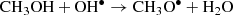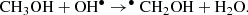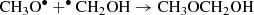| Issue |
A&A
Volume 641, September 2020
|
|
|---|---|---|
| Article Number | A14 | |
| Number of page(s) | 7 | |
| Section | Astrophysical processes | |
| DOI | https://doi.org/10.1051/0004-6361/202037904 | |
| Published online | 01 September 2020 | |
Formation pathways of complex organic molecules: OH• projectile colliding with methanol ice mantle (CH3OH)10
1
Universidad Autónoma de Chile, Facultad de Ingeniería, Núcleo de Astroquímica & Astrofísica, Av. Pedro de Valdivia 425, Providencia, Santiago, Chile
e-mail: natalia.inostroza@uautonoma.cl
2
Universidad de Chile, Facultad de Ciencias Físicas y Matemáticas, Departamento de Astronomía, Camino el observatorio 1515, Las condes, Santiago, Chile
Received:
6
March
2020
Accepted:
12
June
2020
In this article, we simulated the collisions of an OH• projectile impacting on a methanol cluster formed by ten units of methanol to mimic an ice mantle (CH3OH)10. The chemical processes occurring after the impact were studied through Born-Oppenheimer (ab-initio) molecular dynamics. We focus on collisions with initial kinetic impact energy of 10–22 eV, where the richest chemistry happens. We report the formation mechanisms of stable complex organic molecules (COMs) such as methoxymethanol CH3OCH2OH, formic acid HCOOH, formyl radical HCO, formaldehyde H2CO and its elusive HCOH isomer. We show that CH2(OH)2, •CH2OH or +CH2OH are key intermediates to generate H2CO and other COMs. We compare the outcomes using OH• with those using OH− projectiles. These processes are likely relevant to the production of COMs in astrophysical environments. We discuss its formation mechanism and the astrophysical implications of these chemical pathways in star-forming regions.
Key words: astrochemistry / molecular processes / ISM: molecules / ISM: atoms / dust / extinction
© ESO 2020
1. Introduction
The study of the chemistry in the universe has developed strongly since the discovery of CO in 1970, leading to the detection of more than 200 molecules in the interstellar medium (ISM) to date. The start of operations of the ALMA observatory opened the door to study the creation processes of complex organic molecules (COMs) with 6 or more atoms each in the universe, essential to understanding from prebiotic chemistry to the origin of life (Tielens 2013). COMs have been detected in many different astronomical sources (Herbst & Van Dishoeck 2009) including, for example, urea (CO(NH2)2) (Belloche et al. 2019; Inostroza & Senent 2012) and methoxymethanol CH3OCH2OH (McGuire et al. 2017). Even though the formation of COMs has been modeled since 1980s (Tielens & Hagen 1982), these models fail to reproduce the observed abundances in the cold interstellar environments. Laboratory experiments (Öberg et al. 2009) and models (Garrod & Herbst 2006) show that COMs can be formed in the solid state on icy grains, typically following atom-addition or UV-photon absorption processes. For example successive hydrogen addition has been proposed as a route to obtain reduced alcohols from aldehydes starting from a variety of known interstellar molecules: formaldehyde H2CO to methanol (CH3OH); ethenone CH2O to vinyl alcohol CH2CHOH; ethanal CH3CHO to ethanol CH3CH2OH; or glycolaldehyde HOCH2CHO to ethylene glycol HOCH2CH2OH. CH3OH can also be formed via CO hydrogenation (Garrod et al. 2007) or by the reaction of CH4 + OH under cold dense ISM conditions (Qasim et al. 2018). However, in order to reproduce the observed abundances, methanol must be formed on icy dust grains. Since CH3OH has a similar volatility to water ice (Brown & Bolina 2007); it is expected to be abundant on grains in the cold ISM. Methanol can be released from the grain mantles onto the gas phase at low temperatures via non-thermal desorption by energetic photons or particles or by exothermic chemical reactions (Öberg et al. 2011; Charnley et al. 1992). Thus, methanol is thought to be one of the main sources of complex organic molecules in the ISM (Hama & Watanabe 2013; Garrod et al. 2007; Qasim et al. 2018). Dust grains are believed to be covered by molecular icy mantles. Thus, the composition of these mantles are an essential ingredient to understand theoretically and observationally the chemistry on grain surfaces. Dust grain ice mantle composition has been measured using infrared (IR) absorption observations from the ground and space (Öberg et al. 2011). Water ice is found to be the dominant component followed by simple carbon compounds mostly in CO, CO2, and CH3OH (Hama & Watanabe 2013), with smaller amounts of NH3, XCN, (Gibb et al. 2004) and other molecules.
Photo-dissociation experiments on CH3OH ices have shown that radicals are synthesized in situ on the ice surfaces under hot ISM environmental conditions (T of 100–600 K) (Garrod & Herbst 2006; Garrod 2008). In simulations “heavy” radicals like CH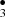 and •CH2OH diffuse within the ice mantles to finally recombine to form complex molecules. Oberg (Öberg et al. 2009) also investigated CH3OH-rich ices in laboratory photochemistry experiments and confirmed the diffusion and recombination of the radicals into more complex species. Chemical reactions that eject products from the surface and energetic processes such as UV Photolysis which produce radicals that quickly react with other molecules play a key role in the synthesis of COMs in the ISM (Öberg et al. 2009). Last year we reported a new route to obtain H2CO and HCO starting from CH3OH-ice-mantles impacted by OH− projectile (Inostroza et al. 2019). We showed how formation of CH
and •CH2OH diffuse within the ice mantles to finally recombine to form complex molecules. Oberg (Öberg et al. 2009) also investigated CH3OH-rich ices in laboratory photochemistry experiments and confirmed the diffusion and recombination of the radicals into more complex species. Chemical reactions that eject products from the surface and energetic processes such as UV Photolysis which produce radicals that quickly react with other molecules play a key role in the synthesis of COMs in the ISM (Öberg et al. 2009). Last year we reported a new route to obtain H2CO and HCO starting from CH3OH-ice-mantles impacted by OH− projectile (Inostroza et al. 2019). We showed how formation of CH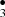 and OCH
and OCH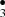 undergo radical-radical reactions to form CH3OCH3. Our main results were in agreement with experiments on CH3OH + OH reactions by Oberg (Öberg et al. 2009). Thus, methanol CH3OH, one of the dominant sources of reactive interstellar organic species, can suffer photo-dissociation (Laas et al. 2011) to produce reactive radicals such as methyl CH
undergo radical-radical reactions to form CH3OCH3. Our main results were in agreement with experiments on CH3OH + OH reactions by Oberg (Öberg et al. 2009). Thus, methanol CH3OH, one of the dominant sources of reactive interstellar organic species, can suffer photo-dissociation (Laas et al. 2011) to produce reactive radicals such as methyl CH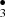 , hydroxymethyl CH2OH• and methoxy CH3O•.
, hydroxymethyl CH2OH• and methoxy CH3O•.
Cernicharo et al. (2012) proposed that the gas-phase reaction CH3OH + OH• is an efficient way to form CH3O• and CH2OH• radicals (see Eqs. (1) and (2)). This helps to understand the lack of detection of CH2OH under ISM conditions, which is the more stable isomer formed on icy grain mantles (Jheeta et al. 2013). More recently, Ocaña et al. (2019) show the reaction of CH3OH + OH• to be a fast and effective source of •CH2OH and CH3O• at the low pressures and temperatures prevalent in the interstellar medium, emphasizing the role of dust-grain mantle reactions are needed to understand gas phase molecular abundances.
From the above isomers, the methoxy radical CH3O• has been detected in space (Cernicharo et al. 2012), although the hydroxymethyl radical, •CH2OH, which is thermodynamically more stable (Bermudez et al. 2017; Nguyen et al. 2019), has not yet been accurately measured in the laboratory, which complicates its detection in the interstellar medium. The direct reaction of CH3O• and •CH2OH can form methoxymethanol CH3OCH2OH (Reaction (3)), which has been observed in the high-mass star-forming region NGC6334 (McGuire et al. 2017). This may explain the non-detection of •CH2OH, since it is an intermediate in the CH3OCH2OH formation process. Thus, CH3OCH2OH would be a molecular tracer for this radical-radical reaction in star-forming regions:
It is known that COMs can be formed in icy grain mantles, which can enhance their gas-phase molecular abundances upon grain surface ejection of COMs. Thus, it is important to study the possible gas or solid phase pathways that are likely to produce one or more of these radicals (McGuire et al. 2017) The reaction between CH3OH + OH• which is involved in the formation of methanol-derived products can help us to better understand the ISM chemistry. We used CH3OH as a target material to mimic an ice mantle because it is highly abundant on the ISM. It can be present in a major variety of astronomical sources. In hot cores it is present in the gas-phase as a dark cloud present on the ice mantle.
Reactions involving OH• are important in the chemistry that occurs in the gas phase of interstellar clouds, as it is highly abundant in the ISM. We analyze how methanol ice mantles colliding with OH• can enrich the chemistry generating COMs. We performed systematic Born-Oppenheimer (ab-initio) molecular dynamics (BOMD) simulations using a decamer of (CH3OH10)-ice mantle impacted by OH• projectiles, to complement our previous calculations using OH− (Inostroza et al. 2019).
The remainder of this paper is organized as follows. In Sect. 2 we describe the calculation methods. The main outcomes of our simulations and discussions are presented in Sect. 3. We present our conclusions in Sect. 4.
2. Computational methods
We used molecular dynamics (BOMD) simulations to study the effect of an OH• projectile impacting with an energy of 10 to 22 eV. As target material we used a methanol cluster formed by ten units of methanol to mimic an ice mantle (CH3OH)10. The details are presented below.
2.1. Simulations
The simulations were performed using the density functional theory (DFT) formalism, under the micro-canonical ensemble approach (also called NEV ensemble), as explained in Paper I Inostroza et al. (2019). We used the long range-corrected hybrid functional of Head-Gordon ωB97X-D (Helgaker et al. 1990; Uggerud & Helgaker 1992; Bolton et al. 1998). This ωB97X-D functional is known to be flexible enough to describe reactive collisions (McBride et al. 2013). We used BOMD at constant energy (i.e., in the NEV ensemble). The NEV ensemble reproduces the conditions where the low concentration of particles hinders the dissipation of thermal energy on timescales of that of the collision (less than 400 fs). The two most relevant electronic states for OH are the ground state X2Π and the first excited state A2Σ+, which is approximately 4 eV higher. The collisions are assumed to happen in the ground state of the species, which is a good approximation because the lowest excitation energy of methanol, 6 eV (Varela et al. 2015) and hydroxyl, 4 eV (Schofield & Kjaergaard 2004) is a large fraction of the kinetic energy of the projectile used during the simulations, which implies a small probability of non-adiabatic dynamics (Inostroza et al. 2019). Moreover, the collision times in our calculations are much shorter than the radiative de-excitation times for both methanol and hydroxyl. Thus, the species remains in its ground state. We follow the simulations until the molecular fragments and reaction products do not change further in time. All calculations were done using the electronic structure package Gaussian 09 (Frisch et al. 2009).
2.2. Chemical model
We use a cluster formed by ten units of methanol to mimic an ice mantle (CH3OH)10. This cluster corresponds to the most stable isomer (Boyd & Boyd 2007). Its size is a good representation between the size of the dust ice-mantle and the computational cost of the BOMD used to simulate the impact of OH•.
The 24 starting points are distributed evenly over a sphere, as depicted in Fig. 1, so that the solid angle subtended by the three closest neighboring points on the surface and the center of the sphere is 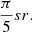 For each impact energy, 24 trajectories were simulated with a time step of 0.5 fs to integrate the equations of motion. This process produces a total of 800 steps for each impact trajectory, or 400 fs. This time step guarantees that the energy is conserved within a few hundreds kcal/mol during the whole simulation, as in Paper I (Inostroza et al. 2019). These trajectories start with the velocity of the O-H axis of the OH• projectile pointing toward the center of mass of the (CH3OH)10-ice mantle. The collision is always the oxygen atom of OH− facing the cluster. The initial conditions of the impact position and kinetic energies are the same as used with the OH− projectile in Inostroza et al. (2019). We focus only on collisions an initial kinetic energy of 10–22 eV, where the richest chemistry takes place.
For each impact energy, 24 trajectories were simulated with a time step of 0.5 fs to integrate the equations of motion. This process produces a total of 800 steps for each impact trajectory, or 400 fs. This time step guarantees that the energy is conserved within a few hundreds kcal/mol during the whole simulation, as in Paper I (Inostroza et al. 2019). These trajectories start with the velocity of the O-H axis of the OH• projectile pointing toward the center of mass of the (CH3OH)10-ice mantle. The collision is always the oxygen atom of OH− facing the cluster. The initial conditions of the impact position and kinetic energies are the same as used with the OH− projectile in Inostroza et al. (2019). We focus only on collisions an initial kinetic energy of 10–22 eV, where the richest chemistry takes place.
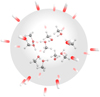 |
Fig. 1. Diagram illustrating initial position of the OH• projectile, whose O–H axis points toward the center of mass of the methanol cluster with its oxygen atom facing the cluster. |
We follow the projectile and methanol decamer during each impact, including all individual chemical bonds created and destroyed during the interactions. New molecules are formed and escape the target in about half of the collisions at low energy and in almost all high energy collisions. The simulations are sensitive enough to allow us to identify transient molecular bonds formed, creating intermediate products that quickly react to form other molecules that escape the decamer. In the next section we explain all the reactions leading to the ejection of new molecules from the decamer.
3. Results
The chemical network with main outcomes obtained from BOMD simulations of (CH3OH)10-ice-mantle hit by hydroxyl radical OH• is summarized in Fig. 2. Stable products like formaldehyde (H2CO) and its isomer hydroxymethylene (HCOH), formic acid (HCOOH), and methoxymethanol (CH3OCH2OH) were found. Additionally, formyl radical (HCO•), methanediol radical (•OCH2OH), methyl radical (CH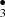 ), hydroxymethylene radical (•CH2OH), and methoxy radical (CH3O•) were also identified.
), hydroxymethylene radical (•CH2OH), and methoxy radical (CH3O•) were also identified.
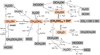 |
Fig. 2. Schematic figure representing the resulting chemical network of COMs obtained after bombardment of the (CH3OH)10-ice-mantle with OH• using impact energies of 10, 12, 15, 18, 20 and 22 eV. For a complete list of reactions and products see Table 1. Radicals are not indicated by dots to simplify the figure. |
Table 1 summarizes the full chemical reaction network found, listing 14 labelled reactions (including sometimes intermediate products). To simplify the table we only indicate the products coming from the reactions (CH3OH)10 + OH•. Each of the twelve relatively complex molecules identified in Table 2 are produced in one or more of these reactions. In addition, simple molecules of H2O, H2, OH−, OH•, and H are also formed and escape the decamer too. Reaction 1 generates the methoxy radical (CH3O•) and water. Reactions 2 and 3 list the outcomes that correspond with the most frequently observed molecules, •CH2OH and CH2(OH)2. Alternatively, +CH2OH can also be formed in a primary process, as shown in reaction 4. This species undergoes a subsequent process to form other stable molecules. Likewise, CH2(OH)2 can release a OH• radical, producing •CH2OH, which can form H2CO and water (see reactions 8, 9, and 12). Methanediol radical (•OCH2OH) is obtained in reactions 5, 6, 10, and 13. By condensation of a methanol of the (CH3OH)10-ice mantle with OH• the stable formic acid HCOOH (reaction 6) or formaldehyde H2CO (reactions 5 and 13) were formed. Methoxymethanol CH3OCH2OH could be produced when the methoxy radical is previously formed (see reaction 14).
Reaction pathways obtained from (CH3OH)10-ice-mantle in collision with a OH• radical.
In the following subsections, we explain the chemical routes observed leading to the primary products H2CO, HCOOH, HCO•, and CH3OCH2OH. The reaction numbers used in the text correspond to those in Table 1.
3.1. Formation pathways of H2CO
Besides being the most stable product, formaldehyde H2CO constitutes an important precursor of the sugar synthesis. Formaldehyde is widely observed in interstellar molecular clouds throughout the Galaxy. Thus, the reactions that produce formaldehyde must be operative under a wide range of physical conditions. H2CO is thought to form through both gas-phase reactions and surface reactions on dust grains. In addition to the proposed gas-phase reaction sequence CH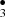 + OH•→ H2CO + H2 (Watanabe & Kouchi 2002), we identify new formation pathways for formaldehyde. Irradiation experiments on pure CH3OH ice at 30 K, found that hydroxymethyl was made and rapidly transformed into other more stable species like formaldehyde H2CO. Molecular products formed within the ice were detected and monitored using continuous Fourier transformed infrared (FT-IR) spectroscopy (Jheeta et al. 2013). They proposed that H2CO may dissociate to a short lived formyl radical (HCO), whose rapid decay leads to the formation of CO and CO2. Our simulations are in agreement with those experimental suggestions.
+ OH•→ H2CO + H2 (Watanabe & Kouchi 2002), we identify new formation pathways for formaldehyde. Irradiation experiments on pure CH3OH ice at 30 K, found that hydroxymethyl was made and rapidly transformed into other more stable species like formaldehyde H2CO. Molecular products formed within the ice were detected and monitored using continuous Fourier transformed infrared (FT-IR) spectroscopy (Jheeta et al. 2013). They proposed that H2CO may dissociate to a short lived formyl radical (HCO), whose rapid decay leads to the formation of CO and CO2. Our simulations are in agreement with those experimental suggestions.
Table 1 lists reactions 5, 8, 9, and 12 as the main routes to obtain formaldehyde. Reaction 5, at 10 eV involves a concerted step, where OH• attached to methanol, creating a new bond between C–O, followed by the creation of a transient C–O⋅H⋅H interaction that led to the formation of a carbonyl double bond C=O and releasing H2. Thus, the reaction produced H2 + •OCH2OH, where the •OCH2OH forms H2CO through a secondary process via the releasing of OH• radical, which can impact on another methanol molecule within the grain mantle. Whilst, in reaction 10, the •OCH2OH radical is the main stable product without the formation of H2CO.
The first step of reaction 8 (at 18 eV) occurs as described above. A second step involves the homolytic rupture of the C–OH bond, releasing a OH• radical, leaving a hydroxymethyl carbon centered radical •CH2OH. Then, the recently formed OH• radical in a vibrationally excited state, provokes the H abstraction from the •CH2OH to generate water. Tertiary processes to produce formaldehyde occur through a kind of SN2 transition state (a bimolecular nucleophilic substitution) in which the rate determining step involves two components with simultaneous bond-making and bond-breaking steps. The transition state (TS) maximum energy in the course of this kind of reactions is reached. The attacked carbon becomes pentacoordinated at the SN2 transition state. In a common SN2 reaction, the resulting product will proceed through the elimination of a group and the inversion of the carbon chirality. Since the SN2 transition state is a radical, the reaction proceeds with the elimination of a H• and their desorption from the mantle.
Reaction 9 also produces H2CO through a tertiary process. This type of reaction was only observed at 20 eV. After the diol formation and H liberation in step 1, step 2a involves the heterolytic rupture of the C-OH bond in the vibrationally excited CH2(OH)2, leaving a hydroxymethyl carbocation +CH2OH and a hydroxyl anion (OH−). Followed by step 2b, this consist of a faster electronic rearrangement of the hydroxymethyl carbocation, [H2C -O−H]+, which concludes with the formation of the carbonyl double bond C=O and liberation of a H+, in step 2c. The last step corresponds to water formation via an OH− + H+ ion association reaction.
Reaction 12 at 20 eV produces H2CO + H2O + H•. However, the mechanism differs from the one observed in reaction 9. In reaction 12, OH• approaches methanol through the C–O axis, whereas in reaction 9 OH• impacts at a different angle. This leads to the elimination of H• in the first step. Then, in the second step occurs the formation of the formaldehyde isomer and water elimination. Finally, HCOH isomerizes to H2CO.
Reaction 4 pointed out the formation of hydroxymethylene, an isomer of H2CO (Koziol et al. 2008) similar to reaction 12, but without the isomerization process. HCOH has not been detected in the gas phase, although HCOH has been seen in rare-gas matrix isolation where it rearranges to formaldehyde with a half-life of about 2 h (Schreiner et al. 2008). Additionally, it was found that this molecule has a tunneling rate sufficiently large so it rapidly forms H2CO in the ISM where there is plenty of time to thermalize into lower energy configurations (Fadilla et al. 2017).
3.2. Formation pathways of HCOOH
Formic acid HCOOH, which is a stable product of the reaction: (CH3OH)10 + OH•, is formed by a secondary process (see reaction 6). Its formation mechanism involves a molecular hydrogen formation followed by a H-elimination from an intermediate •OCHOH species to achieve a stable product. This behavior was observed at 12 and 20 eV.
3.3. Formation pathways of HCO•
We were able to observe the formation of HCO• through a secondary process (reaction 7). This occurs via diol formation as the first step of reaction 8. On the other hand, the first two steps of reaction 13 go through the same pathways as reaction 5. Here, in a tertiary process, HCO• formation occurs via radical abstraction of H from H2CO (see Table 1).
3.4. Formation pathways of CH3OCH2OH
Methoxymethanol CH3OCH2OH was first detected in the ISM in 2017 using the Atacama Large Millimetre/submillimetre Array (ALMA) toward NGC 6334I, a massive protostar (McGuire et al. 2017). Experiments made on temperature-programmed desorption studies, have confirmed that CH3OCH2OH is a photochemistry product of condensed methanol CH3OH (Schneider et al. 2019). Despite that, previous work using interstellar ices under the influence of ionizing radiation have proposed formation pathways for this key molecule (Zhu et al. 2019). Even so, its detection can be a test bed for the existence of its two precursors: methoxy CH3O• and •CH2OH hydroxymethyl isomers. Its mechanism has been proposed to be like that in reaction 3. Our simulations support this hypothesis (Motiyenko et al. 2018). After the collision of OH• with the (CH3OH)10-ice mantle, we observed a diol formation process as the first two steps of reaction 9 to generate +CH2OH + OH− + H. After a short time (5 fs) a hydrogen abstraction by the OH− anion to another methanol molecule in the ice-mantle leads to the formation of the CH3O− anion and H2O. In the third part of this process an ion-ion reaction produces the expected outcome CH3OCH2OH (see reaction 14 and steps therein). These formation pathways can have a relevant role in shocks or photo-dissociation regions produced by high velocity jets and outflows from young stellar objects.
3.5. Comparison of OH• and OH− projectiles
Table 2 lists the main molecular products found using OH• and OH− (Inostroza et al. 2019) projectiles. It indicates the number of each molecule produced summed over the 24 different impact position angles (see Fig. 1). For a given projectile energy, some of the trajectories produced no new chemical species. On the other hand, some collisions produced multiple products. Thus, the sum of the number in each column can be smaller or larger than 24 (the number of different impact trajectories). For example, using an OH• radical projectile with 10 eV, we obtained the following products: seven times CH2OH, five times CH2(OH)2, and once each of CH3O•, OCH2OH, H2CO and HCOH, and H2. The total number of identified new products is thus 17. These occurred in 13 collisions, the other 11 collisions did not form products.
Molecules obtained from simulations of (CH3OH)10-ice-mantle in collision with OH• and OH− projectiles with kinetic energy of 10–22 eV.
The molecular complexity is enriched when the OH• radical impacts the methanol ice-mantle. A general outcome-comparison between both projectiles shows huge differences when we have one additional electron present in the system. For example, in collisions with anion projectile OH− at 10 eV, produced the following reaction CH3OH + OH−→ CH3O• + H2O, where only the methoxy radical (CH3O•) was formed in 17 instances. A single new product compared to seven different molecules produced by the radical OH• under identical conditions. The same tendency can be noticed at 12 eV for the anion projectile OH−.
The most frequently observed products are different in collisions with OH• and OH−. The radical yields predominantly CH2OH and CH2(OH)2, which is infrequently formed at impact energies above 15 eV with an anion projectile. On the other hand, the anion forms predominantly CH3O• at all collision energies, which forms with low frequency in collisions with OH•. This anti-correlation suggests that reactions of OH• + CH3OH produce abundant •CH2OH and +CH2OH, which likely undergo secondary and/or tertiary processes to form COMs that can be released into the gas phase. Thus, •CH2OH and +CH2OH act as key intermediate species in the formation of COMs, disappearing quickly. Our simulations suggest that the charge distribution affects the reactions changing the ratio of CH3O• and •CH2OH.
Adding all the entries in Table 2 for a given projectile will give a rough idea of the relative frequencies of the different molecules formed in an environment with projectiles that have kinetic energies below 22 eV distributed uniformly. However, it is important to note that true abundance predictions are far beyond the scope of these BOMD simulations.
3.6. Astrophysical implications
We identified above new chemical paths for the formation of astrophysical molecules that escape the methanol decamer target after the impact. As a real dust grain mantle is much larger than the decamer we model, the newly formed molecules can escape outwards onto the gas phase or inwards, deeper into the ice mantle. To produce real gas phase molecular abundances requires the use of different simulation techniques, however, it is likely that at least half of the products identified in this work are released onto the gas phase. The most common COMs found when OH• impacts the (CH3OH)10-ice-mantle are CH2OH, CH2(OH)2 and H2CO, in contrast to what we observed in using OH− anion as projectiles (Inostroza et al. 2019), where the methoxy CH3O• was the most frequent product, detected in prestellar cores (Bacmann & Faure 2016).
The reaction of OH• with the methanol molecule CH3OH produces the formation of radicals, through the transition state CH2(OH)2 (Nguyen et al. 2019). Our simulations of impacts of OH• onto a decamer of methanol (CH3OH)10-ice-mantle, show that radicals such as •CH2OH, CH3O• or •OCH2OH are also formed, but subsequently they undergo secondary or tertiary reactions leading to stable molecules. For example, formic acid results in secondary processes due to •OCH2OH. The ALMA detection of interstellar methoxymethanol CH3OCH2OH in NGC 6334I, among other large COMs (McGuire et al. 2017) suggests the need for efficient chemical paths to complexity favoring collisions with the OH• radical.
As cosmic rays have been shown to affect significantly the chemistry on the icy mantles of large grains, producing radicals that quickly react to produce new species (Shingledecker & Herbst 2018), this phenomenon allows some endothermic reactions that do not occur spontaneously in low-temperature ices. In order to observe ion-ice reactions cations must be present in the gas phase and dust grains must be covered by ice-mantles. The impact of energetic projectiles onto the ice-mantles leads to chemical enrichment of the grain mantles. Such grains may then be impacted by astrophysical shock fronts or other highly energetic events that sublimate the grain ice-mantles, enriching the gas phase abundance of COMs, which can then be directly observed using radioastronomical techniques. Thus, ion–ice reactions may be important to enrich the chemistry in molecular clouds (Shingledecker et al. 2017). These molecular projectiles could be produced in regions close to shock fronts arising from powerful protostellar winds. Thus, in protostellar envelopes with large CH3OH ice fractions, where the complex ice chemistry is dominated by pure CH3OH chemistry, similar products as we exposed here would be expected in the gas phase over a large range of objects if only thermal desorption is assumed (Öberg et al. 2009). In dark interstellar clouds, where methanol abundances are higher, desorption through vibrational excitation can also be a plausible alternative mechanism (Geppert et al. 2006).
4. Conclusions
We simulated the interactions of an amorphous cold (CH3OH)10 bombarded by OH• projectiles with kinetic energies of 10–22 eV. We used BOMD simulations to analyze the formation and breaking bond and molecular rearrangements. We were able to keep track of the chemical processes that occur after impact. We confirm that the formation of COMs can be increased where methanol ice-mantle reactions can exist, affecting the chemistry due to the interplay between dust and gas-phase composition (Dulieu et al. 2013).
We pointed out that our previous results in Inostroza et al. (2019) and in this current contribution are in agreement with experimental and theoretical predictions by other authors using different methodologies (Nguyen et al. 2019). We were able to distinguish pathways of stable, intermediary, transition states, and radical molecules. The main molecular outcomes have a similar tendency of formation at impact energies between 10 to 22 eV. The kinetic impact energy is crucial to produce a labile methanol-ice-mantle that can react with the projectile. The resulting COMs use some of this energy to reach highly excited vibrational states that can result in diffusion, accretion, and COM reactions. The most common complex organic molecules produced using the CH3OH10 ice-mantle are: CH3O•, •CH2OH, +CH2OH, CH2(OH)2, •OCH2OH, HCO•, CH3OCH2OH, H2CO, its isomer HCOH, and HCOOH.
We find that H2CO is a product of the reaction of methanol with OH•. In contrast to the early suggestions that it formed through a hydrogenation process (Watanabe & Kouchi 2002), we propose an alternative route to produce H2CO when •CH2OH, +CH2OH or CH2(OH)2 are formed. As experimental work proposed, the CH2OH, rapidly transformed into other more stable species like the formaldehyde H2CO or led to the formation of COMs (Jheeta et al. 2013) as we demonstrated here. Whenever the •CH2OH or CH3O• radicals were formed, they underwent secondary or tertiary processes to form stable products. Formic acid HCOOH is formed in secondary processes due to the radical •OCH2OH.
In reactions on the methanol ice-mantles with energy too large to dissipate, molecules are ejected from the methanol decamer onto the gas-phase. Theses results confirm that dust grains act as interstellar chemical reaction catalysts to form products that are later released into the gas-phase (Garrod 2013), linking solid and gas phase molecular abundances (Dulieu et al. 2013). We suggest these processes are likely relevant in the production of COMs in photo-dissociation regions and in shocks produced by high-velocity jets and outflows from young stellar objects.
The most frequently observed products in collisions with OH• are •CH2OH and CH2(OH)2, while collisions with the anion OH− form mostly CH3O•, which is rare in collisions with OH•. The charge affects interstellar reactions changing that change the ratio of CH3O• and •CH2OH. This can be noticed as reactions with OH• radical, an open shell system, produce richer chemistry (but with low presence of CH3O) than in a collision with the anion. Our results may explain why CH2OH has not been observed since it quickly undergoes secondary or tertiary processes.
Acknowledgments
This research was supported by PCI-CONICYT International Networks for young researchers Grant REDI170243 and PCI-CONICYT Grant RED190113. DM acknowledges support from CONICYT project Basal AFB170002. JG acknowledges support from Fondecyt postdoctoral fellowship 3170768.
References
- Bacmann, A., & Faure, A. 2016, A&A, 587, A130 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Belloche, A., Garrod, R., Müller, H., et al. 2019, A&A, 628, A10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bermudez, C., Bailleux, S., & Cernicharo, J. 2017, A&A, 598, A9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bolton, K., Hase, W. L., & Peslherbe, G. H. 1998, Modern Methods for Multidimensional Dynamics Computations in Chemistry, 143 [CrossRef] [Google Scholar]
- Boyd, S. L., & Boyd, R. J. 2007, J. Chem. Theory Comput., 3, 54 [CrossRef] [Google Scholar]
- Brown, W. A., & Bolina, A. S. 2007, MNRAS, 374, 1006 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Marcelino, N., Roueff, E., et al. 2012, ApJ, 759, L43 [NASA ADS] [CrossRef] [Google Scholar]
- Charnley, S., Tielens, A., & Millar, T. 1992, ApJ, 399, L71 [NASA ADS] [CrossRef] [Google Scholar]
- Dulieu, F., Congiu, E., Noble, J., et al. 2013, Sci. Rep., 3, 1338 [NASA ADS] [CrossRef] [Google Scholar]
- Fadilla, R. N., Aisyah, N. D., Dipojono, H. K., & Rusydi, F. 2017, Proc. Eng., 170, 113 [CrossRef] [Google Scholar]
- Frisch, M. J., Trucks, G. W., & Schlegel, H. B. 2009, Gaussian 09 Revision A.1 (Wallingford CT: Gaussian Inc.) [Google Scholar]
- Garrod, R. 2008, A&A, 491, 239 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T. 2013, ApJ, 765, 60 [Google Scholar]
- Garrod, R. T., & Herbst, E. 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., Wakelam, V., & Herbst, E. 2007, A&A, 467, 1103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Geppert, W. D., Hamberg, M., Thomas, R. D., et al. 2006, Faraday Discuss., 133, 177 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Gibb, E., Whittet, D., Boogert, A., & Tielens, A. 2004, ApJS, 151, 35 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Hama, T., & Watanabe, N. 2013, Chem. Rev., 113, 8783 [CrossRef] [Google Scholar]
- Helgaker, T., Uggerud, E., & Jensen, H. J. A. 1990, Chem. Phys. Lett, 173, 145 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & Van Dishoeck, E. F. 2009, ARA&A, 47, 427 [NASA ADS] [CrossRef] [Google Scholar]
- Inostroza, N., & Senent, M. L. 2012, Chem. Phys. Lett., 524, 25 [NASA ADS] [CrossRef] [Google Scholar]
- Inostroza, N., Mardones, D., Cernicharo, J., et al. 2019, A&A, 629, A28 [CrossRef] [EDP Sciences] [Google Scholar]
- Jheeta, S., Domaracka, A., Ptasinska, S., Sivaraman, B., & Mason, N. 2013, Chem. Phys. Lett., 556, 359 [NASA ADS] [CrossRef] [Google Scholar]
- Koziol, L., Wang, Y., Braams, B. J., Bowman, J. M., & Krylov, A. I. 2008, J. Chem. Phys., 128, 204310 [NASA ADS] [CrossRef] [Google Scholar]
- Laas, J. C., Garrod, R. T., Herbst, E., & Widicus Weaver, S. L. 2011, ApJ, 728, 71 [NASA ADS] [CrossRef] [Google Scholar]
- McBride, E. J., Millar, T. J., & Kohanoff, J. J. 2013, J. Phys. Chem. A, 117, 9666, pMID: 23662836 [Google Scholar]
- McGuire, B. A., Shingledecker, C. N., Willis, E. R., et al. 2017, ApJ, 851, L46 [NASA ADS] [CrossRef] [Google Scholar]
- Motiyenko, R. A., Margulés, L., Despois, D., & Guillemin, J.-C. 2018, Phys. Chem. Chem. Phys., 20, 5509 [NASA ADS] [CrossRef] [Google Scholar]
- Nguyen, T. L., Ruscic, B., & Stanton, J. F. 2019, J. Chem. Phys., 150, 084105 [NASA ADS] [CrossRef] [Google Scholar]
- Öberg, K. I., Garrod, R. T., Van Dishoeck, E. F., & Linnartz, H. 2009, A&A, 504, 891 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Öberg, K. I., Boogert, A. A., Pontoppidan, K. M., et al. 2011, ApJ, 740, 109 [NASA ADS] [CrossRef] [Google Scholar]
- Ocaña, A. J., Blázquez, S., Potapov, A., et al. 2019, Phys. Chem. Chem. Phys., 21, 6942 [CrossRef] [Google Scholar]
- Qasim, D., Chuang, K.-J., Fedoseev, G., et al. 2018, A&A, 612, A83 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Schneider, H., Caldwell-Overdier, A., Coppieters’t Wallant, S., et al. 2019, MNRAS, 485, L19 [NASA ADS] [CrossRef] [Google Scholar]
- Schofield, D. P., & Kjaergaard, H. G. 2004, J. Chem. Phys., 120, 6930 [NASA ADS] [CrossRef] [Google Scholar]
- Schreiner, P. R., Reisenauer, H. P., Pickard Iv, F. C., et al. 2008, Nature, 453, 906 [NASA ADS] [CrossRef] [Google Scholar]
- Shingledecker, C. N., & Herbst, E. 2018, Phys. Chem. Chem. Phys., 20, 5359 [Google Scholar]
- Shingledecker, C. N., Le Gal, R., & Herbst, E. 2017, Phys. Chem. Chem. Phys., 19, 11043 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. 2013, Rev. Mod. Phys., 85, 1021 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A., Hagen, W., et al. 1982, A&A, 114, 245 [Google Scholar]
- Uggerud, E., & Helgaker, T. 1992, J. Am. Chem. Soc., 114, 4265 [CrossRef] [Google Scholar]
- Varela, K., Hargreaves, L. R., Ralphs, K., et al. 2015, J. Phys. B: At. Mol. Opt. Phys., 48, 115208 [CrossRef] [Google Scholar]
- Watanabe, N., & Kouchi, A. 2002, ApJ, 571, L173 [NASA ADS] [CrossRef] [Google Scholar]
- Zhu, C., Frigge, R., Bergantini, A., Fortenberry, R. C., & Kaiser, R. I. 2019, ApJ, 881, 156 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Reaction pathways obtained from (CH3OH)10-ice-mantle in collision with a OH• radical.
Molecules obtained from simulations of (CH3OH)10-ice-mantle in collision with OH• and OH− projectiles with kinetic energy of 10–22 eV.
All Figures
 |
Fig. 1. Diagram illustrating initial position of the OH• projectile, whose O–H axis points toward the center of mass of the methanol cluster with its oxygen atom facing the cluster. |
| In the text | |
 |
Fig. 2. Schematic figure representing the resulting chemical network of COMs obtained after bombardment of the (CH3OH)10-ice-mantle with OH• using impact energies of 10, 12, 15, 18, 20 and 22 eV. For a complete list of reactions and products see Table 1. Radicals are not indicated by dots to simplify the figure. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.