Fig. 1
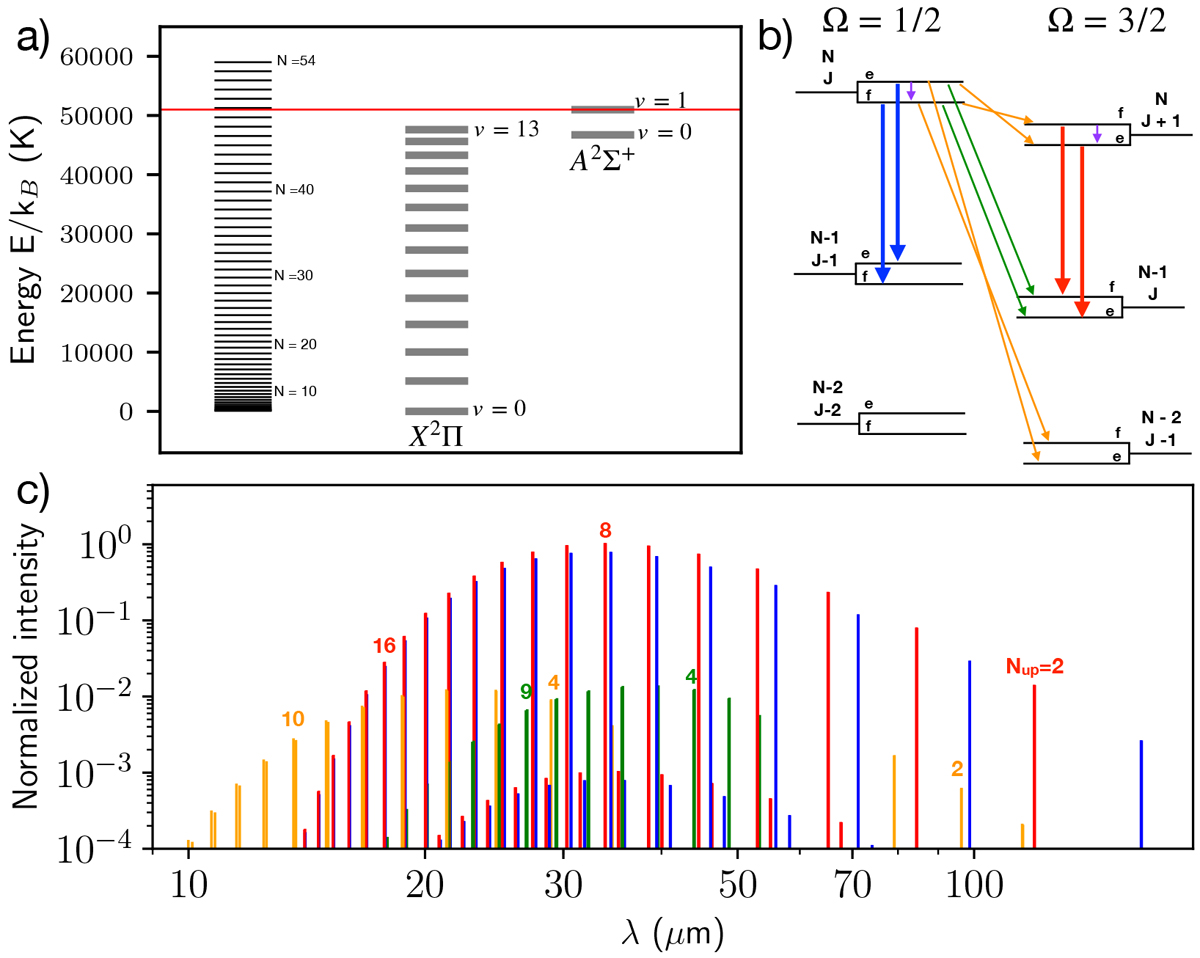
OH model adopted in this work. (a) X2Π and A2 Σ+ electronic levels split into vibrational levels and further split into rotational levels labeled by N (left ladder corresponding the OH(X2Π)(v = 0) state). The red line indicates the dissociation energy of OH(X2Π). (b) Structure of the rotational ladders of OH(X2Π) within a vibrational state that gives rise to mid- and far-IR lines. Each N level is split by the spin-orbit coupling and the Λ-doubling. The two spin-orbit states are labeled by the Ω quantum number and the Λ-doubling states are labeled by their ϵ = e∕f spectroscopic parity. Radiative transitions included in our model and emerging from the four N-levels are also depicted by arrows. There are three kinds of transitions: intra-ladder rotational transitions (blue and red arrows), cross-ladder transitions (green, with ΔN = − 1, and orange, with ΔN = 0, −2), and Λ-doubling transitions (purple). (c) Optically thin LTE spectrum of OH at TK = 750 K. The color code is the same as panel b) and is repeated in Figs. 4 and 9. The upper N level is indicated for some transitions. The Λ-doubling lines are too weak and at longer radio wavelengths to appear in this spectrum. Ro-vibrational lines are at shorter wavelengths than shown here (λ ≲ 2.7 μm).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


