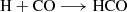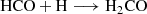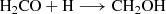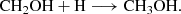| Issue |
A&A
Volume 650, June 2021
|
|
|---|---|---|
| Article Number | A169 | |
| Number of page(s) | 6 | |
| Section | Astrophysical processes | |
| DOI | https://doi.org/10.1051/0004-6361/202140443 | |
| Published online | 25 June 2021 | |
Formation of formaldehyde through methanol-ice-mantle (CH3OH)10 bombardment by OH+ cation
1
Universidad Autónoma de Chile, Facultad de Ingeniería, Instituto de Ciencias Químicas Aplicadas, Núcleo de Astroquímica & Astrofísica, Av. Pedro de Valdivia 425, Providencia, Santiago, Chile
e-mail: natalia.inostroza@uautonoma.cl
2
Universidad Autónoma de Chile, Facultad de Ingeniería, Instituto de Ciencias Químicas Aplicadas, Núcleo de Astroquímica & Astrofísica, Av. Alemania, 01090 Temuco, Chile
3
Universidad de Chile, Facultad de Ciencias Físicas y Matemáticas, Departamento de Astronomía, Camino el Observatorio 1515, Las condes, Santiago, Chile
4
Universidad de Chile, Facultad de Ciencias Químicas y Farmacéuticas, Dr. Carlos Lorca Tobar 964, Independencia, Santiago, Chile
5
Chinese Academy of Sciences South America Center for Astronomy, National Astronomical Observatories, Chinese Academy of Sciences, Beijing 100012, PR China
Received:
28
January
2021
Accepted:
9
April
2021
Context. Formaldehyde H2CO was the first organic polyatomic molecule discovered in the interstellar medium to have been detected in a variety of sources. However, pathways to synthesize this molecule under interstellar conditions have yet to be discussed.
Aims. We carried out a systematic study to analyze the chemical processes that can explain the H2CO formation mechanism toward a decamer of methanol (CH3OH)10 as target material to mimic an ice mantle bombarded by an OH+ cation.
Methods. We performed Born-Oppenheimer (ab initio) molecular dynamics simulations to obtain the formation mechanisms of complex organic molecules (COMs) such as formaldehyde H2CO and its HCOH isomer.
Results. We found that CH2OH+ and CH2(OH)2 are the main precursors to form H2CO and HCOH. We discuss its formation mechanisms and the astrophysical implications in star-forming regions. These processes are likely relevant to the production of COMs.
Key words: astrochemistry / molecular processes / ISM: molecules
© ESO 2021
1. Introduction
Formaldehyde (H2CO) was the first organic polyatomic molecule discovered in the interstellar medium (Snyder et al. 1969). It is abundant in the universe and one of the fundamental molecules for life as H2CO constitutes an important pre-product of sugar synthesis. Formaldehyde is considered the natural precursor of methanol, which is the starting point to produce more complex organic species (Potapov et al. 2017; Allamandola & Hudgins 2003; Andrade et al. 2009; Hollis et al. 2000). It is well-accepted that these organic molecules have to be formed mainly via surface processes on the icy dust to reproduce the observed abundances, which are necessary to produce significant amounts of CH3OH and H2CO. Grain-surface reactions, where dust grains are covered by a molecular ice mantle, act as surface catalysts for many complex organics molecules (COMs; Watanabe et al. 2004) give rise to the interplay between the gas-phase species and solid-phase species.
The H2CO can be formed through successive hydrogenation of CO (carbon monoxide) on ice surfaces (Woon 2002) as follows:
The astrophysical model proposes the hydrogenation of CO to form HCO• and then by successive recombination with an H atom to produce H2CO or dimerize to glyoxal CHOCHO in the presence of a few water molecules in ice surfaces (Woods et al. 2013). This model can work properly in photo-dissociation regions (PDRs) where photo-desorption of H2CO from dust grains is needed to explain the observed H2CO gas-phase abundance since gas-phase chemistry alone does not produce enough H2CO (Guzmán et al. 2011). In contrast, for dense-core regions, the main way to produce H2CO is through gas-phase reactions. Thus, the gas-phase H2CO is accreted onto dust grains, and it proceeds to form CH3OH over molecular ice through hydrogenation (Vichietti et al. 2016; Hiraoka et al. 1994, 2002; Watanabe & Kouchi 2002; Chuang et al. 2017), meaning that both species are intrinsically related, as previously mentioned (reaction 3–4). Methanol molecules are usually detected close to star-forming regions (Minier et al. 2005), being the most abundant alcohol in hot cores (Sjouwerman et al. 2010; Charnley 1995). In PDRs such as the Horsehead nebula, the formation of H2CO and CH3OH occurs throughout reaction (1) and (2) (Guzmán et al. 2013). In this context, to understand the formaldehyde formation process, both gas-phase and grain surface mechanisms must be included in astrophysical models (Potapov et al. 2017; Jin & Garrod 2020).
Even though water is the most common ice in the interstellar medium (ISM) and methanol can be just up to 1–40% with respect to water in cold regions (Basalgète et al. 2021; Gibb et al. 2004), CH3OH is the only COM that has been shown by mid-infrared detection to be a constituent of the icy mantles. Its presence has been confirmed at different star formation stages (Taban et al. 2003; Gibb et al. 2004; Boogert et al. 2015). Further, within methanol detection in a protoplanetary disk for the first time using ALMA (Atacama Large Millimeter/submillimeter Array), formation pathways to explain the detection and formation of larger molecules are certainly a key aspect to be analyzed (Walsh et al. 2016). As mentioned above, we noticed that the formaldehyde formation process could happen from the gas phase, unlike methanol, which suggests that the presence of methanol in the gas phase can only be explained by nonthermal desorption from ice grains (Garrod et al. 2007), as CH3OH is considered a crucial factor to produce larger COMs (Garrod & Herbst 2006; Garrod et al. 2008).
Nonetheless, the mechanisms to form those kinds of COMs are still poorly understood and one of the central issues in current astrochemical studies. Due to the interplay between H2CO/CH3OH, it is essential to study the possible gas or solid-phase pathways that are likely to produce one or more of these molecules (McGuire et al. 2017; Jin & Garrod 2020). Due to this, in this work, we focus on the COMs related to methanol. We study (CH3OH)10 as a target material to mimic an ice mantle in a reaction with OH+, which is a key factor in the chemistry that occurs in the gas phase of interstellar clouds. We selected the OH+ for this work in an X3Σ+ in its ground electric state. This OH+ ion can play a crucial role in PDR regions (Gerin et al. 2016) because it can react efficiently in successive ion-molecule reactions with other species to generate chemical complexity. Further, with ALMA, OH+ has been detected along with galaxies at a higher redshift (greater than 2) at frequencies where Earth’s atmosphere is transparent (Muller et al. 2016). Herschel also detected the OH+, but toward the nuclei of NGC 4418, Arp220, and Mrk 231 where ionization rates are about 10−13 − 10−12 s−1 (González-Alfonso et al. 2013, 2018). As far as we known, ion-molecule reactions are relevant to understand the ISM. An example is the reaction of C+ with H2O producing HCO+ and HOC+ in the gas phase (Martinez et al. 2008). In contrast, the same reactions in ice yields four different products such as HCO+, HOC+, CO + H, and CH(OH)2. This behavior lets us infer about the role that the mantle and the phase can play (McBride et al. 2014). Additionally, a theoretical work by Woon (2011) studied the reaction of OH cation + CO + (H2O)17. In that work, the ion was located near to the H2O-cluster or near CO, and then OH+ was adsorbed to generate different outcomes through barrier-less processes. This information shows us that this cation is involved in many processes in various sources representing a key element to consider in several physical and chemical stellar processes. Considering those facts, we analyze how methanol ice mantles colliding with OH+ generate an alternative route of H2CO formation-grain surface processes.
2. Computational methods
We used Born-Oppenheimer molecular dynamics (BOMD) simulations to study the formation pathways of COMs that happen in a methanol cluster formed by ten units of methanol to mimic an ice mantle (CH3OH)10 under the impact of OH+. We present the details below.
2.1. Simulations
We performed simulations of an ice mantle in the ISM impact by an OH+ projectile. To mimic an ice mantle, we used a methanol cluster consisting of ten methanol molecules stabilized by weak interactions ((CH3OH)10). This cluster has been described previously by Inostroza-Pino et al. (2019, 2020). To simulate the trajectories of the OH+ projectile impacting the methanol ice mantle, we carried out density functional theory (DFT) Born-Oppenheimer molecular dynamics (BOMD) calculations employing the micro-canonical ensemble (NEV ensemble) at energies between 10 to 22 eV.
We employed the long-range-corrected hybrid functional of Head-Gordon ωB97X-D (Helgaker et al. 1990; Uggerud & Helgaker 1992; Bolton et al. 1998; McBride et al. 2013) and the Pople Basis Set 6–31+G(d,p). The initial kinetic energy (10, 12, 15, 18, 20, and 22 eV) and the impact position employed here are the same as those previously used for the OH− and OH• projectiles (Inostroza-Pino et al. 2019, 2020). We observed that impact energies under 10 eV lead only adsorption in the case of OH− and OH•, while impacts above 22 eV result in evaporation of the cluster. In the case of OH+, we found the same behavior.
We set up 24 trajectories with a time step of 0.5 fs. This process produces a total of 800 steps to lead a timescale of 400 fs for each impact trajectory where the OH+ projectile is pointing toward the center of mass of the (CH3OH)10-ice mantle. Thus, we used pure methanol ice to simplify our simulation and to save computation time. We symmetrically distributed the initial projectile positions around a sphere as we previously described (Inostroza-Pino et al. 2019, 2020). All calculations were done using the Gaussian 09 code (Frisch et al. 2009).
2.2. Chemical model
As we have mentioned in our previous work (Inostroza-Pino et al. 2020), the ten-unit-methanol is used to mimic a dust cover via a hypothetical molecular mantle that formed, in this case, by methanol. Using this cluster of methanol, we were able to study different complex organic molecules that can be formed where methanol molecules were present.
We analyzed the mechanisms of the formation of COMs that conduct interstellar chemistry in interstellar ices. According to (Boyd & Boyd 2007), this cluster (CH3OH)10-ice mantle) corresponds to the most stable isomer. As in Inostroza-Pino et al. (2020), the collision will occur with all species in their ground state. We also kept identical initial conditions: the impact position and range of kinetic energy applied previously (Inostroza-Pino et al. 2020). Consequently, we focused only on collisions where the richest chemistry takes place. For each impact energy, these trajectories began with the velocity of the O-H axis of the OH+ projectiles pointing toward the center of mass of the ice mantle (CH3OH)10 to obtain diversity in the impact positions, as we did previously (Inostroza-Pino et al. 2020). To simplify the nomenclature in Table 1, we indicate only outcomes of every reaction of (CH3OH)10 + OH+ that has formaldehyde as a product.
Products obtained from (CH3OH)10-ice mantle in collision with an OH+ projectile with a kinetic energy impact of 10–22 eV.
3. Results
In the context of an alternative route of H2CO formation processes, the purpose of the present work is to focus on H2CO formation produced by bombardment of the methanol icy-mantle (interstellar ice-mantle analogs) via OH+ molecules. The following subsection shows the main processes (see Table 1) obtained through these simulations. The chemical network of (CH3OH)10-ice-mantle + OH+ is presented in Fig. 1.
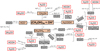 |
Fig. 1. Global chemical network using an OH+ projectile. For a complete list of reactions and products, see Table 1. The precursors are in orange, the intermediates are in gray, and the formaldehyde and the hitherto isomer are outlined in red. |
The most frequent outcome is the methanediol CH2(OH)2. Its search in the gas phase is a high priority (Geppert et al. 2006). In the second place, CH2OH+ leads the main intermediate species in all ranges of kinetic impact energies (see reaction 1 in Table 1). Every time CH2OH+ is formed, there are secondary or even tertiary processes that lead to the formation of stable products such as formaldehyde H2CO. This behavior is independent of the impact energy. The reactions that occur under the impact of OH+ with the (CH3OH)10-ice-mantle cluster were organized according to the precursors from which all the reactions originate. We found the following four key molecules: hydroxymethyl cation (CH2OH+), methanediol (CH2(OH)2), formyl cation (HCO+), and methylene hydroperoxide cation ([H2COOH]+). Their main formation pathways correspond to secondary and tertiary processes shown in Table 1. Nevertheless, slight differences can be due to dipole-dipole attraction or due to other weak interactions present that can unprotect the methyl group to generate CH2OH+ or lead to the production of diol, formyl cation, and [H2COOH]+ as precursors. A remarkable process occurs under reaction 10 and reaction 14, where both isomers HCOH and H2CO are formed as stable products at 22 eV. The reactants in all cases are (CH3OH)10 and OH+. These reactants generate the main outcomes written in Table 1. To simplify the table, we have not included methanol molecules that are not part of the final products whose reaction mechanisms are described below.
3.1. CH2OH+, hydroxymethyl cation
We found the CH2OH+ cation in all ranges of kinetic impact energies. These processes lead to forming a stable product as formaldehyde H2CO. Table 1 shows that the chemistry is even more complex when OH+ reacts with a decamer of methanol in comparison with OH• (Inostroza-Pino et al. 2020) as in our previous reports.
Once the OH+ impacts a (CH3OH)10-ice mantle, a water molecule is produced while a hydroxymethyl cation is formed (see Steps 1.1, 2.1, and 3.1 in Table 1). Subsequently, this CH2OH+ can eliminate a proton to form the formaldehyde (Steps 1.2, 2.2) or the hydroxycarbene (Step 3.2). After release, the proton is stabilized by hydrogen bonds with four methanol molecules participating in it (Steps 1.2, 3.2) or by the formation of a hydronium ion (Step 2.2). It was found for all other subsequent reactions that, when a proton is generated, it is stabilized by transient hydrogen bonds involving two or more methanol molecules, unless otherwise specified, as we depict in Fig. 2. This hydroxymethyl cation can also migrate from the surface of the ice mantle as a stabilized species until it encounters a molecule with which to react.
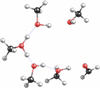 |
Fig. 2. Diagram illustrating transient hydrogen bonds stabilized by two or more methanol molecules during the BOMD simulation. Five methanol molecules stabilize an H+; in the bottom right-hand corner, a formaldehyde molecule is presented. |
On the other hand, the CH2OH+ can react with a second methanol molecule of the ice mantle (Step 4.1 in Table 1), which generates the breakdown of the hydroxymethyl moiety into a carbene (:CH2) and water, while the second methanol molecule decomposes into formaldehyde and a proton that is stabilized via hydrogen bonds with other methanol molecules in a [H…OH…OH…OH]+ fashion (Step 4.2), which is presented in Fig. 2. The processes depicted in Fig. 2 show the stabilization of a hydrogen-bond interaction between H+ and CH3OH-molecules. In contrast, in our previous work (Inostroza-Pino et al. 2020), the proton forms methanol protonated CH3OH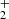 which is released to the gas phase as a final product. The hydroxymethyl cation can also be associated with two nearby methanol molecules at 20 eV (Step 5.1). The elimination of a proton occurs while a redox reaction generates two hydrogen molecules and three formaldehyde molecules (Step 5.2).
which is released to the gas phase as a final product. The hydroxymethyl cation can also be associated with two nearby methanol molecules at 20 eV (Step 5.1). The elimination of a proton occurs while a redox reaction generates two hydrogen molecules and three formaldehyde molecules (Step 5.2).
3.2. CH2(OH)2, methanediol
The second precursor is the methanediol described in interstellar grain surface species (Kent et al. 2003; Ehrenfreund & Charnley 2000) as a result of high-energy UV radiation. We have found that the methanediol molecule can be formed when an OH+ impacts a methanol molecule of the (CH3OH)10-ice mantle, where the first step is to form a reactive protonated methanediol molecule (Step 6.1). This precursor can undergo a dehydration reaction (Step 6.2) generating a CH2OH+ intermediate, which eliminates a proton to yield formaldehyde (Step 6.3). Also, this protonated methanediol can react with a second methanol molecule (Steps 7.1,8.1) to eliminate a dihydrogen molecule via hydride abstraction, resulting in the formation of methanediol and protonated formaldehyde (Steps 7.2,8.2).
From here, the following two possible mechanisms are possible: first, the methanediol eliminates a water molecule to form formaldehyde (Step 7.3). This mechanism is already known and has been described to occur in the gas phase (Kent et al. 2003). At the same time, the CH2OH+ suffers a proton elimination to generate a second formaldehyde molecule (Step 7.4). Second, the methanediol can migrate as a stable molecule from the ice mantle, while the CH2OH+ suffers a proton elimination to generate a second formaldehyde molecule (Step 8.3).
Another decomposition mechanism for the reaction of the protonated methanediol with a second methanol molecule (Steps 9.1, 10.1) consists of several steps. The first protonated methanediol eliminates a dihydrogen molecule via hydride abstraction and a formaldehyde molecule as a primary process. A very reactive OH+, owing to the high energy involved in the process (22 eV), is also generated, which reacts with a second methanol molecule to regenerate a new protonated methanediol (Steps 9.2, 10.2). This second protonated methanediol undergoes a water and proton elimination, producing a second formaldehyde molecule (Step 9.3) or its hitherto isomer (Step 10.3).
3.3. HCO+, formyl cation
The formyl cation has been identified in the pure CH3OH-rich ices experiment with UV (ultraviolet) irradiation (Öberg et al. 2009), showing that photolysis of CH3OH-rich ices and recombination of the fragments can theoretically result in a large number of new species, as we have also shown in this work. In reaction 11, the impact of the OH+ over a methanol molecule produces its decomposition into a formyl cation, molecular hydrogen, and water (Step 11.1). Then, the highly reactive formyl cation interacts with a second methanol molecule, abstracting a hydride from its methyl group to produce formaldehyde, and the CH2OH+. The CH2OH+ recently formed release a proton H+, this H+ is then captured by the water molecule, which releases another proton that is subsequently stabilized by the methanol molecules of the ice mantle Step 11.2).
3.4. [H2COOH]+, methylene hydroperoxide cation
Methylene hydroperoxide cation is another precursor molecule that forms when OH+ impacts a methanol molecule. The oxygen of the OH+ forms an unstable adduct and a protonated methyl hydroperoxide (CH3OOH…H+) that readily experiences an electronic rearrangement that finishes upon the elimination of a dihydrogen molecule and the formation of [H2COOH]+ (Steps 12.1, 13.1, 14.1). The recently formed cation reacts immediately with the nearest methanol molecule, taking one of two possible reaction pathways: (i) formaldehyde is generated via the elimination of a highly energetic OH+ that reacts with the second methanol molecule, rearranging the molecule into a protonated methanediol (H2C(OH)2…H+) that eliminates a proton to form the stable methanediol (Step 12.2); or (ii) formaldehyde is generated via the elimination of a highly energetic OH+ that can abstract a hydride (H:−) from the CH3 group of the second methanol molecule. The latter step produces a water molecule and the CH2OH+ (Steps 13.2,14.2). This cationic intermediate can eliminate a proton to generate a second formaldehyde molecule (Step 13.3 at 20 eV) or the hitherto isomer (Step 14.3 at 2.2 eV). To our knowledge, methylene hydroperoxide cation has not been described elsewhere. The proposed decomposition mechanisms of this new interstellar precursor can be compared with the ones reported by Vaghjiani & Ravishankara (1989), where CH2OOH is described as very unstable and readily falls apart to give us formaldehyde and OH•.
3.5. Astrophysical implications
In cold regions, photons coming from various sources could participate in icy-chemistry reactions, producing a photodesorption mechanism to eject COMs into a gas phase. Also, cosmic rays (CRs) can ionize the environment, producing radicals that quickly react and produce new species (Shingledecker & Herbst 2018; Dartois et al. 2018). This phenomenon allows us to hypothesize which type of reactions one would expect to observe and which do not occur spontaneously at low temperatures on molecular ice mantles. The ion-ice reactions, where this study can apply, are restricted to several conditions such as the following: (1) cations must be present in the gas phase; (2) ice-grain mantles must be present; and (3) the gas phase, which is affected by cosmic rays through its ionization, shocks, or other energetic events, must sublimate the ice, leading to species generated in ice and finally released to the gas phase where they are detected (Dartois et al. 2018).
In dark interstellar clouds, where the methanol abundance is higher, desorption through vibrational excitation can be a plausible alternative mechanism (Geppert et al. 2006). Another well-known mechanism that could explain the presence of COMs in the gas phase is the desorption that exothermic reactions can induce. This mechanism is called chemical desorption (Minissale et al. 2016; Ligterink et al. 2017; Cazaux et al. 2016). In PDRs, the effects of stellar far-ultraviolet (6 eV < hν < 13.6 eV) cause the ionization of molecules with ionization potentials below 13.6 eV. Those processes could also provide a source of larger COMs dominated by photo-desorption. In this context, UV photodesorption experiments by Bertin et al. (2016) in the 7–14 eV range corroborate that methanol UV photodesorption from pure methanol ice is occurring and allows more COMs. Thus, PDRs include all interstellar regions where the gas is predominantly neutral but where far-ultraviolet photons play a significant role in the chemistry and/or heating. Furthermore, any molecular projectile could be produced in regions close to shock fronts, arising from powerful protostellar winds, which drive high drift velocity with respect to the gas when a shock is passing (Van de Sande et al. 2019; Kouchi et al. 1994). Thus, high impact energy can be reached when the relative drift velocity is high. As an example, the C-shock model by Burkhardt et al. (2019) shows that the drift velocity can be up to 12 km s−1 for a shock velocity of 20 km s−1. In an AGB (asymptotic giant branch) outflow, the drift velocity can also reach up to 15 km s−1, as Van de Sande et al. (2019) have shown. For a shock with a higher velocity, the drift velocity can be up to 30 km s−1, corresponding to roughly 20 eV. Additionally, in protoplanetary disks, X-rays are emitted from the central young stellar object (YSO) to irradiate interstellar ices in the disk. In this context, the X-ray spectra in the ISM typically span from 0.1 to at least 10 keV (Dupuy et al. 2018). Therefore, to verify the new reactions, outflows are ideal conditions with high impact energies of 10–22 eV. The four key precursors CH2OH+, CH2(OH)2, HCO+, and H2COOH+ mentioned here are vital species that could participate in the balance of the gas-dust phase. With those species and reactions, the chemical abundances in both the gas phase and solid phase could be changed compared to previous studies, which is needed to be verified by astrochemical models with a full gas-grain reaction network and future observations in various astrophysical conditions (Dartois et al. 2020; Carrascosa et al. 2020).
4. Conclusions
We show new pathways to obtain H2CO through ices chemistry processes. Our results demonstrate that the bombardment of the methanol icy mantle by OH+ cations cause, through primary processes, the formation of four types of precursors such as CH2OH+, CH2(OH)2, HCO+, and H2COOH+ with their further stabilization leading to the formation of formaldehyde in a secondary or tertiary process. We noticed that the H2CO formation is dependent on the kinetic impact energy employed, showing different formation mechanisms via different crucial precursors. In contrast to the early suggestions that H2CO is formed through a hydrogenation process (Watanabe & Kouchi 2002), we propose an alternative route to produce H2CO when CH2OH+, CH2(OH)2, HCO+, or H2COOH+ are formed. In light of our results, we have demonstrated that CH2OH+ underwent deprotonation to generate H2CO, which is released to the gas phase. This cation can be relevant in photo-dissociation regions and/or shocks produced by high-velocity jets and outflows from YSOs. Through the simulations of an OH+ projectile colliding with a cold amorphous methanol decamer ((CH3OH)10) with kinetic impact energies of 10–22 eV, we obtain new formation mechanisms of H2CO affecting the composition of gas and the chemistry of interstellar dust (Dulieu et al. 2013). We found four types of precursors that give rise to several mechanisms that end in the production of H2CO and HCOH. The most frequent precursor obtained is CH2OH+, which is involved in the primary process as a final product, and in secondary and tertiary processes as an intermediate leading to the final product H2CO. These results provide input for ice processes under specific laboratory conditions that can improve molecular assignments. Within these results, we confirm that ice grains act as a catalyst to form more complex outcomes (Dulieu et al. 2013). If these processes are frequent, then ion-ice reactions may become chemically relevant in a molecular cloud (McGuire et al. 2017). Thus, we show in this work that high energy molecular projectiles such as OH+ can also have significant effects on dust grain chemistry. Thus, in protostellar envelopes with large CH3OH ice fractions, where the complex ice chemistry is dominated by pure CH3OH chemistry, similar products as we exposed here would be expected in the gas phase over a wide range of objects if only thermal desorption is assumed (Öberg et al. 2009; Dartois et al. 1999; Carrascosa et al. 2020).
As the OH impacts over a methanol molecule containing only one carbon atom, it is expected that most products have only one carbon atom. In some cases, through the molecular dynamics approach employed here, we observe the interaction of a kinetically excited adduct CH3OH + OH+ with a second CH3OH molecule. We have demonstrated here that the methanol-ice mantle could generate other COMs under further bombardment or another triggering process this information can help us to understand a new set of experiments.
Acknowledgments
This research was supported by FONDECYT-Chile grant 11140770, PCI-ANID International Networks Grant REDES190113 and by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 872081 (ATMOS). DM acknowledges support from ANID project Basal AFB170002. JG acknowledges support from Fondecyt postdoctoral fellowship 3170768. CH acknowledges support from DIUA 225-2021. Credit authorship contribution statement: N. Inostroza-Pino: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Supervision. C. Heyser: Methodology, Investigation, Visualization, Formal analysis, Writing – original draft. D. MacLeod-Carey: Methodology, Writing – review & editing, Writing – original draft, Resources. D.Mardones: Resources, Formal analysis, Writing – review & editing, Writing – original draft, Supervision. J. Ge: Writing – review & editing, Writing – original draft, Resources. C. Espinoza: Writing – original draft, Resources.
References
- Allamandola, L. J., & Hudgins, D. M. 2003, in Solid State Astrochemistry, eds. V. Pirronello, J. Krelowski, & G. Manicò, 120, 251 [Google Scholar]
- Andrade, D., Boechat-Roberty, H., Martinez, R., et al. 2009, Surf. Sci., 603, 1190 [Google Scholar]
- Basalgète, R., Dupuy, R., Féraud, G., et al. 2021, A&A, 647, A36 [EDP Sciences] [Google Scholar]
- Bertin, M., Romanzin, C., Doronin, M., et al. 2016, ApJ, 817, L12 [Google Scholar]
- Bolton, K., Hase, W. L., & Peslherbe, G. H. 1998, Modern Methods for Multidimensional Dynamics Computations in Chemistry, 143 [Google Scholar]
- Boogert, A. A., Gerakines, P. A., & Whittet, D. C. 2015, ARA&A, 53, 541 [Google Scholar]
- Boyd, S. L., & Boyd, R. J. 2007, J. Chem. Theor. Comput., 3, 54 [Google Scholar]
- Burkhardt, A. M., Shingledecker, C. N., Le Gal, R., et al. 2019, ApJ, 881, 32 [Google Scholar]
- Carrascosa, H., Cruz-Díaz, G. A., Muñoz Caro, G. M., Dartois, E., & Chen, Y. J. 2020, MNRAS, 493, 821 [Google Scholar]
- Cazaux, S., Minissale, M., Dulieu, F., & Hocuk, S. 2016, A&A, 585, A55 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Charnley, S. B. 1995, Ap&SS, 224, 251 [Google Scholar]
- Chuang, K. J., Fedoseev, G., Qasim, D., et al. 2017, MNRAS, 467, 2552 [NASA ADS] [Google Scholar]
- Dartois, E., Schutte, W., Geballe, T., et al. 1999, A&A, 342, L32 [Google Scholar]
- Dartois, E., Chabot, M., Id Barkach, T., et al. 2018, A&A, 618, A173 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Dartois, E., Chabot, M., Bacmann, A., et al. 2020, A&A, 634, A103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Dulieu, F., Congiu, E., Noble, J., et al. 2013, Sci. Rep., 3, 1338 [NASA ADS] [CrossRef] [Google Scholar]
- Dupuy, R., Bertin, M., Féraud, G., et al. 2018, Nat. Astron., 2, 796 [Google Scholar]
- Ehrenfreund, P., & Charnley, S. B. 2000, ARA&A, 38, 427 [Google Scholar]
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. 2009, Gaussian09 Revision A.1 (Wallingford, CT: Gaussian Inc.) [Google Scholar]
- Garrod, R. T., & Herbst, E. 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., Wakelam, V., & Herbst, E. 2007, A&A, 467, 1103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., Widicus Weaver, S. L., & Herbst, E. 2008, ApJ, 682, 283 [NASA ADS] [CrossRef] [Google Scholar]
- Geppert, W. D., Hamberg, M., Thomas, R. D., et al. 2006, Faraday Discuss., 133, 177 [Google Scholar]
- Gerin, M., Neufeld, D. A., & Goicoechea, J. R. 2016, ARA&A, 54, 181 [NASA ADS] [CrossRef] [Google Scholar]
- Gibb, E. L., Whittet, D. C. B., Boogert, A. C. A., & Tielens, A. G. G. M. 2004, ApJS, 151, 35 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- González-Alfonso, E., Fischer, J., Bruderer, S., et al. 2013, A&A, 550, A25 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- González-Alfonso, E., Fischer, J., Bruderer, S., et al. 2018, ApJ, 857, 66 [NASA ADS] [CrossRef] [Google Scholar]
- Guzmán, V., Pety, J., Goicoechea, J. R., Gerin, M., & Roueff, E. 2011, A&A, 534, A49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Guzmán, V., Goicoechea, J. R., Pety, J., et al. 2013, A&A, 560, A73 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Helgaker, T., Uggerud, E., & Jensen, H. J. A. 1990, Chem. Phys. Lett., 173, 145 [Google Scholar]
- Hiraoka, K., Ohashi, N., Kihara, Y., et al. 1994, Chem. Phys. Lett., 229, 408 [Google Scholar]
- Hiraoka, K., Sato, T., Sato, S., et al. 2002, ApJ, 577, 265 [Google Scholar]
- Hollis, J. M., Lovas, F. J., & Jewell, P. R. 2000, ApJ, 540, L107 [NASA ADS] [CrossRef] [Google Scholar]
- Inostroza-Pino, N., Mardones, D., Cernicharo, J., et al. 2019, A&A, 629, A28 [CrossRef] [EDP Sciences] [Google Scholar]
- Inostroza-Pino, N., Mardones, D., Ge, J. J. X., & MacLeod-Carey, D. 2020, A&A, 641, A14 [EDP Sciences] [Google Scholar]
- Jin, M., & Garrod, R. T. 2020, ApJ, 249, 26 [Google Scholar]
- Kent, D. R., Widicus, S. L., Blake, G. A., & Goddard, W. A. 2003, J. Chem. Phys., 119, 5117 [Google Scholar]
- Kouchi, A., Yamamoto, T., Kozasa, T., Kuroda, T., & Greenberg, J. M. 1994, A&A, 290, 1009 [Google Scholar]
- Ligterink, N. F. W., Coutens, A., Kofman, V., et al. 2017, MNRAS, 469, 2219 [NASA ADS] [CrossRef] [Google Scholar]
- Martinez, O. J., Betts, N. B., Villano, S. M., et al. 2008, ApJ, 686, 1486 [Google Scholar]
- McBride, E. J., Millar, T. J., & Kohanoff, J. J. 2013, J. Phys. Chem. A, 117, 9666, pMID: 23662836 [Google Scholar]
- McBride, E. J., Millar, T. J., & Kohanoff, J. J. 2014, J. Phys. Chem. A, 118, 6991, pMID: 25090372 [Google Scholar]
- McGuire, B. A., Shingledecker, C. N., Willis, E. R., et al. 2017, ApJ, 851, L46 [Google Scholar]
- Minier, V., Burton, M. G., Hill, T., et al. 2005, A&A, 429, 945 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Minissale, M., Dulieu, F., Cazaux, S., & Hocuk, S. 2016, A&A, 585, A24 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Müller, H. S. P., Black, J. H., et al. 2016, A&A, 595, A128 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Potapov, A., Jäger, C., Henning, T., Jonusas, M., & Krim, L. 2017, ApJ, 846, 131 [Google Scholar]
- Shingledecker, C. N., & Herbst, E. 2018, Phys. Chem. Chem. Phys., 20, 5359 [Google Scholar]
- Sjouwerman, L. O., Murray, C. E., Pihlström, Y. M., Fish, V. L., & Araya, E. D. 2010, ApJ, 724, L158 [Google Scholar]
- Snyder, L. E., Buhl, D., Zuckerman, B., & Palmer, P. 1969, Phys. Rev. Lett., 22, 679 [Google Scholar]
- Taban, I. M., Schutte, W. A., Pontoppidan, K. M., & van Dishoeck, E. F. 2003, A&A, 399, 169 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Uggerud, E., & Helgaker, T. 1992, J. Am. Chem. Soc., 114, 4265 [Google Scholar]
- Vaghjiani, G. L., & Ravishankara, A. R. 1989, J. Phys. Chem., 93, 1948 [Google Scholar]
- Van de Sande, M., Walsh, C., Mangan, T. P., & Decin, L. 2019, MNRAS, 490, 2023 [Google Scholar]
- Vichietti, R. M., Spada, R. F. K., da Silva, A. B. F., Machado, F. B. C., & Haiduke, R. L. A. 2016, ApJS, 225, 2 [Google Scholar]
- Walsh, C., Loomis, R. A., Öberg, K. I., et al. 2016, ApJ, 823, L10 [Google Scholar]
- Watanabe, N., & Kouchi, A. 2002, ApJ, 571, L173 [Google Scholar]
- Watanabe, N., Nagaoka, A., Shiraki, T., & Kouchi, A. 2004, ApJ, 616, 638 [Google Scholar]
- Woods, P. M., Slater, B., Raza, Z., et al. 2013, ApJ, 777, 90 [Google Scholar]
- Woon, D. E. 2002, ApJ, 569, 541 [Google Scholar]
- Woon, D. E. 2011, ApJ, 728, 44 [Google Scholar]
- Öberg, K. I., Garrod, R. T., van Dishoeck, E. F., & Linnartz, H. 2009, A&A, 504, 891 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
All Tables
Products obtained from (CH3OH)10-ice mantle in collision with an OH+ projectile with a kinetic energy impact of 10–22 eV.
All Figures
 |
Fig. 1. Global chemical network using an OH+ projectile. For a complete list of reactions and products, see Table 1. The precursors are in orange, the intermediates are in gray, and the formaldehyde and the hitherto isomer are outlined in red. |
| In the text | |
 |
Fig. 2. Diagram illustrating transient hydrogen bonds stabilized by two or more methanol molecules during the BOMD simulation. Five methanol molecules stabilize an H+; in the bottom right-hand corner, a formaldehyde molecule is presented. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.