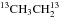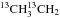| Issue |
A&A
Volume 590, June 2016
|
|
|---|---|---|
| Article Number | A93 | |
| Number of page(s) | 13 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/201628309 | |
| Published online | 20 May 2016 | |
Spectroscopic study and astronomical detection of doubly 13C-substituted ethyl cyanide ⋆
1 Laboratoire de Physique des Lasers, Atomes, et Molécules, UMR CNRS 8523, Université de Lille I, 59655 Villeneuve d’Ascq Cedex, France
e-mail: laurent.margules@univ-lille1.fr
2 Max-Planck-Institut für Radioastronomie, Auf dem Hügel 69, 53121 Bonn, Germany
3 I. Physikalisches Institut, Universität zu Köln, Zülpicher Str. 77, 50937 Köln, Germany
4 Institut des Sciences Chimiques de Rennes, École Nationale Supérieure de Chimie de Rennes, CNRS, UMR 6226, 11 allée de Beaulieu, CS 50837, 35708 Rennes Cedex 7, France
5 Departments of Astronomy and Chemistry, University of Virginia, Charlottesville, VA 22904, USA
Received: 15 February 2016
Accepted: 9 April 2016
Context. We have performed a spectral line survey called Exploring Molecular Complexity with ALMA (EMoCA) toward Sagittarius B2(N) between 84.1 and 114.4 GHz with the Atacama Large Millimeter/submillimeter Array (ALMA) in its Cycles 0 and 1. Line intensities of the main isotopic species of ethyl cyanide and its singly 13C-substituted isotopomers observed toward the hot molecular core Sagittarius B2(N2) suggest that the doubly 13C-substituted isotopomers should also be detectable.
Aims. We want to determine the spectroscopic parameters of all three doubly 13C-substituted isotopologues of ethyl cyanide to search for them in our ALMA data.
Methods. We investigated the laboratory rotational spectra of the three species between 150 GHz and 990 GHz. We searched for emission lines produced by these species in the ALMA spectrum of Sagittarius B2(N2). We modeled their emission and the emission of the 12C and singly 13C-substituted isotopologues assuming local thermodynamic equilibrium.
Results. We identified more than 5000 rotational transitions, pertaining to more than 3500 different transition frequencies, in the laboratory for each of the three doubly 13C-substituted isotopomers. The quantum numbers reach J ≈ 115 and Ka ≈ 35, resulting in accurate spectroscopic parameters and accurate rest frequency calculations beyond 1000 GHz for strong to moderately weak transitions of either isotopomer. All three species are unambiguously detected in our ALMA data. The 12C/13C column density ratio of the isotopomers with one 13C atom to those with two 13C atoms is about 25.
Conclusions. Ethyl cyanide is the second molecule after methyl cyanide for which isotopologues containing two 13C atoms have been securely detected in the interstellar medium. The model of our ethyl cyanide data suggests that we should be able to detect vibrational satellites of the main species up to at least ν19 = 1 at ~1130 K and up to ν13 + ν21 = 2 at ~600 K for the isotopologues with one 13C atom in our present ALMA data. Such satellites may be too weak to be identified unambiguously for isotopologues with two 13C atoms.
Key words: molecular data / methods: laboratory: molecular / techniques: spectroscopic / radio lines: ISM / ISM: molecules / ISM: individual objects: Sagittarius B2(N)
Supplementary text files S1.dat, S2.dat, and S3.dat are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/590/A93
© ESO, 2016
1. Introduction
Investigations of the warm (100−200 K) and dense parts of high mass star-forming regions known as hot cores (or hot corinos for their low-mass star analogs) have unveiled a wealth of complex molecules. These are saturated or nearly saturated organic molecules for the most part, reaching thus far up to 12 atoms (Belloche et al. 2014). Most of these molecules have been detected toward Sagittarius B2 (Sgr B2 for short). The Sgr B2 molecular cloud complex is one of the most prominent star-forming regions in our Galaxy. This complex is located close to the Galactic center and contains two major sites of high-mass star formation, Sgr B2(M) and (N). Sgr B2(N) has a greater variety of complex organic molecules than Sgr B2(M). It contains two dense, compact hot cores that are separated by about 5″ in the north-south direction (Belloche et al. 2008, 2016; Qin et al. 2011). The more prominent hot core is Sgr B2(N1), also known as the Large Molecule Heimat, and Sgr B2(N2) is to the north of it.
We used the Atacama Large Millimeter/submillimeter Array (ALMA) in its Cycles 0 and 1 to perform a spectral line survey of Sagittarius B2(N) between 84.1 and 114.4 GHz. This survey is called Exploring Molecular Complexity with ALMA (EMoCA). The high angular resolution (~1.5–1.8″) achieved in the survey allows us to separate the emission of the two hot cores and reveals that Sgr B2(N2) has relatively narrow linewidths (~5 km s-1), which reduces the line confusion compared to previous single-dish surveys of Sgr B2(N). Therefore, so far we have focused our analysis on this source. One of the first results of EMoCA was the first detection in space of a branched alkyl molecule, iso-propyl cyanide, which is found to be nearly as abundant as its straight-chain isomer n-propyl cyanide (Belloche et al. 2014). The laboratory spectroscopic investigation on iso-propyl cyanide (Müller et al. 2011) was an obvious prerequisite. The decrease in line confusion, however, was also important because this molecule was not detected in our previous single-dish survey of Sgr B2(N) (Belloche et al. 2013; Müller et al. 2011).
The lighter ethyl cyanide, C2H5CN, also known as propionitrile, is considerably more prominent than either iso- or n-propyl cyanide in our ALMA survey. In fact, lines of the three isotopomers with one 13C atom are so strong that we expected to be able to detect those of the three isotopomers with two 13C atoms. The parent isotopic species was detected in the Orion Molecular Cloud and in Sgr B2 (Johnson et al. 1977) soon after the even lighter methyl cyanide was detected (Solomon et al. 1971). The molecule was also detected in the hot corinos of low-mass protostars such as IRAS 16293-2422, NGC 1333-IRAS 2A, and NGC 1333-IRAS 4A (Cazaux et al. 2003; Taquet et al. 2015). Rotational transitions within the two lowest vibrational states were detected in G327.3−0.6 (Gibb et al. 2000) and Sgr B2(N) (Mehringer et al. 2004). Transitions of even higher excited states were detected in the high mass star-forming regions Orion KL and Sgr B2(N) (Daly et al. 2013; Belloche et al. 2013). The 13C isotopomers of ethyl cyanide were detected first in Orion IRc2 (Demyk et al. 2007) and soon thereafter in Sgr B2(N) (Müller et al. 2008). Margulès et al. (2009) reported the detection of C2H5C15N in Orion IRc2.
The rotational spectrum of the parent isotopic species has been extensively studied in its ground vibrational state. The recent work by Brauer et al. (2009) extended the data to 1.6 THz. Information is also available on some low vibrational states (Daly et al. 2013). The ground vibrational states of all singly substituted ethyl cyanide species were studied rather extensively, in particular those with 13C (Demyk et al. 2007; Richard et al. 2012), but also those with D and 15N (Margulès et al. 2009).
No data are available for C2H5CN isotopomers with two 13C atoms. Therefore, we prepared ethyl cyanide samples highly enriched in 13C at two positions, recorded its rotational spectra up to 1 THz and searched for it in our ALMA data toward Sgr B2(N2). Details about the laboratory experiments and the astronomical observations are given in Sect. 2. Section 3 presents the results of the laboratory spectroscopy and the analysis of the astronomical spectra. These results are discussed in Sect. 4 and the conclusions are given in Sect. 5.
2. Experimental details
2.1. Synthesis
Triethylene glycol (20 mL), potassium cyanide (0.7 g, 10.7 mmol), and iodoethane-13C2 (1 g, 6.4 mmol) were introduced into a three-necked flask equipped with a stirring bar, a reflux condenser, and a nitrogen inlet. The mixture was heated to 110 °C and stirred at this temperature for one hour. After cooling to room temperature, the flask was fitted on a vacuum line equipped with two U-tubes. The high boiling compounds were condensed in the first trap cooled at −30 °C and propanenitrile-2, 3-13C2 (13CH313CH2CN) was selectively condensed in the second trap cooled at −90 °C. The reaction was performed starting from stoichiometric amounts of iodoethane-1-13C and K13CN to prepare propanenitrile-1, 2-13C2 (CH313CH213CN), and starting from iodoethane-2-13C and K13CN to prepare propanenitrile-1, 3-13C2 (13CH3CH213CN). The nuclear magnetic resonance data (NMR) of the three isotopologues are given in Appendix A.
2.2. Lille – submillimeter spectra
The measurements in the frequency range under investigation (150−990 GHz) were performed using the Lille spectrometer (Alekseev et al. 2012). A quasi-optic dielectric hollow waveguide of 3 m length containing investigated gas at the required pressure was used as the sample cell in the spectrometer. The measurements were done at typical pressures of 10 Pa and at room temperature. The frequency ranges 150−330, 400−660, and 780−990 GHz were covered with various active and passive frequency multipliers from VDI Inc. and an Agilent synthesizer (12.5−18.25 GHz) was used as the source of radiation. Estimated uncertainties for measured line frequencies are 30 kHz and 50 kHz depending on the observed signal-to-noise ratio and frequency range.
2.3. Observations
Part of the observations used in this article have been briefly described in Belloche et al. (2014). Here we use the full dataset of the EMoCA survey to search for the emission of the three doubly 13C-substituted isotopologues of ethyl cyanide toward Sgr B2(N2) at the equatorial position (α,δ)J2000 = (17h47m19.86s,−28°22′13.4′′). In brief, the survey covers the frequency range 84.1–114.4 GHz with a spectral resolution of 488.3 kHz (1.7 to 1.3 km s-1). The angular resolution ranges from 1.4″ to 2.1″. A detailed account of the observations, reduction, and analysis method of the full dataset is reported in Belloche et al. (2016).
3. Results
3.1. Laboratory spectroscopy
Ethyl cyanide is an asymmetric top rotor with κ = (2B−A−C)/(A−C) = −0.9591 for the parent isotopic species, which is very close to the prolate limit of −1. The cyano group causes a large dipole moment of 3.816 (3) D along the a-inertial axis and a still sizeable 1.235 (1) D along the b-inertial axis (Kraśnicki & Kisiel 2011). As a consequence a-type transitions dominate the room temperature rotational spectrum up to about 0.75 THz. Internal rotation splitting of the CH3 group or hyperfine structure splitting caused by the 14N nucleus are only resolvable in selected transitions at low frequencies. Both types of splitting were not resolved here. Heavy atom substitution changes the spectroscopic parameters only slightly, and the changes in the dipole moment components are very small, such that they are usually neglected.
Spectroscopic parameters of three ethyl cyanide isotopomers with two 13C atoms compared to those of the main isotopologue.
Rotational partition function values of three ethyl cyanide isotopomers with two 13C atoms at selected temperatures compared to those of the main isotopologue.
The initial predictions were obtained from scaling data calculated ab initio to values of known isotopic species. The rotational and quartic centrifugal distortion parameters of the doubly 13C-substituted species were calculated with harmonic force field calculations at B3LYP/6-311G++(3df, 2pd) level. The same type of calculations were performed for the two mono substituted 13CH3CH2CN and CH313CH2CN species, and the differences between the ab initio and experimental values from Richard et al. (2012) were calculated. The scaling values from 13CH3CH2CN were added to 13CH3CH213CN and 13CH313CH2CN parameters, and those of CH313CH2CN to CH313CH213CN. This method permits to obtain first predictions better than 10 MHz in the lowest part of our frequency range. The aR-branch J = 18–17 pattern was easily recognized in the spectra around 150 GHz. The assignment procedure was the same for all three species: transitions obeying a-type selection rules were assigned up to 330 GHz first, then bR- and bQ-branch transitions from 150 to 330 GHz. After these first steps all quartic and sextic distortional parameters could be determined. Transitions from 400 to 990 GHz could be subsequently assigned. Transitions with high Ka values were difficult to assign because these were frequently weaker than transitions pertaining to excited vibrational states and were often blended with these. As is usual for a molecule of this size, many lines remain unassigned because assignments of transitions in excited vibrational states were beyond the scope of the present investigation. Predictions of the spectra were carried out with SPCAT (Pickett 1991), and ASFIT (Kisiel 2001) was employed for fitting. Even though both A and S reduction of the rotational Hamiltonian perform nearly equally well for ethyl cyanide (Richard et al. 2012), we used the S reduction (in the Ir representation) because the molecule is close to the symmetric prolate limit. The final line lists consist of ~6800 transitions for the 1, 2-substituted isotopomer and ~5500 for the 1, 3- and 2, 3-substituted isotopomers. The number of different transition frequencies is smaller, ~4600 and ~3600, respectively, because asymmetry splitting was frequently not resolved, and in some cases accidentally overlapping lines were retained in the fit. The J values reach ~115, and Ka values extend to at least 20 for b-type transitions and to around 35 for a-type transitions. We determined for each isotopomer a full set of up to eighth-order rotational parameters along with three diagonal decic parameters. They are presented in Table 1 together with values for the parent isotopologue from Brauer et al. (2009). Predictions of the rotational spectra of the three isotopomers will be available in the catalog section1 of the Cologne Database for Molecular Spectroscopy (CDMS); the line, parameter, and fit files, along with additional auxiliary files, will be provided in the CDMS archive2. Supplementary text files S1.dat, S2.dat, S3.dat are available at CDS. They contain the transitions used in the fit with experimental frequencies, accuracies and residuals from the fits. Table B.1 provides guidance on these files.
Rotational partition function values of ethyl cyanide and its three isotopomers containing two 13C atoms are provided at selected temperatures in Table 2. The temperatures are the standard temperatures in the CDMS (Müller et al. 2001, 2005) and Jet Propulsion Laboratory (Pickett et al. 1998) catalogs. At the elevated temperatures in hot cores, such as Sgr B2(N2), and under the assumption of local thermodynamic equilibrium (LTE) a considerable part of larger organic molecules is excited to vibrational levels that are higher than the ground vibrational state. At 150 K a vibrational state at an energy of 480 cm-1 (or 691 K) has a population of 0.01 with respect to ν= 0. The fundamental vibrations of ethyl cyanide have been well determined experimentally (Heise et al. 1981), however, only a selection of overtone and combination levels are known. Therefore, we used the harmonic oscillator approximation, as is commonly done, to evaluate contributions of such states. Use of the anharmonic fundamentals accounts in part for the anharmonicity of the vibrations. The resulting vibrational correction factors to the rotational partition function of the main isotopologue are given in Table 3. Isotopic differences are small and most likely within the errors of the harmonic oscillator approximation for such a heavy molecule as ethyl cyanide.
Vibrational correction factors to the rotational partition function of ethyl cyanide at selected temperatures.
 |
Fig. 1 Panels a), b). Population diagram of CH |
3.2. Detection toward Sgr B2(N2)
The LTE modeling of the ALMA spectrum of Sgr B2(N2) reveals the presence of the three doubly 13C-substituted isotopologues of ethyl cyanide; about 8, 7, and 8 lines that do not suffer too much from blending with transitions of other species are clearly detected for CH313CH213CN (Fig. C.1), 13CH3CH213CN (Fig. C.2), and 13CH313CH2CN (Fig. C.3), respectively. The parameters of these detected lines are listed in Tables 4–6, respectively. These tables also contain a few additional lines that we have not counted as formally detected because their wings suffer a bit more from blending with emission from other species (line(s) around 106 434 MHz for CH313CH213CN, 86 938 MHz and 103 906 MHz for 13CH3CH213CN, and 85 727 MHz and 109 313 MHz for 13CH313CH2CN). They are used in the population diagrams described below.
Figure 1 shows the population diagrams of the three isotopologues that were constructed using most of the lines listed in Tables 4–6. The number of lines shown in the population diagrams does not exactly match the number of detected lines reported above for several reasons. First of all, some detected lines are blends of several transitions of the same molecule with different upper-level energies and thus cannot be plotted in a population diagram. Second, some lines plotted in the population diagrams are somewhat more contamined by other species than what we require to qualify them as detected. Still we show them in the population diagrams because we can account for this contamination thanks to our full LTE model that contains all of the species identified so far (see Belloche et al. 2016).
Table 7 lists the results of the rotational temperature fits for the three doubly 13C-substituted isotopologues, along with the results previously obtained for C2H5CN and the three singly 13C-substituted isotopologues (Belloche et al. 2016). The uncertainties on the derived rotational temperatures of the doubly 13C-substituted isotopologues are large because of the small number of transitions and the narrow range spanned by their upper-level energies. Their rotational temperatures are thus poorly constrained but they are consistent within 1–2σ with the value of 150 K that we adopted based on C2H5CN and the three singly 13C-substituted isotopologues (Belloche et al. 2016). Similarly there are too few uncontaminated lines with sufficiently high signal-to-noise ratios to derive the size of the emission accurately on the basis of the integrated intensity maps. Therefore we assume the same source size of 1.2″ as for the more abundant isotopologues. The parameters of the best-fit LTE models are listed in Table 8. Those for C2H5CN, the three singly 13C-substituted isotopologues, and the 15N isotopologue were already reported in Belloche et al. (2016).
Uncertainties are not reported in Table 8. Each doubly 13C-substituted isotopologue has several lines detected with a signal-to-noise ratio close to or above 10 if we consider the peak temperatures, or even 20–40 if we consider the integrated intensities. Therefore, the pure statistical uncertainty on the derived column densities is smaller than 10% if we assume that at least one of the detected lines is free of contamination at its peak or its contamination is well accounted for by our model (otherwise, the column density can only be viewed as an upper limit). Belloche et al. (2016) estimated a calibration uncertainty of 15% for the individual setups of the EMoCA survey. Since the detected lines are distributed among several setups, we do not expect the derived column densities to be biased by the calibration of a particular setup. Therefore the column density uncertainty resulting from the calibration uncertainty should be less than 15%. Taking both sources of uncertainty together (statistics and calibration), the uncertainty on the column density should not be larger than ~15%. The rotational temperatures were assumed to be equal to those of the more abundant isotopologues (Belloche et al. 2016). Provided this assumption is correct, the uncertainty on the rotational temperatures does not have a significant impact on the relative abundance ratios that are discussed in Sect. 4.2. The same is true for the source size and linewidth.
Selection of lines of CH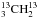 CN covered by the EMoCA survey of Sgr B2(N2).
CN covered by the EMoCA survey of Sgr B2(N2).
Selection of lines of 13CH3CH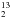 CN covered by the EMoCA survey of Sgr B2(N2).
CN covered by the EMoCA survey of Sgr B2(N2).
Selection of lines of 13CH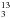 CH2CN covered by the EMoCA survey of Sgr B2(N2).
CH2CN covered by the EMoCA survey of Sgr B2(N2).
4. Discussion
4.1. Laboratory spectroscopy
It can be seen in Table 1 that substitution of two of the three 12C atoms in the main isotopologue of ethyl cyanide by 13C causes only slight decreases in the rotational parameters A, B, and C. It is therefore not surprising that the magnitudes of the centrifugal distortion parameters also change only slightly, in particular those of lower order. It may come as a surprise at first that some of the distortion parameters of the doubly 13C-substituted isotopologues are actually larger than those of the main species. One should, however, keep in mind that one finds empirically that the distortion parameters scale approximately with appropriate powers of A−(B + C)/2, B + C, and B−C. The line lists of the three doubly 13C-substituted ethyl cyanide isotopologues differ somewhat, which easily explains changes in the uncertainties among these isotopologues.
4.2. Astronomical observations
The column densities reported in Table 8 imply a 12C12C/12C13C ratio of ~32 and a 12C13C/13C13C ratio of ~25 for ethyl cyanide in Sgr B2(N2). The 12C13C/13C13C ratio derived for ethyl cyanide is very similar to the 12C13C/13C13C ratio tentatively obtained for HC3N in the same source (~24; see Belloche et al. 2016) and to the 12C/13C ratio derived for methanol and ethanol (~25; see Müller et al. 2016). However, the difference on the order of 25% between the 12C12C/12C13C and 12C13C/13C13C ratios of ethyl cyanide is a priori surprising. Given that the main and singly 13C-substituted isotopologues have a much larger number of detected lines than the doubly 13C-substituted isotopologues, the uncertainty on the column densities of the former should be much smaller than the upper limit of 15% estimated for the latter in Sect. 3.2. Therefore the difference between the two ratios seems to be significant. The two ratios would be reconciled with a value of 28.6 if the column densities of the singly 13C-substituted isotopologues were higher by a factor 1.12, which would correspond to a vertical increment of 0.11 in their population diagrams (see Figs. 7b, 8b, and 9b of Belloche et al. 2016). Such an increment is not completely excluded, but it seems to be marginally consistent with the observed spectra, given the large number of detected transitions and the fact that a few of these are already somewhat overestimated by the current model. The fact that the 12C12C/12C13C ratio is higher than the 12C13C/13C13C ratio cannot be due to an optical depth effect either because saturation of ethyl cyanide transitions, if not properly taken into account, would tend to decrease the apparent 12C12C/12C13C ratio compared to the 12C13C/13C13C ratio. Our analysis takes into account the line optical depth and we excluded the lines that are too opaque (τ< 2.5; see Fig. 6 of Belloche et al. 2016), so we believe that the 12C12C/12C13C ratio is not affected by an opacity bias. Another possibility is that the column density of the doubly 13C-substituted isotopologues is overestimated by a factor ~1.3. Given that the EMoCA spectrum is close to the confusion limit, this cannot be excluded as long as there are still unidentified lines in the survey.
Rotational temperatures derived from population diagrams of ethyl cyanide and its isotopologues toward Sgr B2(N2).
Parameters of our best-fit LTE model of ethyl cyanide and its isotopologues toward Sgr B2(N2).
Finally, we cannot exclude that our assumption of a uniform source structure and the use of the same source size for all isotopologues introduce systematic biases in the derived column densities. Figure 10 of Belloche et al. (2016) shows that the source size actually depends on the upper-level energy of the detected transitions of ethyl cyanide. A more elaborated model taking into account the temperature, density, and possibly abundance gradients in Sgr B2(N2) would be necessary to verify whether the difference between the 12C12C/12C13C and 12C13C/13C13C ratios obtained through our simple analysis is significant or not.
5. Conclusions
We have unambiguously detected the three isotopomers of ethyl cyanide with two 13C atoms in the Sgr B2(N2) hot core. Ethyl cyanide is the second molecule after methyl cyanide (Belloche et al. 2016) for which isotopologues containing two 13C atoms have been securely detected in the interstellar medium. The 12C/13C column density ratio between ethyl cyanide isotopomers with one 13C atom and those with two 13C atoms is ~25, in good agreement with ratios reported for several other molecules in this source. The 12C/13C ratio between the main isotopologue and those with one 13C atom is higher (~32), but it is unclear at this stage whether this is a significant difference or a bias due to our simple assumptions about the physical structure of the source. The signal-to-noise ratios of the detected lines and the derived (rotational) temperature of 150 K suggest that vibrational satellites of the isotopologues with two 13C atoms may be just too weak to be identified unambiguously in our current dataset. We expect, however, to be able to identify vibrational satellites of the isotopologues with one 13C atom up to the three states with ν13 + ν21 = 2 at ~600 K, possibly even those of ν12 = 1 at ~770 K. Vibrational satellites of the main species should be observable up to at least ν19 = 1 at ~1130 K.
Acknowledgments
The present investigations were supported by the CNES and the CNRS program “Physique et Chimie du Milieu Interstellaire” (PCMI). This work was also done under ANR-13-BS05-0008-02 IMOLABS. Support by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the collaborative research grant SFB 956, project B3 is also acknowledged. This paper makes use of the following ALMA data: ADS/JAO.ALMA#2011.0.00017.S, ADS/JAO.ALMA#2012.1.00012.S. ALMA is a partnership of ESO (representing its member states), NSF (USA) and NINS (Japan), together with NRC (Canada), NSC and ASIAA (Taiwan), and KASI (Republic of Korea), in cooperation with the Republic of Chile. The Joint ALMA Observatory is operated by ESO, AUI/NRAO and NAOJ. The interferometric data are available in the ALMA archive at https://almascience.eso.org/aq/.
References
- Alekseev, E. A., Motiyenko, R. A., Margulès, L. 2012, Radio Phys. Radio Astron., 3, 75 [NASA ADS] [CrossRef] [Google Scholar]
- Belloche, A., Menten, K. M., Comito, C., et al. 2008, A&A, 482, 179 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Belloche, A., Müller, H. S. P., Menten, K. M., Schilke, P., & Comito, C. 2013, A&A, 559, A47 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Belloche, A., Garrod, R. T., Müller, H. S. P., & Menten, K. M. 2014, Science, 345, 1584 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Belloche, A., Müller, H. S. P., Garrod, R. T., & Menten, K. M. 2016, A&A, 587, A91 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Brauer, C. S., Pearson, J. C., Drouin, B. J., & Yu, S. 2009, ApJS, 184, 133 [NASA ADS] [CrossRef] [Google Scholar]
- Cazaux, S., Tielens, A. G. G. M., Ceccarelli, C., et al. 2003, ApJ, 593, L51 [NASA ADS] [CrossRef] [Google Scholar]
- Daly, A. M., Bermúdez, C., López, A., et al. 2013, ApJ, 768, 81 [NASA ADS] [CrossRef] [Google Scholar]
- Demyk, K., Mäder, H., Tercero, B., et al. 2007, A&A, 466, 255 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Gibb, E., Nummelin, A., Irvine, W. M., Whittet, D. C. B., & Bergman, P. 2000, ApJ, 545, 309 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Heise, H. M., Winther, F., & Lutz, H. 1981, J. Mol. Spectr., 90, 531 [NASA ADS] [CrossRef] [Google Scholar]
- Johnson, D. R., Lovas, F. J., Gottlieb, C. A., et al. 1977, ApJ, 218, 370 [NASA ADS] [CrossRef] [Google Scholar]
- Kisiel, Z. 2001, Spectroscopy from Space (Dordrecht: Kluwer Academic Publishers), see also PROSPE – Programs for ROtational SPEctroscopy, http://info.ifpan.edu.pl/~kisiel/prospe.htm [Google Scholar]
- Kraśnicki, A., & Kisiel, Z. 2011, J. Mol. Spectr., 270, 83 [NASA ADS] [CrossRef] [Google Scholar]
- Margulès, L., Motiyenko, R., Demyk, K., et al. 2009, A&A, 493, 565 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Mehringer, D. M., Pearson, J. C., Keene, J., & Phillips, T. G. 2004, ApJ, 608, 306 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Thorwirth, S., Roth, D. A., & Winnewisser, G. 2001, A&A, 370, L49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct., 742, 215 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Belloche, A., Menten, K. M., et al. 2008, J. Mol. Spectr., 251, 319 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Coutens, A., Walters, A., et al. 2011, J. Mol. Spectr., 267, 100 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Belloche, A., Xu, L.-H., et al. 2016, A&A, 587, A92 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pickett, H. M. 1991, J. Mol. Spectr., 148, 371 [NASA ADS] [CrossRef] [Google Scholar]
- Pickett, H. M., Poynter, R. L., Cohen, E. A., et al. 1998, J. Quant. Spectr. Rad. Transf., 60, 883 [NASA ADS] [CrossRef] [Google Scholar]
- Qin, S.-L., Schilke, P., Rolffs, R., et al. 2011, A&A, 530, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Richard, C., Margulès, L., Motiyenko, R. A., & Guillemin, J.-C. 2012, A&A, 543, A135 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Solomon, P. M., Jefferts, K. B., Penzias, A. A., & Wilson, R. W. 1971, ApJ, 168, L107 [NASA ADS] [CrossRef] [Google Scholar]
- Taquet, V., López-Sepulcre, A., Ceccarelli, C., et al. 2015, ApJ, 804, 81 [NASA ADS] [CrossRef] [Google Scholar]
Appendix A: Nuclear magnetic resonance data
The NMR type is given first with solvent and the resonance frequency are given in parentheses, the shift δ in ppm with appearance pattern (d, t, q stands for doublet, triplet, quartet), the origin of the pattern is given in case of the 1H NMR, spin-spin coupling parameters 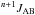 in parentheses, the originating molecule group is given at the end; A and B are the respective nuclei, and n is the number of atoms between A and B.
in parentheses, the originating molecule group is given at the end; A and B are the respective nuclei, and n is the number of atoms between A and B.
Propanenitrile-1, 2- 13 C 2
1H NMR (CDCl3, 400 MHz):
δ 1.29 (ddt, 3H, 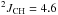 Hz,
Hz, 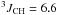 Hz,
Hz, 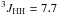 Hz, CH3)
Hz, CH3)
δ 2.35 (ddq, 2H, 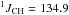 Hz,
Hz, 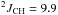 Hz,
Hz, 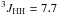 Hz, CH2)
Hz, CH2)
13C NMR (CDCl3, 100 MHz):
δ 10.5 (qdd,  Hz,
Hz, 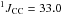 Hz,
Hz, 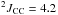 Hz, CH3)
Hz, CH3)
δ 11.1 (td, 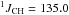 Hz,
Hz, 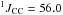 Hz, CH2)
Hz, CH2)
δ 120.8 (d, 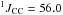 Hz, CN)
Hz, CN)
Propanenitrile-1, 3- 13 C 2
1H NMR (CDCl3, 400 MHz):
δ 1.29 (ddt, 3H,  Hz,
Hz, 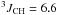 Hz,
Hz, 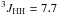 Hz, CH3)
Hz, CH3)
δ 2.35 (ddq, 2H, 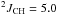 Hz,
Hz, 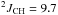 Hz,
Hz, 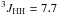 Hz, CH2)
Hz, CH2)
13C NMR (CDCl3, 100 MHz):
δ 10.5 (qd,  Hz,
Hz, 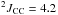 Hz, CH3)
Hz, CH3)
δ 11.1 (tdd, 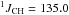 Hz,
Hz, 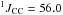 Hz,
Hz, 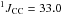 Hz, CH2)
Hz, CH2)
δ 120.8 (d, 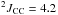 Hz, CN)
Hz, CN)
Propanenitrile-2, 3- 13 C 2
1H NMR (CDCl3, 400 MHz):
δ 1.30 (ddt, 3H,  Hz,
Hz, 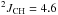 Hz,
Hz, 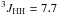 Hz, CH3)
Hz, CH3)
δ 2.35 (dqd, 2H, 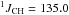 Hz,
Hz, 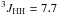 Hz,
Hz, 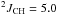 Hz, CH2)
Hz, CH2)
13C NMR (CDCl3, 100 MHz):
δ 10.5 (qd,  Hz,
Hz, 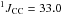 Hz, CH3)
Hz, CH3)
δ 11.1 (td, 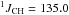 Hz,
Hz, 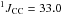 Hz, CH2)
Hz, CH2)
δ 120.8 (dd, 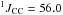 Hz,
Hz, 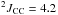 Hz, CN)
Hz, CN)
Appendix B: Experimental data
The experimental transition frequencies of the doubly 13C-substituted isotopomers of ethyl cyanide are available as supplementary material at CDS. The files S1.dat, S2.dat and S3.dat refer to the 1, 2-, 1, 3- and 2, 3-substituted isotopomers, respectively. Only the first 10 and the last 11 lines of the 1, 2-species appear in Table B.1. The files give the rotational quantum numbers J, Ka, and Kc for the upper state followed by those for the lower state. The observed transition frequency is given in megahertz units with its uncertainty and the residual between observed frequency and that calculated from the final set of spectroscopic parameters. Blended transitions are treated by fitting the intensity-averaged frequency, and this weight is also given in the tables. In most cases, the blending is caused by unresolved asymmetry splitting, i.e., the blended transitions agree in terms of their quantum numbers except for Kc (prolate paired transitions) or Ka (oblate paired transitions), and both transitions are equal in intensity. Accidental blending of transitions occured occasionally.
Experimental data for CH CH
CH CN.
CN.
Appendix C: Additional figures
Figures C.1–C.3 show the transitions of the three doubly 13C-substituted isotopologues of ethyl cyanide covered by the EMoCA survey toward Sgr B2(N2).
 |
Fig. C.1 Transitions of CH |
All Tables
Spectroscopic parameters of three ethyl cyanide isotopomers with two 13C atoms compared to those of the main isotopologue.
Rotational partition function values of three ethyl cyanide isotopomers with two 13C atoms at selected temperatures compared to those of the main isotopologue.
Vibrational correction factors to the rotational partition function of ethyl cyanide at selected temperatures.
Rotational temperatures derived from population diagrams of ethyl cyanide and its isotopologues toward Sgr B2(N2).
Parameters of our best-fit LTE model of ethyl cyanide and its isotopologues toward Sgr B2(N2).
All Figures
 |
Fig. 1 Panels a), b). Population diagram of CH |
| In the text | |
 |
Fig. C.1 Transitions of CH |
| In the text | |
 |
Fig. C.2 Same as Fig. C.1 for 13CH3CH |
| In the text | |
 |
Fig. C.3 Same as Fig. C.1 for 13CH |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.