| Issue |
A&A
Volume 688, August 2024
|
|
|---|---|---|
| Article Number | A140 | |
| Number of page(s) | 5 | |
| Section | Astrophysical processes | |
| DOI | https://doi.org/10.1051/0004-6361/202346642 | |
| Published online | 12 August 2024 | |
Formation pathways of formic acid (HCOOH) in regions with methanol ices⋆
1
Universidad Autónoma de Chile, Facultad de Ingeniería, Instituto de Ciencias Aplicadas, Núcleo de Astroquímica & Astrofísica, Av. Pedro de Valdivia 425, Providencia, Santiago, Chile
e-mail: natalia.inostroza@uautonoma.cl
2
Universidad de Chile, Facultad de Ciencias Físicas y Matemáticas, Departamento de Astronomía, Camino el observatorio 1515, Las Condes, Santiago, Chile
3
Xinjiang Astronomical Observatory, Chinese Academy of Sciences, Urumqi, 830011, China.Key Laboratory of Radio Astronomy, Chinese Academy of Sciences, Urumqi 830011, China
Received:
12
April
2023
Accepted:
25
April
2024
We modeled the collisions between OH+ projectiles with kinetic energies ranging from 10 to 22 eV and an amorphous cold (CH3OH)10 substrate using Born-Oppenheimer molecular dynamics (BOMD) simulations. We conducted the simulations for a collision time of 400 femtoseconds (fs), during which we followed multiple bond-forming and breaking reactions. Here, we report four new pathways for the formation of formic acid HCOOH. We find new precursors such as CH3(OH)2+, HC(OH)2+, CH2OH+, and CH2(OH)2, which are essential in these pathways for the formation of formic acid. The methanodiol CH2(OH)2 and hydroxymethyl CH2OH+ cations have previously been identified as key precursors of formaldehyde. These pathways suggest new ways to form formic acid in methanol ice mantles on dust grains, offering alternative mechanisms leading to the formation of complex organic molecules (COMs) in space.
Key words: astrochemistry / accretion / accretion disks / molecular processes / ISM: abundances / ISM: molecules
Movie is available at https://www.aanda.org.
© The Authors 2024
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1. Introduction
In the past decade, there has been a significant increase in the number of discoveries of interstellar complex organic molecules (COMs). More than 300 molecules, including aldehydes, alcohols, acids, amines, and carboxamides, have been detected in the interstellar medium (ISM) or circumstellar shells (Müller et al. 2001, and see the Cologne Database for Molecular Spectroscopy, CDMS1). These detections represent the fundamental functional groups that are essential for initiating the creation of prebiotic molecules and ribonucleic acid (RNA) (Guélin & Cernicharo 2022; Chaabouni et al. 2020; Redondo et al. 2015; Herbst & Garrod 2022); organic acids have been detected in the gas phase in low amounts relative to the H2O detected in various sources. Although they are “rare”, these acids are necessary for the formation of more complex molecules (Ehrenfreund & Charnley 2000). Formic acid, HCOOH, is a particularly important organic acid as it is involved in atmospheric environments, in reactions inside the human body, and in the synthesis of interstellar COMs (Cao et al. 2014; Li et al. 2000).
Formic acid HCOOH has been detected in different regions, including comets (Bennett et al. 2010), star formation regions (Ikeda et al. 2001), (Bisschop et al. 2007), low-mass protostars (Cazaux et al. 2003), hot cores (Liu et al. 2001, 2002) Garrod (2006), Galactic center molecular clouds (Zuckerman et al. 1971), (Requena-Torres et al. 2006), and cold dark clouds (Irvine et al. 1990; Turner et al. 1999; Requena-Torres et al. 2007). HCOOH has fewer than six atoms, and so it is not technically a COM based on the common astronomy classification (Herbst & Garrod 2022; Belloche et al. 2013). Pure gas-phase synthesis is insufficient to account for the measured abundances of HCOOH (Charnley et al. 1992; Mehringer & Snyder 1996). As such, an unresolved astrochemical question is how exactly molecular organic compounds are formed, and how gas detection is linked with solid-phase formation to better reproduce the observed abundances (McGuire et al. 2017), (Herbst & Van Dishoeck 2009). Grain surface chemistry has been suggested as a possible mechanism for producing COMs (Tielens & Hagen 1982; Sandford & Allamandola 1990; Charnley 1995), and this idea is supported by the detection of HCOOH in interstellar ices and meteorites (Keane et al. 2001; Briscoe & Moore 1993) including new James Webb Space Telescope (JWST) detections by Rocha et al. (2024).
It is believed that HCOOH is formed efficiently through surface reactions on dust grains, and is then released into the gas phase where it can be detected. To confirm these reactions, a series of experiments was conducted by Hudson & Moore (1999), showing that HCO, H2CO, HCOOH, CH3OH, CH4, and CO2 could be detected after water-ice and carbon monoxide were bombarded with protons with a kinetic energy of 0.8 MeV. Hudson & Moore (1999) proposed the simplest route for the synthesis of formic acid, which involves the reaction between HCO and OH radicals. More recently, Abplanalp & Kaiser in 2019 detected a variety of molecules through the interaction of ionizing radiation with ice deposited on interstellar dust particles at low temperatures (10 K). Some of these icy components include H2O, CH3OH, CO, CO2, CH4, and NH3. At higher temperatures, such as those found in hot cores and corinos, organic compounds can undergo transformations more easily. However, our understanding of the full extent of the chemical complexity that arises from individual ice components remains limited, as noted in a study of COMs in hot cores by Garrod (2006). Alternative HCOOH formation mechanisms include UV irradiation (Bertin et al. 2023), chemical desorption (Cazaux et al. 2016; Oba et al. 2018), cosmic ray (CR)-induced reactions (Mainitz et al. 2016), and secondary UV photons (Bertin et al. 2016; Cruz-Diaz et al. 2016). According to Goumans et al. (2008), another pathway involving the reaction of hydroxyl radicals with carbon monoxide on carbonaceous surfaces may be relevant. The authors proposed that formic acid is obtained as a result of the stabilization of the HOCO intermediate. However, instead of hydrogenation to form HCOOH, the HOCO intermediate can undergo dissociation to form CO2, as suggested by Ioppolo et al. (2011) and their research on surface reactions. Moreover, in a study by Bennett et al. (2010), the formation of HCOOH can be achieved efficiently via a reaction involving an ice mixture of H2O and CO exposed to highly energetic electrons generated from CRs. The reaction is carried out via unimolecular decomposition of a H2O molecule forming both the hydroxyl radical (OH) and atomic hydrogen (H). The authors found that approximately 75% of the total formic acid studied follows a pathway involving the addition of suprathermal hydrogen atoms to carbon monoxide, forming the formyl radical (HCO). This radical then reacts via a secondary process with another hydroxyl radical to form formic acid HCOOH. The remaining 25% is formed through the hydroxyformyl radical HOCO reacting with atomic hydrogen to yield formic acid (HCOOH). In this pathway, the hydroxyl radicals (OH) reacts with carbon monoxide, producing hydroxy formyl radicals (HO-CO), which then recombine with neighboring hydrogen atoms to produce formic acid HCOOH.
Several studies (Ioppolo et al. 2010; Qasim et al. 2019; Woon 2011) have demonstrated the formation of HCOOH under “nonenergetic” conditions. Ioppolo et al. (2010), examined the reaction pathway involving the HOCO complex, because the pathway via the reaction of HCO + OH radicals was considered inefficient at lower temperatures (Ioppolo et al. 2010). Similarly, Qasim et al. (2019) validate the idea that H + HOCO is the dominant route for HCOOH ice formation in CO-rich ice mixtures. Experiments using purely methanol-containing ices (Öberg et al. 2009; Basalgète et al. 2021; Torres-Díaz et al. 2023) have been performed to explain the COM abundances observed in cold regions. Öberg et al. (2009) was able to confirm the production of HCOOH starting from pure CH3OH ices through UV-photon irradiation. Torres-Díaz et al. (2023), irradiated pure CH3OH ices at 23 K with 505 eV electrons, and confirmed the detection of ice components such as CO, CO2, CH3OH, CH4, H2O, H2CO, and C2H6, as well as other less abundant but more complex organic products.
Theoretical work by Woon (2021) highlights that certain gas-phase cations, such as HCO+, OH+, CH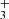 , and C+ can interact with icy grain mantles to create barrier-less ion–ice reactions that exhibit energies comparable to the gas-phase formation processes. Moreover, Woon (2011) demonstrated that HCOOH can be formed by a nonenergetic process using an amorphous cluster of water under interaction with abundant cations. The author of this latter study focus on the process that led to three well-known astronomical molecules: HCOOH, CH3OH, and CO2. Experimental confirmation of the efficacy of cation-ice reactions has been provided (Nakai et al. 2023) in a study of CH
, and C+ can interact with icy grain mantles to create barrier-less ion–ice reactions that exhibit energies comparable to the gas-phase formation processes. Moreover, Woon (2011) demonstrated that HCOOH can be formed by a nonenergetic process using an amorphous cluster of water under interaction with abundant cations. The author of this latter study focus on the process that led to three well-known astronomical molecules: HCOOH, CH3OH, and CO2. Experimental confirmation of the efficacy of cation-ice reactions has been provided (Nakai et al. 2023) in a study of CH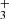 cation reactions with water to form CH3OH. This phenomenon was anticipated in a theoretical study by Woon (2011). Thus, experimental validation aligns with theoretical predictions and underscores the importance of cation–ice studies. Furthermore, the recent identification of CH
cation reactions with water to form CH3OH. This phenomenon was anticipated in a theoretical study by Woon (2011). Thus, experimental validation aligns with theoretical predictions and underscores the importance of cation–ice studies. Furthermore, the recent identification of CH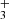 in a protoplanetary disk (Berné et al. 2023) emphasizes the practical significance and relevance of both theoretical and experimental investigation into cation–ice interactions.
in a protoplanetary disk (Berné et al. 2023) emphasizes the practical significance and relevance of both theoretical and experimental investigation into cation–ice interactions.
To gain a better understanding of these processes, we performed a series of simulations using a hypothetical molecular mantle composed of methanol CH3OH (Inostroza-Pino et al. 2020, 2021; Heyser Valencia & Inostroza-Pino 2022). Methanol is widely present in astronomical sources (Spezzano et al. 2022; McClure et al. 2023) and is highly abundant in the ISM (Herbst & Van Dishoeck 2009). It serves as a key precursor for the creation of other COMs as it provides the foundational materials for their formation (Mathew et al. 2022). In the present paper, we focus on HCOOH formation routes through organic molecules in dust ice mantles. We selected as target material a cluster of ten methanol molecules (CH3OH)10, which is bombarded with OH+ projectiles using a Born-Oppenheimer molecular dynamics (BOMD) approach. Our study is not designed to replicate a realistic interstellar ice scenario, but rather to identify possible HCOOH formation pathways within interstellar ices.
2. Computational methods
In this study, we follow on from our previous work (Inostroza-Pino et al. 2020; Heyser Valencia & Inostroza-Pino 2022; Inostroza-Pino et al. 2021, 2019), investigating the OH+ impact on a methanol ice mantle cluster. Impacts with kinetic energies of below 10 eV lead to the adsorption of OH+ onto the surface, while impacts with kinetic energies exceeding 22 eV result in the evaporation and destruction of the methanol ice mantle. Therefore, we focus on a range of projectile kinetic energies (10–22 eV) where the chemistry is rich. We modeled each collision with the OH+ projectile coming from 24 different spherical directions. All calculations were performed using Gaussian 09 (Frisch et al. 2009). An example of our model is shown in Figure 1.
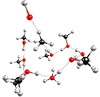 |
Fig. 1. Cluster composed of methanol (CH3OH)10 impacted by OH+ projectiles. |
We used BOMD simulations to investigate a cluster formed by ten units of methanol (CH3OH)10. This cluster was designed to mimic a dust ice mantle and was subjected to impact with OH+. We employed the hybrid functional of Head-Gordon, ωB97XD (Helgaker et al. 1990; Uggerud & Helgaker 1992; Bolton et al. 1998) with the Pople 6-31+G(d,p) basis set (Rassolov et al. 2001) level of theory to perform the geometry optimization. This allowed us to obtain the most stable structure of the (CH3OH)10 cluster, which we then used to simulate the impact of OH+ Boyd & Boyd (2007). We performed our simulations under microcanonical ensemble (NVE), with fixed total energy. Thus, the kinetic impact energy is distributed within the cluster, which allows the formation of intermediate species, such as those listed in Table 1 as reactions 1–4.
Products obtained from (CH3OH)10-ice-mantle in collision with a OH+ projectile with a kinetic energy impact of 10–22 eV.
During the simulation, we observed bond-forming and bond-breaking processes of the reaction of methanol impacted by the OH+ projectile. We selected initial kinetic energies for the OH+ ranging from 10 to 22 eV. The trajectories started with the oxygen atom of OH+ facing the cluster, and the OH+ projectile aligned with the center of mass of the (CH3OH)10-ice-mantle (as shown in Figure 1; additionally, the BO molecular dynamics simulation movie is available onilne). We followed the interactions for 400 femtoseconds (fs) with a time step of 0.5 fs. The chemical outcomes and the formation mechanism depend on the kinetic energy of the OH+ projectile and the impact position of the collision.
3. Results
In Table 1 we only list the outcomes produced after the reaction of (CH3OH)10 + OH+ that yield formic acid in primary, secondary, or tertiary processes. The table includes only the main products generated by the reaction under study. We do not include methanol molecules that are not part of the final products. All listed pathways satisfy mass conservation. We discuss various mechanisms that can proceed from the target (CH3OH)10-ice-mantle) + OH+. In the following paragraphs, we explain how the key organic precursors, such as CH3(OH)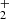 , CH2OH+ and CH2(OH)2 lead to the formation of (HCOOH). The incoming energy makes chemical reactions with higher potential energy barriers accessible. All the reactions we list have energy barriers of less than 100 kcal, accessible at transient temperatures of less than 500 K using equipartition theorem (Pantaleone et al. 2020).
, CH2OH+ and CH2(OH)2 lead to the formation of (HCOOH). The incoming energy makes chemical reactions with higher potential energy barriers accessible. All the reactions we list have energy barriers of less than 100 kcal, accessible at transient temperatures of less than 500 K using equipartition theorem (Pantaleone et al. 2020).
3.1. Formation pathways of HCOOH
Reaction pathway 1 starts with the OH+ approaching one of CH3OH molecules, leading to the formation of a carbocation, which is a labile reactive pentacoordinate carbon CH3(OH)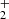 . In the next step (1.2), this highly reactive species undergoes a secondary process to produce a key intermediate, HC(OH)
. In the next step (1.2), this highly reactive species undergoes a secondary process to produce a key intermediate, HC(OH)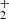 , which loses molecular hydrogen to form the stabilized cation; this step has an energy barrier of 88 Kcal. In step 1.3, the latter proton is stabilized by a hydrogen bridge with a second methanol molecule CH3OH
, which loses molecular hydrogen to form the stabilized cation; this step has an energy barrier of 88 Kcal. In step 1.3, the latter proton is stabilized by a hydrogen bridge with a second methanol molecule CH3OH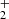 , depicted in Table 1. This pathway is exothermic with a value above −110 kcal/mol.
, depicted in Table 1. This pathway is exothermic with a value above −110 kcal/mol.
Pathway 2 also involves the formation of the same pentacoordinate intermediate, CH3(OH)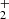 . In step 2.2, the HC(OH)
. In step 2.2, the HC(OH)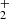 is formed with an energy barrier of about 78 Kcal, which is a key factor in step 2.3, a common reduction process from alcohol to acid function. An additional stabilization process occurs in step 2.4, where dehydration of the HCOOH molecule forms CO + H2O. This pathway is also exothermic, with a value of −70 kcal/mol.
is formed with an energy barrier of about 78 Kcal, which is a key factor in step 2.3, a common reduction process from alcohol to acid function. An additional stabilization process occurs in step 2.4, where dehydration of the HCOOH molecule forms CO + H2O. This pathway is also exothermic, with a value of −70 kcal/mol.
The methanodiol CH2(OH)2 and hydroxymethyl CH2OH+ cation are key precursors of formaldehyde, as shown by Heyser Valencia & Inostroza-Pino (2022). They are also precursors in the formation of HCOOH, as described in pathways 3-4. Initially, a methanol molecule is impacted by OH+, leading to the elimination of the proton to form water as a pseudo-product, plus the CH2OH+ cation. The system is then reorganized, resulting in the elimination of molecular hydrogen H2 and the reduction of alcohol to formic acid HCOOH.
In pathway 4, diol formation CH2(OH)2 and proton elimination occur. The proton then reacts with other molecules to form molecular hydrogen followed by the formation of the key intermediate HC(OH)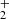 cation. This reactive species then undergoes tertiary processes to stabilize itself through hydrogen elimination and carboxylic bond C=O formation. In all cases exposed in Table 1, the precursors CH3(OH)
cation. This reactive species then undergoes tertiary processes to stabilize itself through hydrogen elimination and carboxylic bond C=O formation. In all cases exposed in Table 1, the precursors CH3(OH)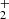 , CH2OH+, and CH2(OH)2 undergo subsequent secondary processes to form the intermediate HC(OH)
, CH2OH+, and CH2(OH)2 undergo subsequent secondary processes to form the intermediate HC(OH)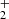 with an energy barrier of about 78 Kcal; this species enables tertiary processes, leading to the generation of stable products. The pathways 3 and 4 are exothermic by −151 and −157 kcal/mol, respectively. These studies contribute to the formation of COMs and provide evidence of likely intermediates that have not yet been successfully detected in laboratory or astronomical searches (Fig. 2).
with an energy barrier of about 78 Kcal; this species enables tertiary processes, leading to the generation of stable products. The pathways 3 and 4 are exothermic by −151 and −157 kcal/mol, respectively. These studies contribute to the formation of COMs and provide evidence of likely intermediates that have not yet been successfully detected in laboratory or astronomical searches (Fig. 2).
 |
Fig. 2. Chemical network obtained using OH+ projectiles. Intermediates are in gray color. Each chemical pathway is indicated by a colored line (pathway 1: blue; pathway 2: red; pathway 3: green; and pathway 4: orange). |
3.2. Astrophysical implications
HCOOH has been detected in high- and low-mass star-forming regions (Liu et al. 2002; Lefloch et al. 2017). This organic acid has also been detected in the protoplanetary disk surrounding the nearby, young, solar-type star TW Hydrae (Favre et al. 2018). The cis-trans conformers of carbonic acid (HOCOOH) have been observed towards the Galactic center molecular cloud G+0.693–0.027 (Sanz-Novo et al. 2023). In addition, CH3OH is abundant in protostellar outflows and has been detected in protoplanetary disks (Walsh et al. 2016). Thus, rich organic chemistry is important in the ISM from early clouds and star formation to late planetary formation processes (Herbst & Van Dishoeck 2009). The present findings could be relevant in all of these ISM structures.
From our results, we are now able to identify chemical precursors and conformers that have been elusive to radio astronomy searches. This may be particularly relevant in regions with moderately high kinetic temperatures, such as Galactic center molecular clouds, or in regions with higher energy processes, including radiation or shock fronts. Thus, identifying the formation process, reactions, and mechanisms that lead to the formation of HCOOH can widen our understanding of the chemistry in a variety of ISM environments.
4. Conclusions
We modeled the collisions between OH+ projectiles with kinetic energies ranging from 10 to 22 eV and an amorphous cold (CH3OH)10 substrate using Gaussian 09 Born-Oppenheimer molecular dynamics (BOMD) simulations. The simulations permit a comprehensive analysis of bond formation, breaking, and molecular rearrangements during and after impact. We identify four new possible pathways of HCOOH formation.
We find labile intermediate species CH3(OH)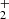 , CH2OH+, and CH2(OH)2, which have not previously been detected in laboratory spectroscopy. The intermediaries may lead to the formation of HCOOH due to the catalytic effects of methanol ice grains on dust grain mantles (Kerkeni et al. 2022). Contrary to earlier suggestions that HCOOH is formed through dissociative recombination of protonated formic acid HCOOH
, CH2OH+, and CH2(OH)2, which have not previously been detected in laboratory spectroscopy. The intermediaries may lead to the formation of HCOOH due to the catalytic effects of methanol ice grains on dust grain mantles (Kerkeni et al. 2022). Contrary to earlier suggestions that HCOOH is formed through dissociative recombination of protonated formic acid HCOOH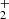 (Leung et al. 1984), our simulations reveal that the formation of HCOOH in the ISM might also occur via pathways that generate HC(OH)
(Leung et al. 1984), our simulations reveal that the formation of HCOOH in the ISM might also occur via pathways that generate HC(OH)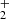 as the key intermediate via secondary processes.
as the key intermediate via secondary processes.
We conclude that OH+ projectiles on methanol ice mantles (a carbonaceous surface) could be important in the formation of COMs, in agreement with previous work by Hudson & Moore (1999) and Goumans et al. (2008). Further research is required to determine the precise physical and chemical parameters of the reactions of these new intermediate species leading to the possible formation of HCOOH on icy dust surfaces.
Movies
Movie 1 (Picture1(2)) Access here
Acknowledgments
This research was supported by FONDECYT Chile Grant No̴ 1241193, PCI-ANID International PCI-ANID Grant REDES190113. DM acknowledges support from ANID project Basal AFB170002.
References
- Abplanalp, M. J., & Kaiser, R. I. 2019, Phys. Chem. Chem. Phys., 21, 16949 [NASA ADS] [CrossRef] [Google Scholar]
- Basalgète, R., Dupuy, R., Féraud, G., et al. 2021, A&A, 647, A35 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Belloche, A., Müller, H. S. P., Menten, K. M., Schilke, P., & Comito, C. 2013, A&A, 559, A47 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bennett, C. J., Hama, T., Kim, Y. S., Kawasaki, M., & Kaiser, R. I. 2010, ApJ, 727, 27 [Google Scholar]
- Berné, O., Martin-Drumel, M.-A., Schroetter, I., et al. 2023, Nature, 621, 56 [CrossRef] [Google Scholar]
- Bertin, M., Romanzin, C., Doronin, M., et al. 2016, ApJ, 817, L12 [NASA ADS] [CrossRef] [Google Scholar]
- Bertin, M., Basalgéte, R., Ocaña, A. J., et al. 2023, Faraday Discuss., 245, 488 [NASA ADS] [CrossRef] [Google Scholar]
- Bisschop, S. E., Jørgensen, J. K., van Dishoeck, E. F., & de Wachter, E. B. M. 2007, A&A, 465, 913 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bolton, K., Hase, W. L., & Peslherbe, G. H. 1998, Mod. Methods Multidimen. Dyn. Comput. Chem., 143 [Google Scholar]
- Boyd, S. L., & Boyd, R. J. 2007, J. Chem. Theory Comput., 3, 54 [Google Scholar]
- Briscoe, J. F., & Moore, C. B. 1993, Meteorit. Planet. Sci., 28, 330 [Google Scholar]
- Cao, Q., Berski, S., Latajka, Z., Räsänen, M., & Khriachtchev, L. 2014, PCCP (Incorporating Faraday Transactions), 16, 5993 [NASA ADS] [Google Scholar]
- Cazaux, S., Tielens, A. G. G. M., Ceccarelli, C., et al. 2003, ApJ, 593, L51 [CrossRef] [Google Scholar]
- Cazaux, S., Minissale, M., Dulieu, F., & Hocuk, S. 2016, A&A, 585, A55 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Chaabouni, H., Baouche, S., Diana, S., & Minissale, M. 2020, A&A, 636, A4 [Google Scholar]
- Charnley, S. B. 1995, Ap&SS, 224, 439 [NASA ADS] [CrossRef] [Google Scholar]
- Charnley, S. B., Tielens, A. G. G. M., & Millar, T. J. 1992, ApJ, 399, L71 [Google Scholar]
- Cruz-Diaz, G. A., Martín-Doménech, R., Caro, G. M., & Chen, Y.-J. 2016, A&A, 592, A68 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ehrenfreund, P., & Charnley, S. B. 2000, ARA&A, 38, 427 [Google Scholar]
- Favre, C., Fedele, D., Semenov, D., et al. 2018, ApJ, 862, L2 [NASA ADS] [CrossRef] [Google Scholar]
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. 2009, Gaussian09 Revision A.1 (Wallingford CT: Gaussian Inc.) [Google Scholar]
- Garrod, T. R., & Herbst, E., 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Goumans, T. P. M., Uppal, M. A., & Brown, W. A. 2008, MNRAS, 384, 1158 [NASA ADS] [CrossRef] [Google Scholar]
- Guélin, M., & Cernicharo, J. 2022, Front. Astron. Space Sci., 9, 787567 [CrossRef] [Google Scholar]
- Helgaker, T., Uggerud, E., & Jensen, H. J. A. 1990, Chem. Phys. Lett, 173, 145 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & Garrod, R. T. 2022, Front. Astron. Space Sci., 8 [Google Scholar]
- Herbst, E., & Van Dishoeck, E. F. 2009, ARA&A, 47, 427 [NASA ADS] [CrossRef] [Google Scholar]
- Heyser Valencia, C., & Inostroza-Pino, N. 2022, A&A, 664, A85 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hudson, R., & Moore, M. 1999, Icarus, 140, 451 [NASA ADS] [CrossRef] [Google Scholar]
- Ikeda, M., Ohishi, M., Nummelin, A., et al. 2001, ApJ, 560, 792 [Google Scholar]
- Inostroza-Pino, N., Mardones, D., Cernicharo, J., et al. 2019, A&A, 629, A28 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Inostroza-Pino, N., Mardones, D., Ge, J., & MacLeod-Carey, D. 2020, A&A, 641, A14 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Inostroza-Pino, N., MacLeod-Carey, D., Heyser, C., et al. 2021, A&A, 650, A169 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ioppolo, S., Cuppen, H. M., van Dishoeck, E. F., & Linnartz, H. 2010, MNRAS, 410, 1089 [Google Scholar]
- Ioppolo, S., Cuppen, H., & Linnartz, H. 2011, Rendiconti Lincei, 22, 211 [CrossRef] [Google Scholar]
- Irvine, W. M., Friberg, P., Kaifu, N., et al. 1990, A&A, 229, L9 [NASA ADS] [Google Scholar]
- Keane, J. V., Tielens, A. G. G. M., Boogert, A. C. A., Schutte, W. A., & Whittet, D. C. B. 2001, A&A, 376, 254 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Kerkeni, B., Duflot, D., & Feautrier, N. 2022, Front. astron. space Sci., 9 [Google Scholar]
- Lefloch, B., Ceccarelli, C., Codella, C., et al. 2017, MNRAS, 469, L73 [Google Scholar]
- Leung, C. M., Herbst, E., & Huebner, W. F. 1984, ApJS, 56, 231 [CrossRef] [Google Scholar]
- Li, Q., Osborne, M. C., & Smith, I. W. M. 2000, Int. J. Chem. Kinet., 32, 85 [CrossRef] [Google Scholar]
- Liu, S.-Y., Mehringer, D. M., & Snyder, L. E. 2001, ApJ, 552, 654 [NASA ADS] [CrossRef] [Google Scholar]
- Liu, S.-Y., Girart, J. M., Remijan, A., & Snyder, L. E. 2002, ApJ, 576, 255 [NASA ADS] [CrossRef] [Google Scholar]
- Mainitz, M., Anders, C., & Urbassek, H. M. 2016, A&A, 592, A35 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Mathew, T., Esteves, P. M., & Prakash, G. K. S. 2022, Front. Astron. Space Sci., 9 [Google Scholar]
- McGuire, B. A., Shingledecker, C. N., Willis, E. R., et al. 2017, ApJ, 851, L46 [Google Scholar]
- McClure, M. K., Rocha, W. R. M., Pontoppidan, K. M., et al. 2023, Nat. Astron., 7, 431 [NASA ADS] [CrossRef] [Google Scholar]
- Mehringer, D. M., & Snyder, L. E. 1996, ApJ, 471, 897 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Thorwirth, S., Roth, D. A., & Winnewisser, G. 2001, A&A, 370, L49 [Google Scholar]
- Nakai, Y., Sameera, W. M. C., Furuya, K., et al. 2023, ApJ, 953, 162 [NASA ADS] [CrossRef] [Google Scholar]
- Oba, Y., Tomaru, T., Lamberts, T., Kouchi, A., & Watanabe, N. 2018, Nat. Astron., 2, 228 [NASA ADS] [CrossRef] [Google Scholar]
- Öberg, K. I., Garrod, R. T., van Dishoeck, E. F., & Linnartz, H. 2009, A&A, 504, 891 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pantaleone, S., Enrique-Romero, J., Ceccarelli, C., et al. 2020, ApJ, 897, 56 [NASA ADS] [CrossRef] [Google Scholar]
- Qasim, D., Lamberts, T., He, J., et al. 2019, A&A, 626, A118 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Rassolov, V. A., Ratner, M. A., Pople, J. A., Redfern, P. C., & Curtiss, L. A. 2001, J. Comput. Chem., 22, 976 [CrossRef] [Google Scholar]
- Redondo, P., Largo, A., & Barrientos, C. 2015, A&A, 579, A125 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Requena-Torres, M. A., Martín-Pintado, J., Rodríguez-Franco, A., et al. 2006, A&A, 455, 971 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Requena-Torres, M. A., Marcelino, N., Jiménez-Serra, I., et al. 2007, ApJ, 655, L37 [CrossRef] [Google Scholar]
- Rocha, W. R. M., van Dishoeck, E. F., Ressler, M. E., et al. 2024, A&A, 683, A124 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Sandford, S. A., & Allamandola, L. J. 1990, Icarus, 87, 188 [NASA ADS] [CrossRef] [Google Scholar]
- Sanz-Novo, M., Rivilla, V. M., Jiménez-Serra, I., et al. 2023, ApJ, 954, 3 [NASA ADS] [CrossRef] [Google Scholar]
- Spezzano, S., Fuente, A., Caselli, P., et al. 2022, A&A, 657, A10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Tielens, A. G. G. M., & Hagen, W. 1982, A&A, 114, 245 [Google Scholar]
- Torres-Díaz, D., Basalgète, R., Bertin, M., et al. 2023, ChemPhysChem, 24, e202200912 [CrossRef] [Google Scholar]
- Turner, B. E., Terzieva, R., & Herbst, E. 1999, ApJ, 518, 699 [NASA ADS] [CrossRef] [Google Scholar]
- Uggerud, E., & Helgaker, T. 1992, J. Am. Chem. Soc., 114, 4265 [Google Scholar]
- Walsh, C., Loomis, R. A., Öberg, K. I., et al. 2016, ApJ, 823, L10 [NASA ADS] [CrossRef] [Google Scholar]
- Woon, D. E. 2011, ApJ, 728, 44 [Google Scholar]
- Woon, D. E. 2021, Acc. Chem. Res., 54, 490 [Google Scholar]
- Zuckerman, B., Ball, J. A., & Gottlieb, C. A. 1971, ApJ, 163, L41 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Products obtained from (CH3OH)10-ice-mantle in collision with a OH+ projectile with a kinetic energy impact of 10–22 eV.
All Figures
 |
Fig. 1. Cluster composed of methanol (CH3OH)10 impacted by OH+ projectiles. |
| In the text | |
 |
Fig. 2. Chemical network obtained using OH+ projectiles. Intermediates are in gray color. Each chemical pathway is indicated by a colored line (pathway 1: blue; pathway 2: red; pathway 3: green; and pathway 4: orange). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


