| Issue |
A&A
Volume 586, February 2016
|
|
|---|---|---|
| Article Number | A17 | |
| Number of page(s) | 6 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201527602 | |
| Published online | 20 January 2016 | |
Rotational spectra of isotopic species of methyl cyanide, CH3CN, in their v8 = 1 excited vibrational states
1 I. Physikalisches Institut, Universität zu Köln, Zülpicher Str. 77, 50937 Köln, Germany
e-mail: hspm@ph1.uni-koeln.de
2 Jet Propulsion Laboratory, California Institute of Technology, Mail Stop 183-301, 4800 Oak Grove Drive, Pasadena, CA 91011-8099, USA
Received: 20 October 2015
Accepted: 26 November 2015
Context. Methyl cyanide is an important trace molecule in space, especially in star-forming regions where it is one of the more common molecules used to derive kinetic temperatures.
Aims. We want to obtain accurate spectroscopic parameters of minor isotopologs of methyl cyanide in their lowest excited ν8 = 1 vibrational states to support astronomical observations, in particular, with interferometers such as ALMA.
Methods. The laboratory rotational spectrum of methyl cyanide in natural isotopic composition has been recorded from the millimeter to the terahertz regions.
Results. Transitions with good signal-to-noise ratios could be identified for the three isotopic species CH313CN, 13CH3CN, and CH3C15N up to about 1.2 THz (J′′ ≤ 66). Accurate spectroscopic parameters were obtained for all three species.
Conclusions. The present data were already instrumental in identifying ν8 = 1 lines of methyl cyanide with one 13C in IRAM 30 m and ALMA data toward Sagittarius B2(N).
Key words: molecular data / methods: laboratory: molecular / techniques: spectroscopic / radio lines: ISM / ISM: molecules / astrochemistry
© ESO, 2016
1. Introduction
The degree of molecular complexity in the interstellar medium (ISM) is particularly large in the warm and dense parts of star-forming regions known as hot cores or hot corinos. A large number of organic molecules with up to 12 atoms have been detected thus far, for example, in the high-mass star-forming region Sagittarius (Sgr for short) B2(N) (Belloche et al. 2009, 2013, 2014). The Orion KL region is another high-mass star-forming region in which molecular complexity has been studied extensively (e.g., Tercero et al. 2015, and references therein). IRAS 16293-2422 (e.g., Jørgensen et al. 2012; Pineda et al. 2012, and references therein) and NGC 1333-IRAS2A (Maury et al. 2014) are corresponding examples of low-mass star-forming regions. The Molecules in Space webpage1 of the Cologne Database for Molecular Spectroscopy, CDMS (Müller et al. 2001, 2005) lists molecules detected in various astronomical sources with detailed information in most cases.
Molecular complexity in the ISM is not only manifest in increasingly larger molecules, but also in excited vibrational states or minor isotopologs of abundant smaller molecules. Corresponding laboratory rotational data are not only necessary to be able to avoid confusing such lines with those of larger and rarer molecules, but these data also have certain diagnostic values. Transitions in excited vibrational states may be used to derive a physical model of a particular source, as done, for example, in the case of CRL 618 and vibrationally excited HC3N isotopologs (Wyrowski et al. 2003). Ratios of isotopic species may provide clues to the formation of a molecule, such as more in the gas phase or more on grain surfaces, as in the case of dimethyl ether (Koerber et al. 2013), among others.
The 12C/13C ratio is particularly low in the vicinity of the Galactic center (~20) (Wilson & Rood 1994; Milam et al. 2005; Müller et al. 2008). Thus, it is not surprising that some 13C containing saturated organic molecules were detected soon after the main species and mainly toward the Galactic center source Sgr B2. These are H13C(O)NH2 (Lazareff et al. 1978), 13CH3OH (Gottlieb et al. 1979), and CH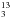 CN (Cummins et al. 1983). Both 13C containing CH3CN isotopomers were detected in a line survey of Orion (Sutton et al. 1985). Detections of molecules containing 13C were often hampered by the lack of laboratory data. In recent years, there have been several reports not only on the laboratory spectroscopy of 13C containing isotopologs, but also on their detections in space. These include ethyl cyanide (Demyk et al. 2007), vinyl cyanide (Müller et al. 2008), methyl formate (Carvajal et al. 2009), and dimethyl ether (Koerber et al. 2013). The 13C containing ethanol isotopomers have also been studied (Bouchez et al. 2012), but only tentatively identified in a line survey of Sgr B2(N) carried out with the IRAM 30 m radio telescope (Belloche et al. 2013). A firm detection has been achieved only very recently (Müller et al. 2016). Further investigations of 13C containing organic molecules include acetaldehyde (Margulès et al. 2015), whose detection has not been reported as far as we know, and methanol (Xu et al. 2014).
CN (Cummins et al. 1983). Both 13C containing CH3CN isotopomers were detected in a line survey of Orion (Sutton et al. 1985). Detections of molecules containing 13C were often hampered by the lack of laboratory data. In recent years, there have been several reports not only on the laboratory spectroscopy of 13C containing isotopologs, but also on their detections in space. These include ethyl cyanide (Demyk et al. 2007), vinyl cyanide (Müller et al. 2008), methyl formate (Carvajal et al. 2009), and dimethyl ether (Koerber et al. 2013). The 13C containing ethanol isotopomers have also been studied (Bouchez et al. 2012), but only tentatively identified in a line survey of Sgr B2(N) carried out with the IRAM 30 m radio telescope (Belloche et al. 2013). A firm detection has been achieved only very recently (Müller et al. 2016). Further investigations of 13C containing organic molecules include acetaldehyde (Margulès et al. 2015), whose detection has not been reported as far as we know, and methanol (Xu et al. 2014).
Methyl cyanide, CH3CN, also known as acetonitrile or cyanomethane, was among the early molecules to be detected by radio astronomical means. Solomon et al. (1971) detected it almost 40 years ago toward the massive star-forming regions Sgr A and B close to the Galactic center. The molecule was also detected in its ν8 = 1 excited vibrational state (Evib = 525 K and even in ν8 = 2 and in ν4 = 1 (Evib = 1324 K, Belloche et al. 2013, 2016). Several rarer isotopic species have been detected. They include CH2DCN in the hot core sources IRc2 in OMC1 and, tentatively, in G34.3 (Gerin et al. 1992) and in Sgr B2(N2) (Belloche et al. 2016). Sgr B2(N2) is to the north of Sgr B2(N1), which is also known as the Large Molecule Heimat (Belloche et al. 2016). Even 13CH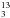 CN was detected in Sgr B2(N), first tentatively (Belloche et al. 2013) and, more recently, with confidence (Belloche et al. 2016). In addition, Belloche et al. (2013, 2016) identified transitions of CH
CN was detected in Sgr B2(N), first tentatively (Belloche et al. 2013) and, more recently, with confidence (Belloche et al. 2016). In addition, Belloche et al. (2013, 2016) identified transitions of CH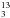 CN and 13CH3CN in their ν8 = 1 excited vibrational states.
CN and 13CH3CN in their ν8 = 1 excited vibrational states.
Methyl cyanide was also among the first molecules to be studied by microwave spectroscopy (Ring et al. 1947). In their rotational and rovibrational study of ν8 = 0, 1, and 2, Müller et al. (2015) revealed and analyzed pronounced interactions between the last two states. In addition, they analyzed an interaction between the first two states. A few years earlier, some of us contributed extensive accounts of the ground vibrational states of several isotopic species of methyl cyanide, including CH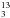 CN, 13CH3CN, and CH3C15N (Müller et al. 2009). Bauer & Maes (1969) and Bauer et al. (1975) reported the rotational spectrum of CH3C15N in its ν8 = 1 excited state up to J = 8−7 below 144 GHz. The only published rotational data of CH
CN, 13CH3CN, and CH3C15N (Müller et al. 2009). Bauer & Maes (1969) and Bauer et al. (1975) reported the rotational spectrum of CH3C15N in its ν8 = 1 excited state up to J = 8−7 below 144 GHz. The only published rotational data of CH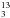 CN and 13CH3CN in their ν8 = 1 states were provided by Tam et al. (1988), who measured transitions up to J = 3−2 below 56 GHz.
CN and 13CH3CN in their ν8 = 1 states were provided by Tam et al. (1988), who measured transitions up to J = 3−2 below 56 GHz.
We have measured rotational spectra of methyl cyanide in natural isotopic composition both in wide and in more limited frequency windows up to 1.63 THz to provide new or updated catalog entries for various isotopologs of methyl cyanide, as well as for excited vibrational states. In the present article we analyzed these and new spectra to derive spectroscopic parameters of 13CH3CN, CH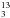 CN, and CH3C15N in their ν8 = 1 excited vibrational states; as usual, unlabeled atoms refer to 12C and 14N. Preliminary results from this study were used to identify lines of the 13C species in 3 mm molecular line surveys of Sgr B2(N) with the IRAM 30 m telescope (Belloche et al. 2013) and with the Atacama Large Millimeter/submillimeter Array (ALMA; Belloche et al. 2016).
CN, and CH3C15N in their ν8 = 1 excited vibrational states; as usual, unlabeled atoms refer to 12C and 14N. Preliminary results from this study were used to identify lines of the 13C species in 3 mm molecular line surveys of Sgr B2(N) with the IRAM 30 m telescope (Belloche et al. 2013) and with the Atacama Large Millimeter/submillimeter Array (ALMA; Belloche et al. 2016).
2. Experimental details
All measurements at the Universität zu Köln were recorded at room temperature in static or very slow flow mode, employing a 7 m long double path Pyrex glass cell equipped with Teflon windows and having an inner diameter of 100 mm. The pressure of CH3CN was in the range of 0.3−0.5 Pa. Source-frequency modulation was used with demodulation at 2f, causing an isolated line to appear close to a second derivative of a Gaussian.
The J = 3−2 transitions of 13CH3CN and CH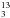 CN in their ν8 = 1 excited vibrational states around 53.8 and 55.3 GHz, respectively, were recorded with an Agilent E8257D microwave synthesizer as source and a Schottky diode detector. In addition, J = 6−5 transitions of 13CH3CN and CH3C15N around 107.4 GHz and the J = 5−4 and 6−5 transitions of CH
CN in their ν8 = 1 excited vibrational states around 53.8 and 55.3 GHz, respectively, were recorded with an Agilent E8257D microwave synthesizer as source and a Schottky diode detector. In addition, J = 6−5 transitions of 13CH3CN and CH3C15N around 107.4 GHz and the J = 5−4 and 6−5 transitions of CH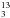 CN around 92.2 and 110.6 GHz, respectively, were recorded with essentially the same instrument. Source frequencies were generated using a Virginia Diodes, Inc. (VDI) tripler driven by the synthesizer mentioned above. The measurements were similar to the ones of 1,2-propanediol (Bossa et al. 2014) and mono-deuterated ethanol (Walters et al. 2015) taken at lower frequencies.
CN around 92.2 and 110.6 GHz, respectively, were recorded with essentially the same instrument. Source frequencies were generated using a Virginia Diodes, Inc. (VDI) tripler driven by the synthesizer mentioned above. The measurements were similar to the ones of 1,2-propanediol (Bossa et al. 2014) and mono-deuterated ethanol (Walters et al. 2015) taken at lower frequencies.
We assigned uncertainties from 5 to 20 kHz to these lines because the lines were so narrow at these low frequencies and because of the good signal-to-noise ratios (S/Ns). Hyperfine structure caused by the 14N nucleus was resolved partially for several of these transitions.
The majority of the data were extracted from broad frequency scans taken with the JPL cascaded multiplier spectrometer (Drouin et al. 2005). Generally, a multiplier chain source is passed through a one to two-meter path length flow cell and is detected by a silicon bolometer cooled to near 1.7 K. The cell is filled with a steady flow of reagent grade acetonitrile and the pressure and modulation are optimized to enable good S/Ns with narrow lineshapes. With a gas with very strong transitions, such as the K< 7 transitions of the main isotopolog of acetonitrile, the S/N was optimized for a higher K transition (e.g., K = 12), such that the lower K transitions exhibit saturated line profiles. This procedure enables a better dynamic range for the extraction of line positions for rare isotopologs and highly excited vibrational satellites. The frequency ranges covered most of the 400 to 1200 GHz region. Spectra around 1600 GHz were not considered in the present work because the ground state transitions of the three isotopic species were already too weak to be used in the final fits (Müller et al. 2009). These measurements are similar to recent ones of mono-deuterated ethane (Daly et al. 2015), with one important difference being the very small dipole moment of the ethane isotopolog.
The efficiency of frequency multipliers usually changes strongly with frequency. In addition, recording conditions and sensitivities of detectors can have strong influences on the quality of the spectra. Uncertainties of 50 to 100 kHz were assigned to typical lines. Larger uncertainties of up to 200 kHz were assigned to weaker lines or lines that were not isolated, conversely, smaller uncertainties down to 20 kHz were assigned for isolated lines with very good S/N and a very symmetric line shape.
3. Results, analyses, and discussion
The basic features of the rovibrational energy level structure of CH3CN in its three lowest vibrational states (ν8 ≤ 2) have been given by Müller et al. (2015). The main isotopolog of methyl cyanide and the ones investigated in the present work are prolate symmetric rotors with C3v symmetry. Since the three light H atoms are the only ones not on the symmetry axis, the A rotational parameter is much larger than B, ~5.27 cm-1 versus 0.307 cm-1. The rotational energy increases therefore rapidly with K and less so with J. The intensities of transitions involving higher K levels drop quickly in intensity as a consequence. The rotational energies of the highest K levels accessed in the present study correspond to ~750 cm-1 or ~1080 K. The ΔK = 0 selection rules for rotational transitions cause transitions with the same J to occur in a comparatively narrow frequency range with the spacing increasing quite regularly with K in the ground vibrational state. This is why rotational transitions of CH3CN are frequently used to infer the temperature in the dense ISM. The spacing between two specific K values also increases with J.
The lowest vibrationally excited state in CH3CN is the doubly degenerate bending state ν8 = 1 with Evib = 365 cm-1 or 525 K for the main isotopic species. Levels having the same K (> 0) repel each other because of the Coriolis interaction, lifting the degeneracy. The K levels in the l = −1 stack (i.e., k = K × sign(l) < 0) are shifted to higher energies, those in the l = + 1 stack (i.e., k> 0) to lower energies. The Coriolis parameter ζ is ~0.877, which is close to the limiting case of ζ = 1, causing levels with ΔK = Δl = 2 to be close in energy. The parameter q22 causes these levels to repel each other more, because it induces interactions between levels differing in ΔK = Δl = 2. The strength of the interaction depends strongly on J, as well as on K. The parameter q22 is often abbreviated as q.
These two interactions together lead to a more irregular pattern of the transitions in ν8 = 1 with the same J but differing K compared with the quite regular pattern in ν= 0. Those with k ≤ 0 occur for lower values of J at decreasing frequencies with fairly regularly increasing spacing. Transitions with k> 0 occur less regularly to lower frequencies with k = + 2 being higher in frequency than K = 0. Transitions with ΔK = Δl = 2 are often close in frequency at higher K in the absence of additional perturbations. The q22 interaction leads to an asymmetry-like A1/A2 splitting for the K = 1, l = + 1 level. The A1 component is always highest in frequency for each J, whereas the A2 component occurs near k = −6 for lower values of J. Figure 1 shows a part of the rotational spectrum of CH3CN, with transitions of the 13C isotopomers in their ν8 = 1 excited states.
 |
Fig. 1 Section of the submillimeter spectrum of CH3CN. Transitions of 13CH3CN (J = 36−35) and CH |
Spin statistics involving the three equivalent H atoms lead to A and E symmetry levels with ortho and para spin states, respectively. The K levels with K−l ≡ 0 mod 3 have A symmetry with twice the spin weight of the E symmetry K levels with the important exception of K = 0 in vibrational (sub-) states with l ≡ 0 mod 3, in particular in the ground vibrational state. If the K levels show A1/A2 splitting (e.g., k = + 1 in ν8 = 1 of the present CH3CN isotopologs), each level carries half the spin weight, which means they appear in intensity as if they were E-level transitions.
The large dipole moment of methyl cyanide (3.92197 (13) D, Gadhi et al. 1995) leads to a very strong rotational spectrum, such that CH2DCN and even 13CH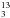 CN are observable in natural isotopic composition under favorable conditions (Müller et al. 2009). The dipole moment was also determined for CH3C15N, yielding an essentially identical value (3.9256 (7) D, Mito et al. 1984a). Dipole moments have also been determined for this isotopic species in excited vibrational states (Mito et al. 1984a,b). Excitation to ν8 = 1 leads to a value of 3.9073 (13) D.
CN are observable in natural isotopic composition under favorable conditions (Müller et al. 2009). The dipole moment was also determined for CH3C15N, yielding an essentially identical value (3.9256 (7) D, Mito et al. 1984a). Dipole moments have also been determined for this isotopic species in excited vibrational states (Mito et al. 1984a,b). Excitation to ν8 = 1 leads to a value of 3.9073 (13) D.
The terrestrial 12C/13C and 14N/15N ratios are about 90 and 270, respectively (Berglund & Wieser 2011). Transitions in the ν8 = 1 excited state are weaker by almost a factor of six than those of the ground vibrational state at room temperature. Thus, CH3C15N transitions in ν8 = 1 are more than five times stronger than 13CH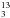 CN transitions in ν= 0. Initially, we searched for transitions of CH3C15N because attempts to assign 13C isotopomers in their ν8 = 1 states in astronomical data (Belloche et al. 2013, 2016) based on the previous laboratory data (Tam et al. 1988) already displayed deviations of up to 2 MHz in the 3 mm wavelength region.
CN transitions in ν= 0. Initially, we searched for transitions of CH3C15N because attempts to assign 13C isotopomers in their ν8 = 1 states in astronomical data (Belloche et al. 2013, 2016) based on the previous laboratory data (Tam et al. 1988) already displayed deviations of up to 2 MHz in the 3 mm wavelength region.
We adjusted the previous ν8 = 1 spectroscopic parameters of CH3C15N (Bauer & Maes 1969; Bauer et al. 1975) to those used for the main isotopic species (Müller et al. 2015) and estimated higher order parameters for the 15N species as described below. After fitting the previously reported transition frequencies with their reported uncertainties and creating predictions for higher frequencies, assignments could be made rather easily in the 446−483 GHz region (J′′ = 24−26). Subsequently, we estimated spectroscopic parameters for the CH3CN isotopomers containing one 13C isotope in their ν8 = 1 states from their ground-state parameters (Müller et al. 2009), along with the CH3CN and CH3C15N parameters in ν8 = 0 and 1. These assignments could be made relatively easily in essentially the same frequency window.
Subsequently, we increased the assignments in several steps to higher J and K values for all three isotopologs. Eventually, the J range covered 24 to 66 (23 to 64 for CH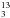 CN for which the B value is almost the same as for the main species). Around 20 transition frequencies could be assigned for each k and each of the two 13C isotopomers between k = −6 and +10 with assigments decreasing to higher K because of decreasing intensity. The number of assigned transition frequencies for each k of CH3C15N were very similar for the lowest energy k values, but decreased earlier because of the lower abundance of the isotopolog compared to the two with one 13C isotope. Assignments reached k = + 13 and initially k = −9 for all three isotopic species. Based on intensities, we expected to be able to assign at least some transitions with k = −10 and − 11, but these were not found to be sufficiently close to the predictions. We suspected that these rather weak transitions were perturbed by K = 10 and 11 of ν8 = 2, l = + 2 via a strong Δν8 = ± 1, ΔK = 0, Δl = ± 3 Fermi resonance with the strongest effects at K = 14 for the main isotopic species (Müller et al. 2015). Including estimates of low-order spectroscopic parameters for ν8 = 2 and the ν8 = 1/ν8 = 2 interaction parameters into the fit, we were able to assign some transitions with k = −10 and − 11 for all three isotopologs that displayed perturbations of up to 1 MHz and around 2 MHz, respectively. In the late stages of the project, we also made assignments at millimeter wavelengths for the three isotopic species. These covered J′′ = 2, 4 and 5 transitions for CH
CN for which the B value is almost the same as for the main species). Around 20 transition frequencies could be assigned for each k and each of the two 13C isotopomers between k = −6 and +10 with assigments decreasing to higher K because of decreasing intensity. The number of assigned transition frequencies for each k of CH3C15N were very similar for the lowest energy k values, but decreased earlier because of the lower abundance of the isotopolog compared to the two with one 13C isotope. Assignments reached k = + 13 and initially k = −9 for all three isotopic species. Based on intensities, we expected to be able to assign at least some transitions with k = −10 and − 11, but these were not found to be sufficiently close to the predictions. We suspected that these rather weak transitions were perturbed by K = 10 and 11 of ν8 = 2, l = + 2 via a strong Δν8 = ± 1, ΔK = 0, Δl = ± 3 Fermi resonance with the strongest effects at K = 14 for the main isotopic species (Müller et al. 2015). Including estimates of low-order spectroscopic parameters for ν8 = 2 and the ν8 = 1/ν8 = 2 interaction parameters into the fit, we were able to assign some transitions with k = −10 and − 11 for all three isotopologs that displayed perturbations of up to 1 MHz and around 2 MHz, respectively. In the late stages of the project, we also made assignments at millimeter wavelengths for the three isotopic species. These covered J′′ = 2, 4 and 5 transitions for CH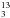 CN, J′′ = 2 and 5 for 13CH3CN, and J′′ = 5 for CH3C15N. Hyperfine structure caused by the 14N nucleus was resolved partially for several of the transitions in the millimeter-wave range.
CN, J′′ = 2 and 5 for 13CH3CN, and J′′ = 5 for CH3C15N. Hyperfine structure caused by the 14N nucleus was resolved partially for several of the transitions in the millimeter-wave range.
Prediction and fitting of the rotational spectra were carried out with the SPCAT/SPFIT program suite (Pickett 1991). In the CH3C15N fits, we used the previously recorded laboratory data (Bauer & Maes 1969; Bauer et al. 1975), except for the J′′ = 5 transition frequencies, which were replaced by our more accurate data. The ν8 = 1 and ν8 = 20 vibrational energies were taken from Duncan et al. (1978) and corrected for the differences with respect to values determined for the main species by Müller et al. (2015). The correction was only 0.02 cm-1 in the latter case because the sharp Q branch of the parallel 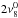 band permit accurate determinations of the vibrational energies. The absence of a single sharp Q branch in the perpendicular ν8 band led to a larger correction of 0.31 cm-1. Scaling of the vibrational energies of ν8 = 20 with the ratio E(ν8 = 22) /E(ν8 = 20) of the main isotopolog was used to estimate E(ν8 = 22) values for the three minor isotopic species.
band permit accurate determinations of the vibrational energies. The absence of a single sharp Q branch in the perpendicular ν8 band led to a larger correction of 0.31 cm-1. Scaling of the vibrational energies of ν8 = 20 with the ratio E(ν8 = 22) /E(ν8 = 20) of the main isotopolog was used to estimate E(ν8 = 22) values for the three minor isotopic species.
The purely axial parameters A (or A−B), DK, HK, etc., of a symmetric top molecule cannot be determined by rotational spectroscopy in the absence of perturbations. Moreover, rovibrational spectroscopy is only able to determine the differences with respect to the ground vibrational state unless ground-state ΔK = 3 loops are formed from rovibrational spectra involving two degenerate states, as was done initially for CH3CN (Anttila et al. 1993), or through perturbations, or through a combination of both, which we used in our study on ν8 ≤ 2 states of the main isotopolog (Müller et al. 2015).
The ground-state spectroscopic parameters of the minor isotopic species were initially taken from Müller et al. (2009). Because of slight changes in the ground-state parameters of CH3CN in our recent study (Müller et al. 2015), we adjusted the purely axial parameters, as well as the estimates of some higher order parameters, and redetermined the remaining ground-state spectroscopic parameters from the data in Müller et al. (2009). Initial ν8 = 1 spectroscopic parameters of CH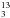 CN and 13CH3CN or changes thereof, as well as higher order values for CH3C15N, were derived from corresponding values for CH3CN by scaling them with appropriate powers of the ratio of the B rotational constants, as was done earlier for the ground vibrational data (Müller et al. 2009). Later, the distortion corrections η to Aζ were also scaled with the Aζ ratio, and the distortion corrections to q were scaled equivalently. Later, lower order spectroscopic parameters of ν8 = 2 were estimated in a similar way. The Fermi parameter F, which described the Δν8 = ± 1, ΔK = 0, Δl = ± 3 interaction between ν8 = 1 and 2, was assumed to be identical for all isotopologs. The hyperfine structure was reproduced well by the ground-state parameters of the respective isotopologs combined with the changes from the ground to the ν8 = 1 state of the main isotopic species.
CN and 13CH3CN or changes thereof, as well as higher order values for CH3C15N, were derived from corresponding values for CH3CN by scaling them with appropriate powers of the ratio of the B rotational constants, as was done earlier for the ground vibrational data (Müller et al. 2009). Later, the distortion corrections η to Aζ were also scaled with the Aζ ratio, and the distortion corrections to q were scaled equivalently. Later, lower order spectroscopic parameters of ν8 = 2 were estimated in a similar way. The Fermi parameter F, which described the Δν8 = ± 1, ΔK = 0, Δl = ± 3 interaction between ν8 = 1 and 2, was assumed to be identical for all isotopologs. The hyperfine structure was reproduced well by the ground-state parameters of the respective isotopologs combined with the changes from the ground to the ν8 = 1 state of the main isotopic species.
Throughout, we tried to fit the rotational spectra of the three methyl cyanide isotopologs with as few spectroscopic parameters being floated as possible. Starting from the lowest order parameters, we searched at each intermediate fit for the parameter that reduced the rms error (also known as the reduced or weighted χ squared) of the fit most. The rms error of the fit is a measure of the quality of the fit, and it should be close to 1.0 ideally, preferably slightly smaller. We tried to avoid floating parameters that changed from the initial value by too much.
Initially, our fits did not include any distortion corrections to F. However, we were unable to reproduce transitions with k = −10 and − 11 satisfactorily. Floating F yielded relatively small changes from the initial values compared to floating other parameters, but the values were increased by about 15% to 20%. Trial fits, with fixed FJ values scaled with the ratio of the B rotational constants included, reduced the values of F to around that of the main isotopic species. Even though including FK and FJJ had effects within the uncertainties of F, they were retained in the fits. The final set of ν8 = 1 spectroscopic parameters of the three methyl cyanide isotopic species is given in Table 1, along with values for the main species from Müller et al. (2015). The adjusted ground-state parameters are provided in Table 2. These parameters were kept fixed in all ν8 = 1 fits. Finally, the low-order ν8 = 2 parameters are summarized in Table 3, along with those describing the Fermi interaction between ν8 = 1 and 2.
The rms errors of all fits are around 0.8, so the experimental transition frequencies have been reproduced within uncertainties on average, and the uncertainties even appear to be slightly conservative. The ν8 = 1 parameters in Table 1 are quite similar among the isotopic species, in particular those of 13CH3CN and CH3C15N, which have rather similar vibrational energies and values of ΔB, Aζ, and q. The ground-state parameters of three minor isotopic species change only slightly with respect to our previous values because of relatively modest changes in the estimates of PJK and somewhat larger changes in LJ. The change in HK has no effect on the not purely axial parameters. The only floated parameter in Table 3 is the main Fermi term F. The values of the minor isotopic species differ slightly from those of the main species. Assumptions on the purely axial parameters, as well as the truncation of the ν8 = 2 parameters, may have non-negligible effects on these values. On the other hand, Müller et al. (2015) pointed out that F appeared to scale roughly with q for the isoelectronic molecules CH3CN, CH3CCH (Pracna et al. 2004) and CN3NC (Pracna et al. 2011). Interestingly, q and F of CH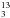 CN are both slightly greater than the values of the main species, whereas both are less by very similar amounts for 13CH3CN and CH3C15N, and, remarkably, basically identical with q = 16.78926 (19) MHz and F = 51 745 (3) MHz of CH3CCH (Pracna et al. 2004). However, the agreement with the CH3CCH values may be coincidental.
CN are both slightly greater than the values of the main species, whereas both are less by very similar amounts for 13CH3CN and CH3C15N, and, remarkably, basically identical with q = 16.78926 (19) MHz and F = 51 745 (3) MHz of CH3CCH (Pracna et al. 2004). However, the agreement with the CH3CCH values may be coincidental.
4. Conclusion
We have analyzed rotational transitions for three minor isotopologs of methyl cyanide in their ν8 = 1 excited vibrational states up to 1.2 THz and combined these data with existing ones in the case of CH3C15N. While the prospects of detecting such lines for this isotopic species are uncertain at present, transitions pertaining to 13CH3CN and CH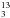 CN have already been identified in astronomical spectra with the help of preliminary results from this study (Belloche et al. 2013, 2016).
CN have already been identified in astronomical spectra with the help of preliminary results from this study (Belloche et al. 2013, 2016).
Predictions generated from the present data should be sufficient for observations with ALMA, and even more so with other arrays or single-dish radio telescopes. These predictions will be available in the catalog section2 of the Cologne Database for Molecular Spectroscopy3 (Müller et al. 2001, 2005). The complete line, parameter, and fit files will be deposited in the Spectroscopy Data section of the CDMS. Updated or new JPL catalog (Pickett et al. 1998) entries4 will also be available.
The very good S/N of our spectra should permit analyses of rotational transitions of 13CH3CN and CH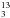 CN, possibly even of CH3C15N in their ν8 = 2 and ν4 = 1 excited vibrational states. Such transitions may well be detectable in astronomical spectra in case of the 13C containing isotopomers.
CN, possibly even of CH3C15N in their ν8 = 2 and ν4 = 1 excited vibrational states. Such transitions may well be detectable in astronomical spectra in case of the 13C containing isotopomers.
The use of enriched samples will permit accessing energy levels having even higher J and K quantum numbers, which will yield an improved description of the ν8 = 1 and 2 interactions. However, such a study will also require recording and analyses of ν8 and 2ν8 infrared spectra.
Acknowledgments
The measurements in Köln were supported by the Deutsche Forschungsgemeinschaft (DFG) through the collaborative research grant SFB 956, project area B3. We would like to thank Dr. Bernd Vowinkel for making Schottky detectors available for our measurements. The portion of this work, which was carried out at the Jet Propulsion Laboratory, California Institute of Technology, was performed under contract with the National Aeronautics and Space Administration (NASA).
References
- Anttila, R., Horneman, V.-M., Koivusaari, M., & Paso, R. 1993, J. Mol. Spectr., 157, 198 [NASA ADS] [CrossRef] [Google Scholar]
- Bauer, A., & Maes, S. 1969, J. Phys. (Paris), 30, 169 [CrossRef] [EDP Sciences] [Google Scholar]
- Bauer, A., Tarrago, G., & Remy, A. 1975, J. Mol. Spectr., 58, 111 [Google Scholar]
- Belloche, A., Garrod, R. T., Müller, H. S. P., et al. 2009, A&A, 499, 215 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Belloche, A., Müller, H. S. P., Menten, K. M., Schilke, P., & Comito, C. 2013, A&A, 559, A47 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Belloche, A., Garrod, R. T., Müller, H. S. P., & Menten, K. M. 2014, Science, 345, 1584 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Belloche, A., Müller, H. S. P., Garrod, R. T., & Menten, K. M. 2016, A&A, in press, DOI: 10.1051/0004-6361/201527268 [Google Scholar]
- Berglund, M., & Wieser, M. E. 2011, Pure Appl. Chem., 83, 397 [CrossRef] [Google Scholar]
- Bossa, J.-B., Ordu, M. H., Müller, H. S. P., Lewen, F., & Schlemmer, S. 2014, A&A, 570, A12 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bouchez, A., Walters, A., Müller, H. S. P., et al. 2012, J. Quant. Spectr. Rad. Transf., 113, 1148 [NASA ADS] [CrossRef] [Google Scholar]
- Carvajal, M., Margulès, L., Tercero, B., et al. 2009, A&A, 500, 1109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cummins, S. E., Green, S., Thaddeus, P., & Linke, R. A. 1983, ApJ, 266, 331 [NASA ADS] [CrossRef] [Google Scholar]
- Daly, A. M., Drouin, B. J., Groner, P., Yu, S., & Pearson, J. C. 2015, J. Mol. Spectr., 307, 27 [NASA ADS] [CrossRef] [Google Scholar]
- Demyk, K., Mäder, H., Tercero, B., et al. 2007, A&A, 466, 255 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Drouin, B. J., Maiwald, F. W., & Pearson, J. C. 2005, Rev. Sci. Instr., 76, 093113 [NASA ADS] [CrossRef] [Google Scholar]
- Duncan, J. L., McKean, D. C., Tullini, F., Nivellini, G. D., & Perez Peña, J. 1978, J. Mol. Spectr., 69, 123 [NASA ADS] [CrossRef] [Google Scholar]
- Gadhi, J., Lahrouni, A., Legrand, J., & Demaison, J. 1995, J. Chim. Phys. Phys-Chim. Biol., 92, 1984 [CrossRef] [EDP Sciences] [Google Scholar]
- Gerin, M., Combes, F., Wlodarczak, et al. 1992, A&A, 259, L35 [NASA ADS] [Google Scholar]
- Gottlieb, C. A., Ball, J. A., Gottlieb, E. W., & Dickinson, D. F. 1979, ApJ, 227, 422 [NASA ADS] [CrossRef] [Google Scholar]
- Koerber, M., Bisschop, S. E., Endres, C. P., et al. 2013, A&A, 558, A112 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Jørgensen, J. K., Favre, C., Bisschop, S. E., et al. 2012, ApJ, 757, L4 [NASA ADS] [CrossRef] [Google Scholar]
- Lazareff, B., Lucas, R., & Encrenaz, P. 1978, A&A, 70, L77 [NASA ADS] [Google Scholar]
- Margulès, L., Motiyenko, R. A., Ilyushin, V. V., & Guillemin, J. C. 2015, A&A, 579, A46 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Maury, A. J., Belloche, A., André, P., et al. 2014, A&A, 563, L2 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Milam, S. N., Savage, C., Brewster, M. A., Ziurys, L. M., & Wyckoff, S. 2005, ApJ, 634, 1126 [NASA ADS] [CrossRef] [Google Scholar]
- Mito, A., Sakai, J., & Katayama, M. 1984a, J. Mol. Spectr., 103, 26 [Google Scholar]
- Mito, A., Sakai, J., & Katayama, M. 1984b, J. Mol. Spectr., 105, 410 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Thorwirth, S., Roth, D. A., & Winnewisser, G. 2001, A&A, 370, L49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Schlöder, F., Stutzki, J., & Winnewisser, G. 2005, J. Mol. Struct, 742, 215 [CrossRef] [Google Scholar]
- Müller, H. S. P., Belloche, A., Menten, K. M., et. al. 2008, J. Mol. Spectr., 251, 319 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Drouin, B. J., & Pearson, J. C. 2009, A&A, 506, 1487 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Brown, L. R., Drouin, B. J., et al. 2015, J. Mol. Spectr., 312, 22 [NASA ADS] [CrossRef] [Google Scholar]
- Müller, H. S. P., Belloche, A., Xu, L.-H., et al. 2016, A&A, in press, DOI: 10.1051/0004-6361/201527470 [Google Scholar]
- Pickett, H. M. 1991, J. Mol. Spectr., 148, 371 [NASA ADS] [CrossRef] [Google Scholar]
- Pickett, H. M., Poynter, R. L., Cohen, E. A., et al. 1998, J. Quant. Spectr. Rad. Transf., 60, 883 [NASA ADS] [CrossRef] [Google Scholar]
- Pineda, J. E., Maury, A. J., Fuller, G. A., et al. 2012, A&A, 544, L7 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pracna, P., Müller, H. S. P., Klee, S., & Horneman, V.-M. 2004, Mol. Phys., 102, 1555 [NASA ADS] [CrossRef] [Google Scholar]
- Pracna, P., Urban, J., Votava, O., et al. 2011, Mol. Phys., 109, 2237 [NASA ADS] [CrossRef] [Google Scholar]
- Ring, H., Edwards, H., Kessler, M., & Gordy, W. 1947, Phys. Rev., 72, 1262 [NASA ADS] [CrossRef] [Google Scholar]
- Solomon, P. M., Jefferts, K. B., Penzias, A. A., & Wilson, R. W. 1971, ApJ, 168, L107 [NASA ADS] [CrossRef] [Google Scholar]
- Sutton, E. C., Blake, G. A., Masson, C. R., & Phillips, T. G. 1985, ApJS, 58, 341 [NASA ADS] [CrossRef] [Google Scholar]
- Tam, H., An, I., & Roberts, J. A. 1988, J. Mol. Spectr., 129, 202 [Google Scholar]
- Tercero, B., Cernicharo, J., López, A., et al. 2015, A&A, 582, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Walters, A., Schäfer, M., Ordu, M. H., et al. 2015, J. Mol. Spectr., 314, 6 [NASA ADS] [CrossRef] [Google Scholar]
- Wilson, T. L., & Rood, R. 1994, ARA&A, 32, 191 [NASA ADS] [CrossRef] [Google Scholar]
- Wyrowski, F., Schilke, P., Thorwirth, S., Menten, K. M., & Winnewisser, G. 2003, ApJ, 586, 344 [NASA ADS] [CrossRef] [Google Scholar]
- Xu, L.-H., Lees, R. M., Hao, Y., et al. 2014, J. Mol. Spectr., 303, 1 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Section of the submillimeter spectrum of CH3CN. Transitions of 13CH3CN (J = 36−35) and CH |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


