 923 [NASA ADS] [CrossRef] [Google Scholar]
- Moore, M. H., Khanna, R., & Donn, B. 1991, J. Geophys. Res., 96, 17541 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Nummelin, A., Whittet, D. C. B., Gibb, E. L., Gerakines, P. A., & Chiar, J. E. 2001, ApJ, 558, 185 [NASA ADS] [CrossRef] [Google Scholar]
- Öberg, K. I., Fayolle, E. C., Cuppen, H. M., van Dishoeck, E. F., & Linnartz, H. 2009, A&A, 505, 183 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Palumbo, M. E., Baratta, G. A., Brucato, J. R., et al. 1998, A&A, 334, 247 [NASA ADS] [Google Scholar]
- Pontoppidan, K. M., Fraser, H. J., Dartois, E., et al. 2003, A&A, 408, 981 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pontoppidan, K. M., Boogert, A. C. A., Fraser, H. J., et al. 2008, ApJ, 678, 1005 [NASA ADS] [CrossRef] [Google Scholar]
- Poteet, C. A., Megeath, S. T., Watson, D. M., et al. 2011, ApJ, 733, L32 [NASA ADS] [CrossRef] [Google Scholar]
- Poteet, C. A., Pontoppidan, K. M., Megeath, S. T., et al. 2013, ApJ, 766, 117 [NASA ADS] [CrossRef] [Google Scholar]
- Raut, U., & Baragiola, R. A. 2011, ApJ, 737, L14 [NASA ADS] [CrossRef] [Google Scholar]
- Roser, J. E., Vidali, G., Manicò, G., & Pirronello, V. 2001, ApJ, 555, L61 [NASA ADS] [CrossRef] [Google Scholar]
- Sandford, S. A., & Allamandola, L. J. 1990, ApJ, 355, 357 [NASA ADS] [CrossRef] [Google Scholar]
- Satorre, M. A., Palumbo, M. E., & Strazzulla, G. 2000, Ap&SS, 274, 643 [Google Scholar]
- Slanger, T. G., Wood, B. J., & Black, G. 1972, J. Chem. Phys., 57, 233 [NASA ADS] [CrossRef] [Google Scholar]
- Talbi, D., Chandler, G. S., & Rohl, A. L. 2006, Chem. Phys., 320, 214 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. G. G. M., & Hagen, W. 1982, A&A, 114, 245 [NASA ADS] [Google Scholar]
- Tso, T., & Lee, E. 1985, J. Phys. Chem., 89, 1612 [CrossRef] [Google Scholar]
- van Broekhuizen, F. A., Groot, I. M. N., Fraser, H. J., van Dishoeck, E. F., & Schlemmer, S. 2006, A&A, 451, 723 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- van Dishoeck, E. F., Helmich, F. P., de Graauw, T., et al. 1996, A&A, 315, L349 [NASA ADS] [Google Scholar]
- Watanabe, N., & Kouchi, A. 2002, ApJ, 567, 651 [NASA ADS] [CrossRef] [Google Scholar]
- Watanabe, N., Nagaoka, A., Shiraki, T., & Kouchi, A. 2004, ApJ, 616, 638 [NASA ADS] [CrossRef] [Google Scholar]
- Watanabe, N., Nagaoka, A., Hidaka, H., et al. 2006, Planet. Space Sci., 54, 1107 [NASA ADS] [CrossRef] [Google Scholar]
- White, D. W., Gerakines, P. A., Cook, A. M., & Whittet, D. C. B. 2009, ApJS, 180, 182 [NASA ADS] [CrossRef] [Google Scholar]
- White, D. W., Mastrapa, R. M. E., & Sandford, S. A. 2012, Icarus, 221, 1032 [NASA ADS] [CrossRef] [Google Scholar]
- Whittet, D. C. B., Gerakines, P. A., Tielens, A. G. G. M., et al. 1998, ApJ, 498, L159 [NASA ADS] [CrossRef] [Google Scholar]
- Whittet, D. C. B., Shenoy, S. S., Bergin, E. A., et al. 2007, ApJ, 655, 332 [NASA ADS] [CrossRef] [Google Scholar]
- Whittet, D. C. B., Cook, A. M., Chiar, J. E., et al. 2009, ApJ, 695, 94 [NASA ADS] [CrossRef] [Google Scholar]
- Zasowski, G., Kemper, F., Watson, D. M., et al. 2009, ApJ, 694, 459 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
High-resolution (0.1 cm-1) band positions
(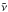 ) and linewidths (FWHM) in pure
CO2 ice.
) and linewidths (FWHM) in pure
CO2 ice.
All Figures
 |
Fig. 1 Schematic drawing of the experimental setup for preparation and spectroscopic characterization of the cryogenic CO2 samples. |
| In the text | |
 |
Fig. 2 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the 12CO2 (ν2) bending mode. The displayed spectra are obtained by smoothing through superposition of Gaussians. |
| In the text | |
 |
Fig. 3 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the 12CO2 and 13CO2 (ν3) stretching modes. The zoom-in of the latter is shown in Fig. 4. The displayed spectra are baseline corrected. Gaseous CO2 absorption features have been subtracted. |
| In the text | |
 |
Fig. 4 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the 13CO2 (ν3) stretching mode. The displayed spectra are baseline corrected. |
| In the text | |
 |
Fig. 5 High-resolution (0.1 cm-1) solid-state IR spectra of pure CO2 ice at 15–75 K showing the (ν1 + ν3) and (2ν2 + ν3) combination bands. The displayed spectra are baseline corrected. |
| In the text | |
 |
Fig. 6 Spectral corrections performed on the CO2 (ν3) mode. Rotational fine-structure of gaseous CO2 (green trace), overlapping with the CO2 (ν3) ice band, is subtracted from the raw spectrum (black trace). The corrected spectrum is shown in red. |
| In the text | |
 |
Fig. 7 Relative peak position (in cm-1), width, peak intensity and integrated area of the CO2 ice features with respect to the 15 K values as a function of temperature. |
| In the text | |
 |
Fig. 8 Dip-to-peak ratio at different temperatures derived from the 0.1 cm-1 (filled squares) and 1.0 cm-1 (open triangles) resolution spectra of the CO2 bending mode. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


