Table 3
Spectral-line results for ammonia, hydrogen sulphide, hydroxyl, and silicon monoxide.
| Species and | Rotational | Eupp/k† | Rest freq. | Setting & | rms‡ | Peak | Area | Veloc. range | |
| elec./vibr. state | quantum nums. | (K) | (GHz) | sideband | (mK) | (mK) | (K km s-1) | LSR (km s-1) | Comments |
|
|
|||||||||
| ortho-NH3v = 0 | JK =
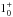 – –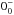 |
27 | 572.498 | 14 USB | 6.6 (1.05) | 308 | 15.5 | [–50; +65] | |
JK =
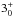 – –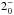 |
170 | 1763.525 | 19 LSB | 77.8 (1.02) | 1441 | 68.9 | g-fitted | ||
| para-NH3v = 0 | JK =
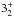 – –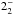 |
127 | 1763.821 | 19 LSB | 77.8 (1.02) | 450 | 21.0 | g-fitted | |
JK =
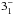 – –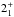 |
143 | 1763.602 | 19 LSB | 77.8 (1.02) | ≲100 | ≲4.5 | |||
| ortho-H2S v = 0 | JKa,Kc = 31,2–22,1 | 117 | 1196.012 | 05 LSB | 88.8 (1.01) | 177 | 10.3 | g-fitted | |
| para-H2S v = 0 | JKa,Kc = 31,3–20,2 | 103 | 1002.779 | 09 USB | 33.1 (1.05) | 97 | 4.22 | g-fitted | tent. detec. |
| JKa,Kc = 52,4–51,3 | 303 | 1862.436 | 01 LSB | 90.9 (0.97) | 590 | 11.8 | g-fitted | ||
OH 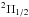 v = 0 v = 0 |
J = 3/2–1/2 | 270 | 1834.747 | 16 LSB | 55.5 (0.98) | 1768 | 85.5 | [–40; +90] | |
| 28SiO v = 0 | J = 14–13 | 219 | 607.608 | 13 LSB | 5.4 (0.99) | 370 | 15.5 | [–40; +105] | |
| J = 16–15 | 283 | 694.294 | 17 USB | 13.4 (1.08) | 384 | 15.1 | [–35; +85] | ||
| v = 1 | J = 13–12 | 1957 | 560.325 | 14 LSB | 6.6 (1.08) | 76 | 2.13 | [–15; +67] | |
| J = 15–14 | 2017 | 646.429 | 12 LSB | 7.8 (0.93) | 108 | 3.01 | [–20; +60] | ||
| J = 23–22 | 2340 | 990.355 | 09 LSB | 33.1 (1.06) | 178 | 5.12 | g-fitted | ||
| J = 27–26 | 2550 | 1161.945 | 06 USB | 59.5 (1.03) | 190 | 5.36 | g-fitted | ||
| J = 40–39 | 3462 | 1717.236 | 04 USB | 64.5 (1.05) | 160 | 4.98 | g-fitted | tent. detec. | |
| v = 2 | J = 26–25 | 4282 | 1111.235 | 07 USB | 22.0 (1.08) | 140 | 3.43 | g-fitted | |
| J = 27–26 | 4297 | 1153.804 | 06 LSB | 59.5 (1.04) | 925 | 25.3 | g-fitted | maser ? | |
| J = 28–27 | 4355 | 1196.353 | 05 LSB | 88.8 (1.01) | 50 | 2.50 | g-fitted | tent. detec. | |
| 29SiO v = 0 | J = 13–12 | 187 | 557.179 | 14 LSB | 6.6 (1.08) | 138 | 5.73 | [–15; +75] | |
| J = 26–25 | 722 | 1112.798 | 07 USB | 22.0 (1.08) | 203 | 7.07 | g-fitted | ||
| v = 1 | J = 16–15 | 2036 | 680.865 | 17 LSB | 13.4 (1.10) | 35 | 0.97 | g-fitted | |
| 30SiO v = 0 | J = 26–25 | 713 | 1099.708 | 07 LSB | 22.0 (1.09) | 231 | 6.47 | [–20; +70] | |
| J = 42–41 | 1832 | 1771.251 | 19 USB | 77.8 (1.02) | 153 | 3.68 | g-fitted | tent. detec. | |
Notes.
Since ortho- and para-NH3 behave as different chemical species, for
para-NH3 the level excitation energies are relative to that of the
lowest para level (JK =
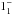 ), while
for ortho-NH3 are given w.r.t. the
JK =
), while
for ortho-NH3 are given w.r.t. the
JK =
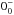 level.
Since the ortho and para spin isomer variants of hydrogen sulphide behave as
different chemical species, for ortho-H2S the level excitation energy is
relative to its lowest (ortho) energy level, the
JKa,Kc
= 10,1, while for the para species they are given w.r.t. the
JKa,Kc
= 00,0 ground level.
level.
Since the ortho and para spin isomer variants of hydrogen sulphide behave as
different chemical species, for ortho-H2S the level excitation energy is
relative to its lowest (ortho) energy level, the
JKa,Kc
= 10,1, while for the para species they are given w.r.t. the
JKa,Kc
= 00,0 ground level.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


