Table B.1.
List of reactions included in our chemical model.
| Tag | Reaction | Rate coefficient (cm3 s−1, cm6 s−1, s−1) | Ref. | |
|---|---|---|---|---|
| H01H_01 | H+ + e− → H + γ | krec = CH ⋅ αH; | 1 | |
| αH = F ⋅ a1 ⋅ 10−19ta2/(1.0 + a3ta4), | ||||
| F = 1.14, a1 = 4.309, a2 = −0.6166, | ||||
| a3 = 0.6703, a4 = 0.5300, t = T/104 | ||||
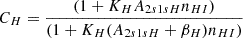 |
||||
| βH = αH ⋅ (2πmekBTR/h2)3/2exp(−E2s/kTR) | ||||
| KH ≡ λLyα3/(8πH(z)), λLyα = 121.5682 nm | ||||
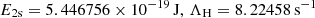 |
||||
| H02H_02 | H + γ → H+ + e− | kion = CH ⋅ βH ⋅ exp(−E2s1s/kTM); | 1 | |
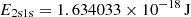 |
||||
| H03H_03 | H + e− → H− + γ | dex[−17.845 + 0.762logT | T ≤ 6000 K | 2 |
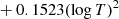 |
||||
![$ \phantom{ \mathrm{dex}[}- 0.03274 (\log{T})^{3}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq39.gif) |
||||
| dex[−16.4199 + 0.1998(logT)2 | T > 6000 K | |||
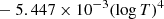 |
||||
![$ \phantom{ \mathrm{dex}[} + 4.0415 \times 10^{-5} (\log{T})^{6}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq41.gif) |
||||
| H04H_04 | H− + γ → H + e− | 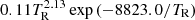 |
3 | |
| H05H_05 | H− + H → H2 + e− | 1.5 × 10−9 | T < 300.0 | 3 |
| 4.0 × 10−9T−0.17 | T ≥ 300.0 | |||
| H06H_06 | H− + H+ → H + H | 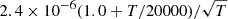 |
4 | |
| H07H_07 | H + H+ → H2+ + γ | dex[−19.38 − 1.523logT | 5 | |
![$ \phantom{\mathrm{dex}[} + 1.118 (\log{T})^{2} - 0.1269 (\log{T})^{3}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq44.gif) |
||||
| H08H_08 | H2+ + γ → H + H+ | 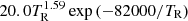 |
v = 0 | 3 |
| 1.63 × 107exp(−32400.0/TR) | LTE | |||
| H09H_09 | H + H2+ → H2 + H+ | 6.4 × 10−10 | 6 | |
| H10H_10 | H2 + H+ → H2+ + H | [ − 3.3232183 × 10−7 | 7 | |
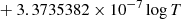 |
||||
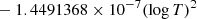 |
||||
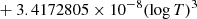 |
||||
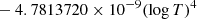 |
||||
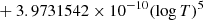 |
||||
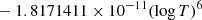 |
||||
![$ \phantom{[} + 3.5311932 \times 10^{-13} (\log{T})^7 ] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq52.gif) |
||||
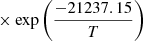 |
||||
| H11H_11 | H+ + H− → H2+ + e− | 6.9 × 10−9T−0.35 | T ≤ 8000 K | 8 |
| 9.6 × 10−7T−0.90 | T > 8000 K | |||
| H12H_12 | H2+ + e− → H + H | 4.2278 × 10−14 − 2.3088 × 10−17T + 7.3428 × 10−21T2 | ||
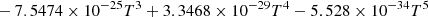 |
9 | |||
| H13H_13 | H2+ + H2 → H3+ + H | 2.0 × 10−9 | 10 | |
| H14H_14 | H2 + γ → H2+ + e− | 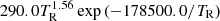 |
3 | |
| H15H_15 | H2 + γ → H + H | 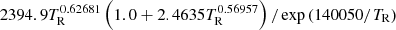 |
TR < 15000 | 11 |
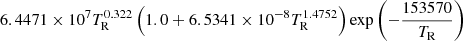 |
TR ≥ 15000 | |||
| H16H_16 | H3+ + H → H2+ + H2 | 7.7 × 10−9exp(−17560.0/TM) | 3 | |
| H17H_17 | H3+ + e− → H2 + H | 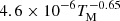 |
3 | |
| H18H_18 | H2 + H+ → H3+ + γ | 10−16 | 3 | |
| H19H_19 | H2 + H → H + H + H | ![$ 6.67 \times 10^{-12} T^{0.5} \exp \left[-(1+ \frac{63593}{T}) \right] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq59.gif) |
v=0 | 12 |
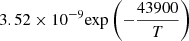 |
LTE | 13 | ||
| H20H_20 | H + e− → H+ + e− + e− | exp[−3.271396786 × 101 | 14 | |
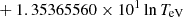 |
||||
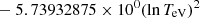 |
||||
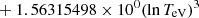 |
||||
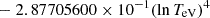 |
||||
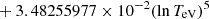 |
||||
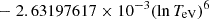 |
||||
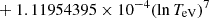 |
||||
![$ \phantom{\exp[} - 2.03914985 \times 10^{-6} (\ln T_{\mathrm{eV}})^{8}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq68.gif) |
||||
| H21H_21 | H3+ + γ → H2 + H+ | 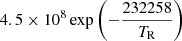 |
15 | |
| H22H_22 | H3+ + e− → 3 H | the same as for blueH_17 | ||
| H23H_23 | H + e− → H+ + 2e− | 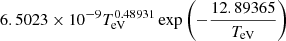 |
16 | |
| H24H_24 | H+ + H + H → H+ + H2 | 1.145 × 10−29T−1.12 | 17 | |
| H25H_25 | H + H + H → H2 + H | 1.14 × 10−31T−0.38 | T < 300 K | 18 |
| 3.9 × 10−30T−1 | T ≥ 300 K | |||
| H26H_26 | H2+ + γ → 2H+ + e− | 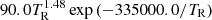 |
3 | |
| H27H_27 | H2 + e− → H− + H | 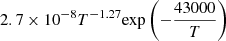 |
19 | |
| H28H_28 | H2 + e− → H + H + e− | 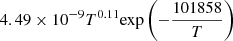 |
v = 0 | 20 |
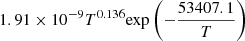 |
LTE | 20 | ||
| H29H_29 | H2 + H2 → H2 + H + H | 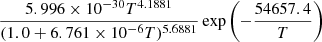 |
v = 0 | 21 |
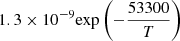 |
LTE | 22 | ||
| H30H_30 | H2 + He → H + H + He | ![$ \mathrm{dex} \left[ -27.029 + 3.801 \log{T} - \frac{29487}{T} \right] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq77.gif) |
v = 0 | 23 |
![$ \mathrm{dex} \left[ -2.729 -1.75 \log{T} - \frac{23474}{T} \right] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq78.gif) |
LTE | 23 | ||
| H31H_31 | H− + e− → H + e− + e− | exp[−1.801849334 × 101 | 14 | |
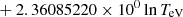 |
||||
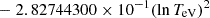 |
||||
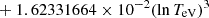 |
||||
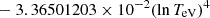 |
||||
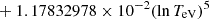 |
||||
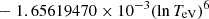 |
||||
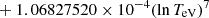 |
||||
![$ \phantom{\exp [} - 2.63128581\times 10^{-6} (\ln T_{\mathrm{eV}})^{8} ] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq86.gif) |
||||
| H32H_32 | H− + H → H + H + e− | 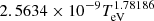 |
TeV ≤ 0.1 eV | 14 |
| exp[−2.0372609 × 101 | TeV > 0.1 eV | |||
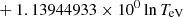 |
||||
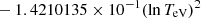 |
||||
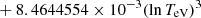 |
||||
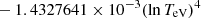 |
||||
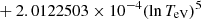 |
||||
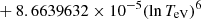 |
||||
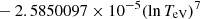 |
||||
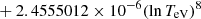 |
||||
![$ \phantom{\exp [} -8.0683825\times 10^{-8} (\ln T_{\mathrm{eV}})^{9}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq96.gif) |
||||
| H33H_33 | H− + H2+ → H2 + H | 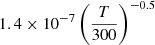 |
24 | |
| H34H_34 | H− + H2+ → H + H + H | 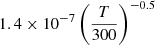 |
24 | |
| H35H_35 | H + H + H2 → H2 + H2 | 0.143 × 10−31T−0.38 | T < 300 K | 18 |
| 0.486 × 10−30T−1 | T ≥ 300 K | 18 | ||
| H36H_36 | H + H + He → H2 + He | 6.9 × 10−32T−0.4 | 25 | |
| H37H_37 | H2+ + H → 2H + H+ | exp(−32.912+6.9498×10−5T−3.3248×104/T | 9 | |
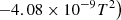 |
||||
| H38H_38 | H2 + H+ → 2H + H+ | exp(−33.404+2.0148×10−4T−5.2674×104/T | 9 | |
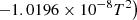 |
||||
| H39H_39 | H3+ + H2 → H3+ + 2H | 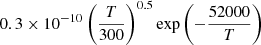 |
26 | |
| H40H_40 | H3+ + e− → H2+ + H + e− | 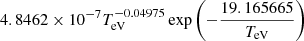 |
16 | |
| H41H_41 | H2+ + e− → H+ + H + e− | 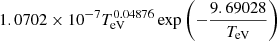 |
16 | |
| H42H_42 | H + H → H + H+ + e− | 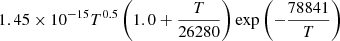 |
27 | |
| H43H_43 | H+ + H + H → H3+ + H | 1.238 × 10−29T−1.046 | 17 | |
| H44H_44 | H3+ + H− → H2 + H2 | 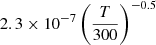 |
28 | |
| He01He_01 | He+ + e− → He + γ | krec = CHeI ⋅ αHeI; | 1 | |
![$ \phantom{k_{1} = } \alpha_{\mathrm{HeI}} =q\left[\sqrt{T_{\mathrm{M}}\over T_2}\left(1+\sqrt{T_{\mathrm{M}}\over T_2}\right)^{1-p} \left(1+\sqrt{T_{\mathrm{M}}\over T_1}\right)^{1+p}\right]^{-1}\! \mathrm{m^{3}s^{-1}}, $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq106.gif) |
||||
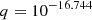 , p = 0.711, T1 = 105.114 K, T2 = 3 K. , p = 0.711, T1 = 105.114 K, T2 = 3 K. |
||||
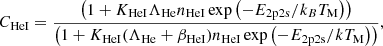 |
||||
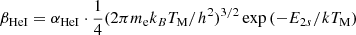 |
||||
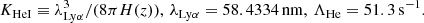 |
||||
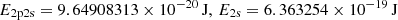 |
||||
| He02He_02 | He + γ → He+ + e− | kion = CHeI ⋅ βHeIexp(−E2s1s/kBTM); | 1 | |
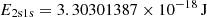 |
||||
| He03He_03 | He + H+ → HeH+ + γ | 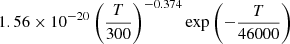 |
29 | |
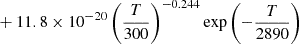 |
||||
| He04He_04 | HeH+ + H → He + H2+ | 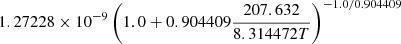 |
30 | |
| He05He_05 | HeH+ + γ → He + H+ | 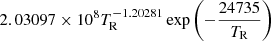 |
31 | |
| He06He_06 | He+ + +e− → He+ + γ | 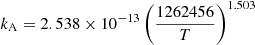 |
Case A | 32 |
![$ \phantom{k_{\mathrm{B}} = } \times [1.0+ \left(\frac{2418500}{T}\right)^{0.470}]^{-1.923} $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq118.gif) |
||||
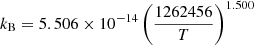 |
Case B | 32 | ||
![$ \phantom{k_{\mathrm{B}} = } \times \left[1.0+ \left(\frac{460752}{T}\right)^{0.407}\right]^{-2.242} $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq120.gif) |
||||
| He07He_07 | He+ + γ → He+ + +e− | 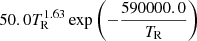 |
3 | |
| He08He_08 | He + H+ → He+ + H | 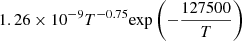 |
T ≤ 10000 K | 33 |
| 4.0 × 10−37T4.74 | T > 10000 K | |||
| He09He_09 | He+ + H → He + H+ | 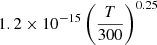 |
34 | |
| He10He_10 | He + H2+ → HeH+ + H | 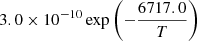 |
35 | |
| He11He_11 | He+ + H → HeH+ + γ | 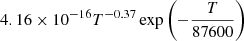 |
59 | |
| He12He_12 | HeH+ + e− → He + H | 3.0 × 10−8T−0.5 | 28 | |
| He13He_13 | HeH+ + H2 → H3+ + He | 1.80 × 10−9 | 28 | |
| He14He_14 | HeH+ + γ → He+ + H | 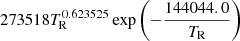 |
31 | |
| He15He_15 | He + e− → He+ + 2e− | exp[−4.409864886 × 101 | 14 | |
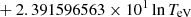 |
||||
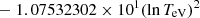 |
||||
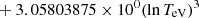 |
||||
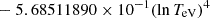 |
||||
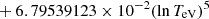 |
||||
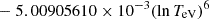 |
||||
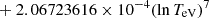 |
||||
![$ \phantom{\exp[} - 3.64916141 \times 10^{-6} (\ln T_{\mathrm{eV}})^{8}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq134.gif) |
||||
| He16He_16 | He+ + e− → He+ + +2e− | exp[−6.87104099 × 101 | 14 | |
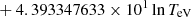 |
||||
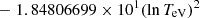 |
||||
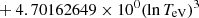 |
||||
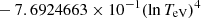 |
||||
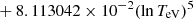 |
||||
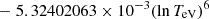 |
||||
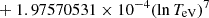 |
||||
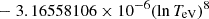 |
||||
| He17He_17 | He+ + H− → He + H | 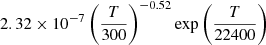 |
36 | |
| He18He_18 | He + H+ + γ → HeH+ + 2γ | 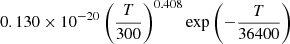 |
35 | |
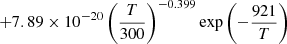 |
||||
| He19He_19 | H2 + He+ → He + H + H+ | 10−13 ⋅ (−0.4732142857+0.0369047619⋅T | T < 78 K | 37 |
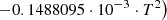 |
||||
| 10−13 ⋅ (1.623809524−0.1587301587⋅10−2⋅T) | 78 K ≤ T < 330 K | |||
| 10−13 ⋅ (0.111111524+0.1555555413⋅10−2⋅T | T ≥ 330 K | |||
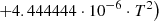 |
||||
| He20He_20 | H2 + He+ → H2+ + He | 7.2 × 10−15 | 38 | |
| He21He_21 | He + H− → He + H + e− | 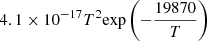 |
39 | |
| He22He_22 | He+ + H2 → He+ + 2H | 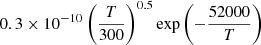 |
26 | |
| D01D_01 | D+ + e− → D + γ | the same as for blueH_01 | ||
| D02D_02 | D + γ → D+ + e− | the same as for blueH_02 | ||
| D03D_03 | D + H+ → H + D+ | 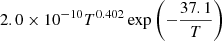 |
T ≤ 2 ⋅ 105 K | 40 |
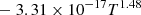 |
||||
| 3.44 × 10−10T0.35 | T > 2 ⋅ 105 K | |||
| D04D_04 | H + D+ → D + H+ | 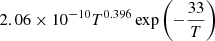 |
40 | |
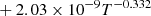 |
||||
| D05D_05 | H2 + D+ → HD + H+ | [0.417+0.846logT−0.137(logT)2] × 10−9 | 41 | |
| D06D_06 | HD + H+ → H2 + D+ | 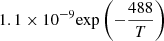 |
41 | |
| D07D_07 | H + D → HD + γ | 10−25[2.80202 − 6.63697lnT | T ≤ 200 K | 42 |
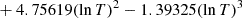 |
||||
![$ \phantom{10^{-25} [} + 0.178259 (\ln T)^{4} - 0.00817097 (\ln T)^{5} ] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq156.gif) |
||||
| 10−25exp[507.207 − 370.889lnT | T > 200 K | |||
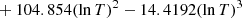 |
||||
![$ \phantom{10^{-25} \exp } + 0.971469 (\ln T)^{4} - 0.0258076 (\ln T)^{5} ] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq158.gif) |
||||
| D08D_08 | H2 + D → HD + H | dex[−56.4737+5.88886logT | T ≤ 2000 K | 43 |
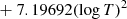 |
||||
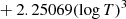 |
||||
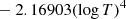 |
||||
![$ \left. \phantom{\mathrm{dex}[} + 0.317887 (\log{T})^{5} \right] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq162.gif) |
||||
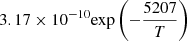 |
T > 2000 K | |||
| D09D_09 | HD+ + H → HD + H+ | the same as for blueH_09 | ||
| D10D_10 | HD + H → H2 + D | 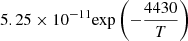 |
T ≤ 200 K | 44 |
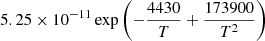 |
T > 200 K | |||
| D11D_11 | D + H+ → HD+ + γ | 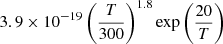 |
45 | |
| D12D_12 | H + D+ → HD+ + γ | 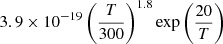 |
45 | |
| D13D_13 | HD+ + γ → H + D+ | the same as for blueH_08 | ||
| D14D_14 | HD+ + e− → H + D | 7.2 × 10−8T−0.5 | 46 | |
| D15D_15 | HD+ + H2 → H2D+ + H | the same as for blueH_13 | ||
| D16D_16 | D + H3+ → H2D+ + H | 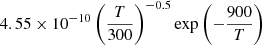 |
28 | |
| D17D_17 | H2D+ + e− → 2H + D | 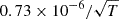 |
47 | |
| D18D_18 | H2D+ + e− → H2 + D | 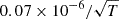 |
47 | |
| D19D_19 | H2D+ + e− → HD + H | 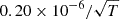 |
47 | |
| D20D_20 | H2D+ + H → H3+ + D | 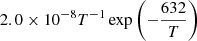 |
58 | |
| D21D_21 | HD+ + H → H2+ + D | 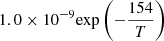 |
48 | |
| D22D_22 | D + H− → HD + e− | 7.5 × 10−10 | T < 300 | 49 |
| 2.0 × 10−9T−0.17 | T ≥ 300 | 49 | ||
| D23D_23 | H + D− → HD + e− | 7.5 × 10−10 | T < 300 | 49 |
| 2.0 × 10−9T−0.17 | T ≥ 300 | 49 | ||
| D24D_24 | HD + H+ → H2+ + D | 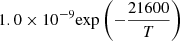 |
50 | |
| D25D_25 | HD+ + H → H2 + D+ | 1.0 × 10−9 | 50 | |
| D26D_26 | HD+ + γ → H+ + D | the same as for blueH_08 | ||
| D27D_27 | H2+ + D → HD+ + H | 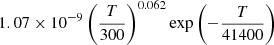 |
51 | |
| D28D_28 | D + e− → D− + γ | dex[−17.845 + 0.762logT | T ≤ 6000 K | 2 |
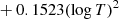 |
||||
![$ \phantom{ \mathrm{dex}[} - 0.03274 (\log{T})^{3}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq177.gif) |
||||
| dex[−16.4199 + 0.1998(logT)2 | T > 6000 K | |||
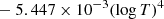 |
||||
![$ \phantom{ \mathrm{dex}[} + 4.0415 \times 10^{-5} (\log{T})^{6}] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq179.gif) |
||||
| D29D_29 | H + D− → D + H− | 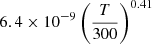 |
48 | |
| D30D_30 | D + H− → H + D− | 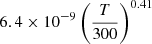 |
48 | |
| D31D_31 | D + D− → D2 + e− | the same as for blueH_05 | ||
| D32D_32 | H+ + D− → HD+ + e− | 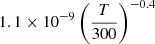 |
45 | |
| D33D_33 | D+ + H− → HD+ + e− | 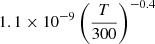 |
45 | |
| D34D_34 | D+ + D− → D2+ + e− | 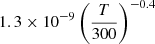 |
45 | |
| D35D_35 | H+ + D− → D + H | 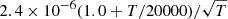 |
52 | |
| D36D_36 | D + D+ → D2+ + γ | 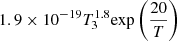 |
45 | |
| D37D_37 | D + H2+ → H2 + D+ | the same as for blueH_09 | ||
| D38D_38 | H2+ + D → HD + H+ | 1.0 × 10−9 | 50 | |
| D39D_39 | HD+ + D → D2+ + H | 1.0 × 10−9 | 53 | |
| D40D_40 | HD+ + D → D2 + H+ | 1.0 × 10−9 | 50 | |
| D41D_41 | H + D2+ → D2 + H+ | the same as for blueH_09 | ||
| D42D_42 | D2+ + H → HD+ + D | 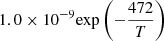 |
53 | |
| D43D_43 | D2+ + H → HD + D+ | 1.0 × 10−9 | 50 | |
| D44D_44 | HD + D+ → D2 + H+ | 1.0 × 10−9 | 53 | |
| D45D_45 | D2 + H+ → HD+ + D | 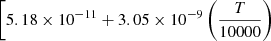 |
54 | |
![$ \phantom{[} \left. -5.42 \times 10^{-10} \left(\frac{T}{10000}\right)^{2} \right]{\exp \left(-\frac{20100}{T}\right)} $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq189.gif) |
||||
| D46D_46 | D2 + H+ → D2+ + H | the same as for blueH_10 | 52 | |
| D47D_47 | HD + D → D2 + H | 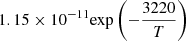 |
44 | |
| D48D_48 | D2 + H → HD + D | dex[−86.1558+4.53978logT | T ≤ 2200 K | 43 |
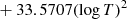 |
||||
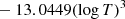 |
||||
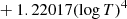 |
||||
![$ \left. \phantom{\mathrm{dex}[} + 0.0482453 (\log{T})^{5} \right] $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq194.gif) |
||||
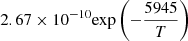 |
T > 2200 K | |||
| D49D_49 | HD + γ → H + D | 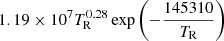 |
55 | |
| D50D_50 | D2+ + γ → D + D+ | the same as for blueH_08 | ||
| D51D_51 | D2 + γ → D2+ + e− | the same as for blueH_14 | ||
| D52D_52 | D2 + γ → D + D | the same as for blueH_15 | ||
| D53D_53 | D− + γ → D + e− | the same as for blueH_04 | ||
| D54D_54 | HD + H3+ → H2 + H2D+ | 1.0 × 10−9(2.1−0.4⋅log(T)) | 3 | |
| D55D_55 | D2 + e− → D + D + e− | 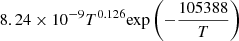 |
v = 0 | 20 |
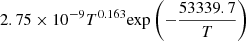 |
LTE | |||
| D56D_56 | HD+ + H2 → H3+ + D | the same as for blueH_13 | ||
| D57D_57 | H2D+ + H2 → H3+ + HD | 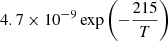 |
T < 100 K | 3 |
| 5.5 × 10−10 | T ≥ 100 K | |||
| D58D_58 | D + e− → D+ + e− + e− | the same as for blueH_20 | 52 | |
| D59D_59 | HD + e− → D + H− | 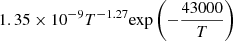 |
56 | |
| D60D_60 | HD + e− → H + D− | 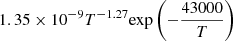 |
56 | |
| D61D_61 | HD + e− → H + D + e− | 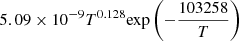 |
v = 0 | 57 |
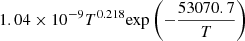 |
LTE | |||
| D62D_62 | H2 + D+ → H2+ + D | the same as for blueH_10 | 52 | |
| D63D_63 | H2 + D+ → HD+ + H | 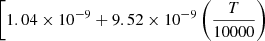 |
54 | |
![$ \left. \;-1.81 \times 10^{-9} \left(\frac{T}{10000}\right)^{2} \right]{\exp \left(-\frac{21000}{T}\right)} $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq205.gif) |
||||
| D64D_64 | HD + H+ → HD+ + H | the same as for blueH_10 | 52 | |
| D65D_65 | HD + H → H + D + H | the same as for blueH_19 | 18 | |
| D66D_66 | HD + He → H + D + He | the same as for blueH_30 | 18 | |
| D67D_67 | HD + He+ → HD+ + He | the same as for blueHe_20 | 52 | |
| D68D_68 | HD + He+ → He + H+ + D | 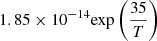 |
49 | |
| D69D_69 | HD + He+ → He + H + D+ | 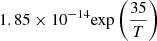 |
49 | |
| D70D_70 | HD+ + D → HD + D+ | the same as for blueH_09 | 52 | |
| D71D_71 | D2 + e− → D + D− | 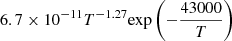 |
56 | |
| D72D_72 | D− + e− → D + e− + e− | the same as for blueH_31 | 52 | |
| D73D_73 | D− + H → D + H + e− | the same as for blueH_32 | 52 | |
| D74D_74 | D+ + H− → D + H | the same as for blueH_06 | 52 | |
| D75D_75 | D+ + D− → D + D | the same as for blueH_06 | 52 | |
| D76D_76 | H2+ + D− → H2 + D | 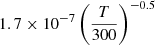 |
45 | |
| D77D_77 | H2+ + D− → H + H + D | 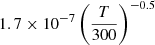 |
45 | |
| D78D_78 | HD+ + H− → HD + H | 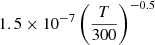 |
45 | |
| D79D_79 | HD+ + H− → D + H + H | 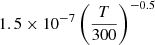 |
45 | |
| D80D_80 | HD+ + D− → HD + D | 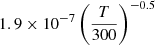 |
45 | |
| D81D_81 | HD+ + D− → D + H + D | 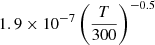 |
45 | |
| D82D_82 | D2+ + H− → D2 + H | 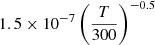 |
45 | |
| D83D_83 | D2+ + H− → D + D + H | 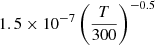 |
45 | |
| D84D_84 | D2+ + D− → D2 + D | 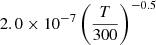 |
45 | |
| D85D_85 | D2+ + D− → D + D + D | 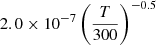 |
45 | |
| D86D_86 | D + D2+ → D2 + D+ | the same as for blueH_09 | 52 | |
| D87D_87 | HD + D+ → HD+ + D | the same as for blueH_10 | 52 | |
| D88D_88 | HD + D+ → D2+ + H | 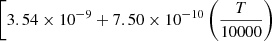 |
54 | |
![$ \left. \;-2.92 \times 10^{-10} \left(\frac{T}{10000}\right)^{2} \right]{\exp \left(-\frac{21100}{T}\right)} $](/articles/aa/full_html/2025/06/aa52761-24/aa52761-24-eq220.gif) |
||||
| D89D_89 | D2 + H+ → HD + D+ | 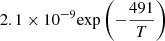 |
53 | |
| D90D_90 | D2 + D+ → D2+ + D | the same as for blueH_10 | 52 | |
| D91D_91 | HD + H2 → H + D + H2 | the same as for blueH_29 but with ncr → 100ncr | 18 | |
| D92D_92 | D2 + H → D + D + H | the same as for blueH_19 | 52 | |
| D93D_93 | D2 + H2 → D + D + H2 | the same as for blueH_29 | 52 | |
| D94D_94 | He + D+ → D + He+ | 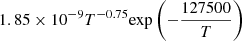 |
T ≤ 10000 K | 45 |
| 5.9 × 10−37T4.74 | T > 10000 K | |||
| D95D_95 | He+ + D → D+ + He | 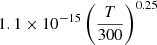 |
45 | |
| D96D_96 | D− + He → D + He + e− | 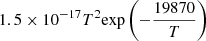 |
45 | |
| D97D_97 | He+ + D− → He + D | 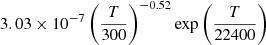 |
45 | |
| D98D_98 | D2 + He+ → D2+ + He | 2.5 × 10−14 | 53 | |
| D99D_99 | D2 + He+ → He + D+ + D | 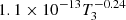 |
53 | |
| D100D_100 | D2 + He → D + D + He | the same as for blueH_30 | 52 |
Notes. T and TeV are the gas temperature in units of K and eV respectively. References are to the primary source of data for each reaction. Reactions with a peak contribution to the synthesis or destruction of any reactant exceeding 0.1 % are bolded.References. 1: Seager et al. (1999), 2: Wishart (1979), 3: Galli & Palla (1998), 4: Croft et al. (1999), 5: Ramaker & Peek (1976), 6: Karpas et al. (1979), 7: Savin et al. (2004), 8: Poulaert et al. (1978), 9: Coppola et al. (2011a), 10: Theard & Huntress (1974), 11: Novosyadlyj et al. (2022), 12: Mac Low & Shull (1986), 13: Lepp & Shull (1983), 14: Janev et al. (1987), 15: this paper: Fit based on cross-section from https://home.strw.leidenuniv.nl/ ewine/photo/, 16: Méndez et al. (2006), 17: Janev et al. (2003), 18: Glover & Abel (2008), 19: Schulz & Asundi (1967), 20: Trevisan & Tennyson (2002a), 21: Martin et al. (1998), 22: Shapiro & Kang (1987), 23: Dove et al. (1987), 24: Dalgarno & Lepp (1987), 25: Walkauskas & Kaufman (1975), 26: Albertsson et al. (2014), 27: Soon (1992), 28: Database of kinetic data of interest for astrochemical (KIDA, kida.astrochem-tools.org), 29: Courtney et al. (2021), 30: De Fazio (2014), 31: Coppola et al. (2017), 32: Ferland et al. (1992), 33: Kimura et al. (1993), 34: Zygelman et al. (1989), 35: Black (1978), 36: Peart & Hayton (1994), 37: this paper: Fit to data from Schauer et al. (1989); Johnsen et al. (1980) 38: Glover & Abel (2008): Barlow S. G., 1984, PhD thesis, Univ. Colorado 39: Huq et al. (1982), 40: Savin (2002), 41: Lindinger et al. (1982), 42: Dickinson (2005), 43: Fit of Glover & Abel (2008) to data from Mielke et al. (2003), 44: Shavitt (1959), 45: Same as corresponding H reaction, but scaled by D reduced mass (see Glover & Abel (2008)), 46: Strömholm et al. (1995), 47: Datz et al. (1995); Larsson et al. (1996), 48: Dalgarno & McDowell (1956), scaled by D reduced mass (see Glover & Abel (2008)), 49: Same as corresponding H reaction, with branching ratio assumed uniform (see Glover & Abel (2008)), 50: estimate from Glover & Abel (2008), 51: Linder et al. (1995), 52: Same as corresponding H reaction (see Glover & Abel (2008)), 53: Walmsley et al. (2004), 54: Fit of Glover & Abel (2008) based on cross-section from Wang & Stancil (2002) 55: Coppola et al. (2011b), 56: Xu & Fabrikant (2001), 57: Trevisan & Tennyson (2002b), 58: Adams & Smith (1985), 59: Stancil et al. (1998).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


