| Issue |
A&A
Volume 550, February 2013
|
|
|---|---|---|
| Article Number | A36 | |
| Number of page(s) | 13 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201220465 | |
| Published online | 22 January 2013 | |
The UMIST database for astrochemistry 2012⋆
1
Astrophysics Research Centre, School of Mathematics and Physics, Queen’s
University Belfast, Belfast, BT7
1NN
UK
e-mail: dmcelroy03@qub.ac.uk
2
Jodrell Bank Centre for Astrophysics, School of Physics and
Astronomy, University of Manchester, Manchester
M13 9PL,
UK
3
Astrochemistry Laboratory and the Goddard Center for Astrobiology,
Mailstop 691, NASA Goddard Space
Flight Center, 8800 Greenbelt Road, Greenbelt, MD
20770,
USA
4
Institute for Astrophysics and Computational Sciences, The
Catholic University of America, Washington, DC
20064,
USA
Received:
28
September
2012
Accepted:
20
December
2012
We present the fifth release of the UMIST Database for Astrochemistry (UDfA). The new reaction network contains 6173 gas-phase reactions, involving 467 species, 47 of which are new to this release. We have updated rate coefficients across all reaction types. We have included 1171 new anion reactions and updated and reviewed all photorates. In addition to the usual reaction network, we also now include, for download, state-specific deuterated rate coefficients, deuterium exchange reactions and a list of surface binding energies for many neutral species. Where possible, we have referenced the original source of all new and existing data. We have tested the main reaction network using a dark cloud model and a carbon-rich circumstellar envelope model. We present and briefly discuss the results of these models.
Key words: astrochemistry / molecular data / molecular processes / ISM: molecules / circumstellar matter
All codes, along with reaction networks and data files, are accessible at http://www.udfa.net.
© ESO, 2013
1. Introduction
Chemical models are an important tool in helping us understand various physical and chemical processes in space. The need for accurate models of chemical evolution of astrophysical environments is of ever-increasing importance as a new generation of ground-based and space-borne facilities opens up spectroscopic windows at high spatial and spectral resolution. The concurrent development of improved receivers and laboratory spectroscopy has led to the identification of more than 150 molecular species (ignoring isotopologues) and to the realisation that a full understanding of the physics and chemistry in molecular sources requires a detailed understanding of chemical kinetics and, in particular, reaction rate coefficients over a wide range of temperatures: from 10 K or less in pre-stellar cores, to several hundred Kelvin in hot molecular cores, to several thousand Kelvin in post-shock gas.
Although it has been widely recognised over the past decade that grain-surface chemistry can play a significant role in molecular synthesis, the many uncertainties associated with this has prevented the development of accurate quantitative models for surface chemistry. Thus, we do not attempt to include surface chemistry, and focus on describing gas-phase chemistry as accurately as possible.
In this paper, we present the fifth release of the UMIST Database for Astrochemistry,
Rate12 (previous releases: Rate91 – Millar et al. 1991; Rate95 – Millar
et al. 1997a; Rate99 – Le Teuff et al.
2000; Rate06 – Woodall et al.
2007). We have undertaken a major revision of the database. The gas-phase chemistry
is now described by 6173 reactions among 467 species, of which, 47 of these are new
additions, composed of 13 elements. We have a new website1 from which the reaction network and associated data and codes can be downloaded.
We also include state-specific rate coefficients for deuterium fractionation of
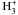 , a complete, singly-deuterated version of
Rate12 and surface binding energies of neutral species. We have made updates to
data across all reaction types and, in particular, have sought out original references to
the data, giving the DOI (Digital Object Identifier) code, where available.
, a complete, singly-deuterated version of
Rate12 and surface binding energies of neutral species. We have made updates to
data across all reaction types and, in particular, have sought out original references to
the data, giving the DOI (Digital Object Identifier) code, where available.
We use the entire gas-phase reaction network to model the chemistry in two environments: a dark cloud and a carbon-rich circumstellar envelope (CSE) surrounding an AGB star. Our results show that the data is sufficiently comprehensive to use in a range of astrophysical environments without the need to omit specific reactions or indeed to include additional reactions. Other environments for which Rate12 can be used without modification, along with careful treatment of molecular hydrogen and CO self shielding, are photodissociation regions (PDRs), hydrodynamic shock regions and diffuse clouds. Protoplanetary discs and hot molecular cores can be modelled with the addition of grain-surface chemistry.
In Sect. 2 we briefly describe the format of the data and in Sect. 3, we list the updates we have made. In Sect. 4 we outline some of the new features of Rate12, while in Sect. 5 we present the results of our model calculations which we also compare with observations of TMC-1 and IRC+10216.
Format of the colon-separated reaction network file.
Code, reaction type, and the number of each reaction type in Rate12.
2. Species and related data
We present the interstellar chemistry of 467 species, including 268 cations, 28 anions and 171 neutral species, in the form of the Rate12 reaction network, which is available electronically as a colon-separated file. The format of each line is explained in Table 1. Where available, Digital object identifiers (DOI) are used for the reference codes (REF). In cases where a DOI has not been found or does not exist, we adopt the Rate06 referencing method allocating a 4 digit code to each source. The reaction type codes are listed in Table 2.
For reactions where it has not been possible to define a single formula to fit the available data, the “NE” field gives the number of different temperature ranges for which we supply a different formula fitting the data. Care has been taken not to have any discontinuities in the rate coefficient between temperature ranges. Thus, if a particular rate coefficient is best fitted using two separate Arrhenius expressions for the temperature ranges 10–100 K and 100–1000 K, both expressions will give the same value at 100 K. In order to evaluate the rate coefficient outside the given temperature range, we recommend that the user chooses the expression that is closest to the temperature of interest. While there is no guarantee that this will give the correct rate coefficient, we have taken care to ensure that, when evaluated at low temperatures (<50 K), no rate coefficient will become unphysically large. We discuss this issue further in Sect. 3.8.
2.1. Calculation of the reaction rate coefficient using α, β, and γ
For two-body reactions the rate coefficient is given by the usual Arrhenius-type formula
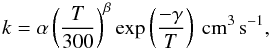 (1)where T (K) is the gas
temperature. For direct cosmic-ray ionisation (type = CP),
(1)where T (K) is the gas
temperature. For direct cosmic-ray ionisation (type = CP),
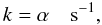 (2)whereas for cosmic-ray-induced photoreactions
(type = CR),
(2)whereas for cosmic-ray-induced photoreactions
(type = CR),  (3)where α is the cosmic-ray
ionisation rate, γ is the efficiency of the cosmic-ray ionisation event
as defined in Eq. (8) of Gredel et al. (1989), and
ω is the dust-grain albedo in the far ultraviolet (typically 0.4–0.6 at
150 nm). The cosmic-ray ionisation rates listed here are normalised to a total rate for
electron production from cosmic-ray ionisation (primarily from H2 and He in
dark clouds) of ζ0 = 1.36 × 10-17 s-1.
Rates for both direct cosmic-ray ionisation and cosmic-ray-induced photoreactions can be
scaled to other choices of the ionisation rate, ζ, by multiplying the
appropriate rate coefficients by ζ/ζ0. For
interstellar photoreactions (type = PH), the rate coefficient is parameterised as,
(3)where α is the cosmic-ray
ionisation rate, γ is the efficiency of the cosmic-ray ionisation event
as defined in Eq. (8) of Gredel et al. (1989), and
ω is the dust-grain albedo in the far ultraviolet (typically 0.4–0.6 at
150 nm). The cosmic-ray ionisation rates listed here are normalised to a total rate for
electron production from cosmic-ray ionisation (primarily from H2 and He in
dark clouds) of ζ0 = 1.36 × 10-17 s-1.
Rates for both direct cosmic-ray ionisation and cosmic-ray-induced photoreactions can be
scaled to other choices of the ionisation rate, ζ, by multiplying the
appropriate rate coefficients by ζ/ζ0. For
interstellar photoreactions (type = PH), the rate coefficient is parameterised as,
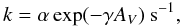 (4)where α represents the rate
coefficient in the unshielded interstellar ultraviolet radiation field,
AV is the dust extinction at visible wavelengths and
γ is the parameter used to take into account the increased dust
extinction at ultraviolet wavelengths.
(4)where α represents the rate
coefficient in the unshielded interstellar ultraviolet radiation field,
AV is the dust extinction at visible wavelengths and
γ is the parameter used to take into account the increased dust
extinction at ultraviolet wavelengths.
3. Updates since RATE06
3.1. Anion reactions
In 2006, C6H− was discovered in the Taurus Molecular Cloud 1 (TMC-1) by McCarthy et al. (2006). Since then, it and other anions have been detected in a variety of sources (Brünken et al. 2007; Remijan et al. 2007; Sakai et al. 2010b; Cordiner et al. 2011). Rate12 includes 22 new anions, which are involved in 1280 reactions. The anion reaction network comes from Walsh et al. (2009) with rate coefficients updated to include more recent laboratory measurements.
Anions are formed primarily via radiative electron attachment,
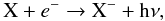 (5)and destruction mechanisms include mutual
neutralization with abundant cations, photodetachment reactions and reactions with atoms:
H, C, O and N. Of the new anion reactions, 950 are “mutual neutralisation” reactions
(A− + B + → A + B). This new reaction type, labelled “MN”,
replaces the Rate06 ion-molecule and ion-ion neutralisations reaction types (IM
and II). We have included reactions of anions with the 20 most abundant cations in a dark
cloud chemical model, along with the top 10 most abundant cations in the CSEs surrounding
both an oxygen-rich and a carbon-rich AGB star. These reactions are all assumed to have
the same rate coefficient,
k = 7.51 × 10-8 (T/300)-0.5
cm3 s-1, as included in the model of Walsh et al. (2009). The products are obtained, where appropriate, by
simple electron transfer. For cations with no direct neutral equivalent e.g.,
(5)and destruction mechanisms include mutual
neutralization with abundant cations, photodetachment reactions and reactions with atoms:
H, C, O and N. Of the new anion reactions, 950 are “mutual neutralisation” reactions
(A− + B + → A + B). This new reaction type, labelled “MN”,
replaces the Rate06 ion-molecule and ion-ion neutralisations reaction types (IM
and II). We have included reactions of anions with the 20 most abundant cations in a dark
cloud chemical model, along with the top 10 most abundant cations in the CSEs surrounding
both an oxygen-rich and a carbon-rich AGB star. These reactions are all assumed to have
the same rate coefficient,
k = 7.51 × 10-8 (T/300)-0.5
cm3 s-1, as included in the model of Walsh et al. (2009). The products are obtained, where appropriate, by
simple electron transfer. For cations with no direct neutral equivalent e.g.,
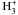 , we assume the neutralisation of the
cation results in the same products as its dissociative recombination e.g.
, we assume the neutralisation of the
cation results in the same products as its dissociative recombination e.g.
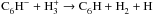 .
.
3.2. Dissociative recombination reactions
Since the release of Rate06, many new dissociative recombination (DR) rate coefficients have been accurately measured, along with branching ratios, at the CRYRING storage ring experiment in Stockholm. In Rate12, we include new DR data for 18 species, resulting in the addition of 58 new reactions. This brings the total DR product channels to 532 (compared with 486 in Rate06), 146 of these are measured.
3.3. Neutral-neutral reactions
There are 74 new or updated neutral-neutral reactions in Rate12 which have been measured in the laboratory. These include rate coefficient measurements down to 24 K using the CRESU (Cinitique de Reaction en Ecoulement Supersonique Uniforme or Reaction Kinetics in Uniform Supersonic Flow) method, and measurements of the reactions of many hydrocarbons (e.g. Carty et al. 2001; Canosa et al. 2007; Loison & Bergeat 2009; Berteloite et al. 2010).
3.4. Cation-neutral reactions
There are 174 new, and 30 updated, ion-neutral (IN) reactions in Rate12. The majority of the new cation-neutral reactions include neutral species new to Rate12. In Rate06 we included a separate “dipole-enhanced” reaction network, which enhanced rate coefficients at low temperatures in cases where the neutral species has a large, permanent dipole moment. Such enhancements, due to “dipole locking” effects (Troe 1987; Herbst & Leung 1986), result in rate coefficients which have a T−1/2 dependence at low temperatures. In our reaction file we included this power-law behaviour in all reactions for which (i) the neutral has a dipole moment in excess of 0.9 Debye; (ii) the reaction does not already have a temperature dependence; and (iii) the reaction does not have a measured rate coefficient at low temperatures. Woon & Herbst (2009) present an alternative approach, based on the Su-Chesnavich expression (Su & Chesnavich 1982). In Rate12, we offer only the “dipole-enhanced” reaction network which has been shown to give a much better fit to observations at low temperatures while not significantly influencing results at high temperature (≲1000 K).
3.5. Radiative association
There are just 17 updated or new radiative association (RA) rate coefficients in
Rate12. Although these are not many in number, several important reactions have
been revisited, measured or calculated. These include reactions of
C
 ,
,  and C+ with molecular hydrodgen
and the radiative association of H and H+.
and C+ with molecular hydrodgen
and the radiative association of H and H+.
3.6. Cosmic-ray-induced photoreactions
Rate12 includes at least one cosmic-ray photoreaction rate coefficient for all neutral and anionic species in the reaction network.
3.7. Photoprocesses
Since the last release of the database, many new photodissociation and photoionisation rates, along with depth-dependent rates, have been calculated (van Hemert & van Dishoeck 2008). These rates along with some corrections and unpublished data, have been incorporated into the database (van Dishoeck 2011, priv. comm.). We have also recalculated anion photorates using the Draine interstellar radiation field (Draine 1978), and the power law described in Millar et al. (2007). Rate12 now includes at least one photoprocess for every neutral species in the reaction network.
3.8. Refits to problematic reaction rates
In Rate06, there was a list of reaction ID’s for which it was recommended that the rate coefficients be set to zero at 10 K. This is because the best fit for the available data had a negative value for γ, which, in some cases, led to unrealistic divergent behaviour at low temperatures. Röllig (2011) addressed this problem and refitted many of the problematic reactions in Rate06. He fixed the value of the rate coefficient at 10 K to a particular value and used a fitting algorithm to find a new Arrhenius-type expression for the reaction. That paper also gave new fits for reactions which have discontinuities at certain temperatures, or temperature ranges in which no rate coefficient is defined in Rate06. He did this because, when using chemical models, it is often preferable to have a reaction rate that is continuous within the errors, than one that has discontinuities. In Rate12 we incorporate many of the changes suggested by Röllig (2011), and we use a similar method to fit some of our new reaction rate coefficients.
3.9. HNCO isomers
Three of the four isomers of isocyanic acid (HNCO) have been detected in space (Marcelino et al. 2008; Brünken et al. 2010). A comprehensive gas and grain reaction set was compiled and modelled by Quan et al. (2010). We have included all of the gas-phase reactions from Quan et al. (2010) in Rate12.
4. What’s new in RATE12
4.1. Deuterium chemistry
In order to accurately model deuterium chemistry, all D-bearing analogues of H-bearing species need to be included in a chemical model. This can increase the number of reactions by roughly an order of magnitude as well as increasing the number of ODEs to be solved. In order to reduce the number of reactions, specific rules can be adopted to limit the types of reaction that can occur (Roberts & Millar 2000). In addition, branching ratios and rates can differ significantly from their H equivalent and are often unmeasured. For these reasons, we are providing a list of important deuterium exchange reactions as well as a singly-deuterated version of Rate12 for download from our website.
Flower et al. (2004) have shown that the
H2 ortho/para ratio has a strong influence on the deuterium fractionation in
interstellar clouds. We also include, for download, a set of state-specific deuterated
reactions involving isotopologues of  ,
,  , and
, and
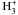 , mostly taken from Flower et al. (2004), Walmsley et al.
(2004) and Hugo et al. (2009).
, mostly taken from Flower et al. (2004), Walmsley et al.
(2004) and Hugo et al. (2009).
4.2. Reaction notes files
We have recorded detailed sets of notes for each new and updated reaction, giving comments on possible fits and reasons for particular choices regarding rate coefficients. These are available on the website.
4.3. Data source references
Where possible, we have cited the original source of data for each reaction. Correct citing of the data is important, not only to gauge the accuracy or reliability of a particular reaction rate coefficient, but also to ensure the original authors are correctly cited in future publications. All new and updated reactions are referenced with a DOI, where available, which allows website users to link directly to the paper’s webpage if they require further information on the data. We have also provided DOI codes, where available, for existing sources and located references for over 1200 reactions which were lacking source information in earlier versions of the database. In Appendix A, we list separately all original sources of laboratory and literature data.
4.4. INCHI codes
We have assigned all species in the database with an IUPAC International Chemical Identifiers (InChI). The InChI is a layered, variable length ASCII identifier which allows each species to be uniquely described. The Standard versions of InChI and InChIKey have been generated, where possible, for species in Rate12 and many of these have been cross-referenced against InChI data in other databases2.
4.5. VAMDC collaboration
UDfA is a part of the VAMDC project. VAMDC, the Virtual Atomic and Molecular Data Centre, aims to build a reliable, open, flexible interface to existing atomic and molecular (A&M) data hosted in various databases worldwide (Dubernet et al. 2010; Rixon et al. 2011). It will provide the wide community of both European and global users with access to a comprehensive, federated set of A&M data and application resources3.
4.6. Surface binding energies
In order to model grain-surface chemistry accurately, many factors need to be taken into account; the grain-surface morphology, composition, layering and grain size all effect how the chemistry progresses (Cuppen & Herbst 2005). In addition, under certain conditions, normal rate equation methods for modelling surface chemistry are inadequate and stochastic approaches such as Monte-Carlo, modified rate-equation and master equation methods are needed to model grain-surface chemistry more accurately (Charnley 1998; Caselli et al. 1998; Biham et al. 2001).
Surface binding-energies can be used for determining thermal and cosmic-ray-induced desorption rates from interstellar dust-grains and also for estimating the diffusion barriers between grain surface sites (Hasegawa et al. 1992). In Rate12, we have included a list of surface binding energies for 206 species. The binding energies in the list are a mixture of theoretical work, estimates and measurements (Hasegawa et al. 1992; Hasegawa & Herbst 1993; Tielens & Allamandola 1987; Allen & Robinson 1977; Garrod & Herbst 2006). Whittet et al. (1998) observed that water ice is the main constituent of interstellar dust-grain ice mantles and so, where possible, we have listed the values measured in water ice. We include recent measurements from Collings et al. (2004), Öberg et al. (2009b), Öberg et al. (2009c) and Edridge (2010).
4.7. New website
Our website4 has been significantly updated for this release of the database. In addition to being able to download the entire Rate12 reaction network, users can also now download the control models presented here, together with instructions on how to run and modify them. A singly-deuterated version of the whole reaction network, a state-specific deuterium chemistry and a list of surface binding energies are also available to download.
Comprehensive searching of the database by chemical species is, of course, possible. In addition, we have integrated content previously available5. This means that the entry for each species contains considerably more information than just those of reactions involving it. We now also present the control model data for both the dark cloud and circumstellar envelope models and the user can also explore the effects of switching on or off specific reactions. In addition, it is now possible to view results from a sensitivity analysis on a species by species basis. This analysis follows the method of Wakelam et al. (2010b), where many models are run with the reaction rate coefficients varied within the quoted errors using a log-normal distribution. The analysis provides information on the variation of the mean value of the fractional abundance of each species with time, and the first and second standard deviations away from the mean, for every species in the database and for both control models.
For each reaction, as much information as possible is presented regarding the evolution of rate coefficient data between previous releases of UDfA and the this one. In most cases where a rate coefficient has changed between Rate06 and Rate12, there is now an explanatory note. In the future, changes will not be made without these notes, and the version of the database at the time of publication will remain available, even if it is not the current one.
Finally, users of the website can comment directly on any species or reaction, for example, to discuss the values we have adopted or to alert us and the community to new laboratory or theoretical data.
5. Results
In this section, we use the complete Rate12 reaction network in two models: a dark cloud, and a circumstellar envelope (CSE) of a mass-losing, carbon-rich AGB star. Although we shall compare our results with observations towards two archetypical and well-studied objects, TMC-1 (CP) and IRC+10216, our purpose here is not to reproduce the observations in detail, but to check (i) whether our global rate file gives a reasonable first approximation to the chemistry in these objects and (ii) whether there are particular species for which our new gas-phase model dramatically fails to reproduce observations. The latter item will highlight particular species or reactions which require further investigation or whether additional chemical processes are required, e.g., grain-surface chemistry.
5.1. Dark cloud model
We model the gas-phase chemistry of a dark cloud by treating it as an homogeneous, isotropic cloud with constant physical parameters: n(H2) = 104 cm-3, T = 10 K, AV = 10 mag, a dust-grain albedo in the far ultraviolet of 0.6 and a cosmic-ray ionisation rate of 1.3 × 10-17 s-1. No grain-surface chemistry is included in this model except the formation of H2 via the association of two H atoms. This occurs at a rate of (5.2 × 10-17)(T/300)0.5nH n(H) cm-3 s-1 which assumes that all H atoms that stick to a grain surface will recombine to form H2. Our initial elemental abundances, which are listed in Table 3, are identical to those in Garrod et al. (2008), these are the low-metal abundances of Graedel et al. (1982), updated with more recent diffuse cloud values for He, C+, N and O.
Initial abundances relative to total H nuclei, nH.
5.1.1. Model results
Although we are not constructing a model of a particular dark cloud, it is instructive nonetheless, to compare a simple model with observations of TMC-1 (CP), the cyanopolyyne peak in the TMC-1 ridge, a dark cloud region in which over 60 molecules have been detected. In order to compare our model results with TMC-1 (CP) observed values, we used a simple method where we select the best-fit time as the time at which the most modelled abundances “match” those observed. We define a match for a particular species when its calculated abundance agrees within an order of magnitude of the observed value. This definition of a match, while simple, has the advantage of giving equal weight to all species. Thus, species which are known to have an incorrect or incomplete chemistry do not skew the agreement. For example, if we used the “distance” method of Wakelam et al. (2006), the calculation of the “best time” would be completely dominated by CH3OH, whose modelled abundance is more than 3 orders of magnitude lower than its observed value at all times in the model. It is well known that gas-phase models do not reproduce the methanol abundances seen in dark clouds, so this method would not give a good estimate of how well the model fits observations.
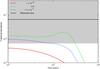 |
Fig. 1 Plot of C2H fractional abundance, relative to H2 abundance, as a function of time using different values of the rate coefficient for the reaction, C2H + O → CO + CH. The shaded region corresponds to an order of magnitude error on the observed abundance in TMC-1 (CP). |
As our model does not include grain-surface chemistry, we do not expect those species thought to form in, and on, grain mantles, such as methanol, to have an accurately modelled abundance. Our models are also clearly unphysical for times greater than about 1–2 million years since freeze out onto grain surfaces will dominate the evolution of the gas-phase abundances. Table 4 shows the observed and computed best-time abundances for 63 species at our “best-fit time” (defined above), ~2 × 105 years, when 41 of 63 species have abundances falling within an order of magnitude of those observed. A further 14 species match at some time between 104 and 108 years. In the table, the species listed in bold font are those for which the modelled abundance never falls within an order of magnitude of the observed value. Some of these species, for example, methanol and acetaldehyde, are generally thought to be produced mainly through grain-surface processes. However, other species, for instance, C2, C2H and C4H, are not thought to be influenced greatly by grain chemistry. The fact that these three species fall significantly below their observed values and the abundances calculated with Rate06, indicates that the updated Rate12 chemistry results in reduced abundances of C2-bearing species and some of the higher-order carbon chains, whose abundances depend strongly on the C2 chemistry.
One reaction which makes a significant difference to how well our results agree with observations, is C2H + O → CO + CH, one of the “key reactions” in the KIDA database (KInetic Database for Astrochemistry6, Wakelam et al. 2012). Rate12 adopts the same high value for the rate coefficient, 10-10 cm3 s-1, as recommended in the KIDA datasheets; the Rate06 value for this reaction is 1.7 × 10-11 cm3 s-1 at 10 K, on the recommendation of Baulch et al. (1992), based on laboratory measurements at room temperature and higher. Subsequently, Baulch (2005) recommended a rate coefficient about an order of magnitude larger with a constant value over a wide temperature range, based on a single measurement at 600 K. As it turns out, using the “old” lower value gives a better agreement with observations for a number of species such as C2H, C4H, CH3CHCH2 and SO2, while setting the rate coefficient to zero makes the agreement even better, this can be seen for C2H in Fig. 1. While one cannot accurately infer a rate coefficient from astrophysical data, this example highlights the importance of knowing rate coefficients accurately at low temperatures.
Observed fractional abundances, relative to H2, in TMC-1 (CP) and corresponding modelled “best fit” values.
In order to see how Rate12 compares with the KIDA database, we ran a dark cloud model, using the reaction network “kida.uva.2011”, with the same physical conditions and initial abundances as above. This reaction network is the most recent reaction network from the KIDA website for use in dense interstellar clouds.
As expected, the model results are largely similar to Rate12. Using the same
criteria as above, we find that of the 62 modelled species that have been observed in
TMC1 (CP), 38 agree at a “best fit” time of 2.5 × 105 years. In Table 5, we list those species for which we find either
Rate12 only or kida.uva.2011 only agree with observations. Of the species for
which Rate12 shows a better fit, those that stand out include acetaldehyde,
propene and formic acid. The differences between the kida.uva.2011 and Rate12
results for acetaldehyde and formic acid can be explained by the newly measured
dissociative recombination rate coefficients for the reactions 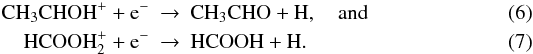 These rate coefficients are larger than
their previous values and are each the main formation mechanism for their respective
products (Vigren et al. 2010a; Hamberg et al. 2010). Similarly, kida.uva.2011 is
missing reactions that synthesise propene efficiently. In Rate12, propene
formation is initiated by the dissociative recombination of
C
These rate coefficients are larger than
their previous values and are each the main formation mechanism for their respective
products (Vigren et al. 2010a; Hamberg et al. 2010). Similarly, kida.uva.2011 is
missing reactions that synthesise propene efficiently. In Rate12, propene
formation is initiated by the dissociative recombination of
C
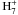 as outlined in Herbst et al. (2010). One species that is highly underabundant in
Rate12 is C2S, caused by the large rate coefficient of
1 × 10-10 cm-3 s-1 for its destruction by atomic
oxygen. This reaction has been deemed likely to proceed without a barrier by Loison et al. (2012), and is another example of how
lack of low temperature data can have a large impact on calculated abundances.
as outlined in Herbst et al. (2010). One species that is highly underabundant in
Rate12 is C2S, caused by the large rate coefficient of
1 × 10-10 cm-3 s-1 for its destruction by atomic
oxygen. This reaction has been deemed likely to proceed without a barrier by Loison et al. (2012), and is another example of how
lack of low temperature data can have a large impact on calculated abundances.
The major difference between Rate12 and Rate06 is the inclusion of anion reactions. Walsh et al. (2009) investigated the effect of anions on the chemistry in dark clouds. They used the Rate06 reaction network and added a set of carbon-chain anion reactions. They found that the addition of these anion reactions resulted in enhanced abundances of several families of carbon-chain molecules and better agreement with the observed values of cyanopolyyne molecules in TMC-1 (CP). Many of the same reactions and species are included in Rate12 and, as expected, the main differences between Rate06 and Rate12 are the same as those found by Walsh et al. (2009). Other differences not previously discussed are an improved agreement with abundances for both propyne and OCS. The former may be attributed to Rate12 having a more comprehensive treatment of allene (CH2CCH2) and propyne (CH3CCH) than Rate06, with the latter due to the inclusion of the newly reviewed and updated OCS chemistry of Loison et al. (2012).
List of species whose observed abundances are matched by only one of Rate12 or kida.uva.2011.
5.2. Carbon-rich circumstellar envelope model
In this section, we report the results of a simple chemical model for the CSE of a carbon-rich AGB star, using the new Rate12 reaction network to compute the chemical evolution. For a constant mass-loss rate, the star has a uniform, spherically symmetric outflow which can be easily modelled (Millar 1998).
5.2.1. CSE model details
The model for the CSE is based on that of Millar et al. (2000) using parameters appropriate to IRC+10216 but ignoring the presence of dust and gas shells in the outflow, the inclusion of which gives a significant improvement in agreement between models and observation in both column densities and spatial distributions (Cordiner & Millar 2009). The cool, expanding outer envelope of the carbon-rich AGB star, IRC+10216, is one of the most important extra-terrestrial environments for the study of gas-phase chemical kinetics due to the abundance and diversity of the different molecules detected in the envelope (e.g. Cernicharo et al. 2000). IRC+10216 has therefore been the subject of intensive ongoing astrochemical study (e.g. Bieging & Rieu 1988; Guélin et al. 1999; Cordiner & Millar 2009; De Beck et al. 2012; Agúndez et al. 2012), with over 80 different molecules detected in this source to date. We assume the central star loses mass at a uniform rate of 1.5 × 10-5M⊙ yr-1 (De Beck et al. 2012). The ejected matter expands in a spherically-symmetric outflow at a radial velocity of 14.5 km s-1, resulting in a gas density distribution that falls as 1/r2, where r is the distance from the central star. The adopted temperature profile in the envelope is based on an empirical fit to the gas kinetic temperature profile derived by Crosas & Menten (1997), the form of which is given in Eq. (1) of Cordiner & Millar (2009). Parent species (with abundances taken from Agúndez et al. 2012; and Cordiner & Millar 2009; see Table 6), are injected at the inner radius, ri = 2 × 1015 cm, where the molecular hydrogen number density is nH2 = 3.2 × 106 cm-3, and the kinetic temperature of the gas is 221 K. The standard interstellar radiation field (Draine 1978) is assumed to impinge on the outside of the envelope from all directions, and is attenuated by a radial visual extinction of AV = 6.9 mag at ri. The radiation flux inside the envelope is calculated assuming purely absorbing grains. Extinction of the incident radiation is derived using the Bohlin et al. (1978) standard interstellar gas-to-dust ratio and the extinction curve tabulated by Savage & Mathis (1979). Photodissociation of CO is assumed to occur through absorption of 100 nm photons, using a single-band approximation to account for self-shielding (Morris & Jura 1983).
The chemical kinetic equations are solved as a function of radius as material traverses the CSE, until it reaches the final radius, rf = 7 × 1017 cm, at which point the density has decreased to nH2 = 26 cm-3, the radial extinction is AV = 0.02 mag and the majority of molecules (apart from self-shielded H2) are dissociated.
Initial abundances of parent species relative to H2.
5.2.2. CSE model results
In Table 7 we list calculated and observed column densities for those species detected in IRC+10216. We obtain a match between observed and calculated column densities (to within an order of magnitude, as denoted by the “Agree” column) for 31 out of 46 of those species. In general, agreement is very good for the hydrocarbons, nitriles and anions, but is poor for CH3CCH and for the phosphorus-bearing species, which indicates that our understanding of the chemistry of these species in carbon-rich CSEs is not yet complete.
Compared with the previous models for large molecules in IRC+10216 by Millar et al. (2000, MHB00) and Cordiner & Millar
(2009, CM09), the new Rate12 chemical
network results in significantly better agreement between calculated and observed column
densities of the larger cyanopolyynes (HC2n+1N;
n = 2, 3, 4). The physical model and initial conditions for the CSE
in the present model are more similar to those of CM09 than MHB00, so comparison with
the results of CM09 is more relevant. Whereas, the calculated column densities for
HC5N, HC7N and HC9N from CM09 were about an
order-of-magnitude too large, we find that using the Rate12 model the column
densities of these species are accurately reproduced, within about a factor of two. The
marked differences between the cyanopolyyne abundances calculated by the two chemical
networks are shown in Fig. 2 (top left panel),
where the dotted lines show the results of the CM09 network and the solid lines are
using Rate12 (in this figure, the same physical model and initial conditions
are used for both networks). Using the CM09 network, the cyanopolyyne abundances peak
closer to the star and reach higher peak values. This is because CM09/MHB00 include the
following class of neutral-neutral reactions (for n = 1–11) that are
absent from Rate12 (except for the case of n = 1, which is
included and has an energy barrier of 770 K),
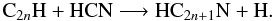 (8)These reactions dominate the synthesis of
cyanopolyynes in MHB00 and CM09 whereas in the Rate12 model, they are produced
mainly from the reaction of CN with (poly-)acetylenes,
(8)These reactions dominate the synthesis of
cyanopolyynes in MHB00 and CM09 whereas in the Rate12 model, they are produced
mainly from the reaction of CN with (poly-)acetylenes,  (9)For clarity, HC3N is not shown
in Fig. 2 as it exhibits the opposite behaviour to
the larger cyanopolyynes; its production rate in both models is dominated by Reaction
9, whereas in CM09, a larger
photodissociation rate for the molecule, as well as the inclusion of additional
destruction reactions with polyynes, leads to a reduction in the overall HC3N
abundance.
(9)For clarity, HC3N is not shown
in Fig. 2 as it exhibits the opposite behaviour to
the larger cyanopolyynes; its production rate in both models is dominated by Reaction
9, whereas in CM09, a larger
photodissociation rate for the molecule, as well as the inclusion of additional
destruction reactions with polyynes, leads to a reduction in the overall HC3N
abundance.
Calculated and observed column densities (cm-2) for a carbon-rich CSE.
 |
Fig. 2 Top left: plot of the fractional abundances, relative to H2, of cyanopolyynes as a function of envelope radius using the Rate12 model (solid lines) compared with the results from CM09 (dotted lines). Top right: plot of fractional abundances of polyynes as a function of envelope radius for the Rate12 model including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). Bottom left: plot of fractional abundances of polyyne anions as a function of envelope radius. Bottom right: comparison of the fractional abundances of various cations and electrons, including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). The C6H− fractional abundance for the “anions included” model is shown, for reference, with a dot-dashed line. |
Other species with improved matches, when compared with previous models, include
C3N and C3O, which are greater by factors of 20 and 200,
respectively, using the new network. This brings these into better agreement with
observations (see Table 7). In CM09 and MHB00,
C3N was produced primarily from HC3N photolysis, whereas in
Rate12 the following reaction dominates its synthesis,
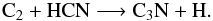 (10)The carbon-chain oxide, C3O, is
assumed to originate via the recombination of H3C3O+
and H2C3O+ in Rate12 and CM09. However, the
production of H3C3O+ is much more rapid in
Rate12 due to the inclusion of the radiative association reaction,
(10)The carbon-chain oxide, C3O, is
assumed to originate via the recombination of H3C3O+
and H2C3O+ in Rate12 and CM09. However, the
production of H3C3O+ is much more rapid in
Rate12 due to the inclusion of the radiative association reaction,
 (11)which is absent from CM09. The resulting
enhanced H3C3O+ abundance then leads to a substantially
increased C3O yield.
(11)which is absent from CM09. The resulting
enhanced H3C3O+ abundance then leads to a substantially
increased C3O yield.
The sulphuretted carbon chain, C4S, is another species for which the
calculated abundance has changed significantly from CM09. The abundance is an order of
magnitude less when using the new chemistry. In the CM09 model, C4S was
synthesised mainly via the sulphur exchange reaction,
 (12)for which the analogous reaction involving
C2H was measured in the laboratory by Smith et al. (2004). The unmeasured C4H + S reaction is not
included in Rate12, and the primary C4S production mechanism is via
the dissociative recombination of HC4S+.
(12)for which the analogous reaction involving
C2H was measured in the laboratory by Smith et al. (2004). The unmeasured C4H + S reaction is not
included in Rate12, and the primary C4S production mechanism is via
the dissociative recombination of HC4S+.
5.3. Impact of anions on the CSE chemistry
Polyyne anions have been included in models of circumstellar envelopes since the work of
Millar et al. (2000). Produced predominantly by
radiative electron attachment to neutral polyynes, the polyyne anions become abundant
inside the main molecular shell at a radius,
r ~ 1016−1017 cm (see Fig. 2, bottom left panel). C6H− is the
most abundant anion in the CSE as a result of the large C6H abundance and its
large electron attachment rate (the bare carbon chain anion,
C , also reaches a similar abundance).
, also reaches a similar abundance).
The presence of polyyne anions boosts the abundances of neutral polyynes towards larger
radii (~1017 cm), as shown by the solid lines in Fig. 2 (top right panel). The main source of polyynes at these radii is from
photodetachment of their respective anions (as studied in the laboratory by Best et al. 2011),
 (13)The presence of free electrons can thus have
a shielding effect on the polyynes because in the presence of UV radiation, anions tend to
undergo electron detachment rather than photodissociation.
(13)The presence of free electrons can thus have
a shielding effect on the polyynes because in the presence of UV radiation, anions tend to
undergo electron detachment rather than photodissociation.
In molecular clouds, polyyne anions can have significant effects on the carbon-chain
chemistry as a result of their high reactivity (Walsh
et al. 2009; Cordiner & Charnley
2012). In contrast, the presence of anions in a carbon-rich circumstellar envelope
has a greater impact on the chemistry as a result of their effect on the ionisation
balance. Anions affect the ionisation balance because they can remove a significant
fraction of free electrons from the gas. For
r ≈ 3 × 1016−1 × 1017 cm, the presence of anions
reduces the free electron number density by over an order of magnitude. A reduction in the
electron number density leads to reduced recombination rates, and consequently more
cations build up in the CSE (as shown by comparison of the solid and dashed lines for a
selection of the more abundant cations in the lower-right panel of Fig. 2). Within the main molecular shell, the majority of
cations exhibit increased abundances, enhanced by factors ~10–1000. For atomic
cations, however, the opposite effect occurs because mutual neutralisation is more rapid
than radiative recombination, such that, when the abundance of anions becomes sufficiently
large, the abundance of atomic cations fall. This is illustrated for the case of
C+ in Fig. 2 (lower-right panel). The
abundance of C H− is also shown for reference;
it exceeds the free electron abundance by about a factor of 4 at radial distances
(3−4) × 1016 cm, at which point it is the dominant charge carrier in the
envelope.
H− is also shown for reference;
it exceeds the free electron abundance by about a factor of 4 at radial distances
(3−4) × 1016 cm, at which point it is the dominant charge carrier in the
envelope.
The increased abundances of cations have knock-on effects on the chemistry, for example,
in the abundances of their respective recombination products. The
C H
H and
C
and
C H
H cations recombine to C2H (plus
products), so their elevated abundances give rise to the enhancement in C2H
seen at around (1−3) × 1017 cm in Fig. 2
(top-right panel). Other examples include C2H3 (produced from
recombination of C
cations recombine to C2H (plus
products), so their elevated abundances give rise to the enhancement in C2H
seen at around (1−3) × 1017 cm in Fig. 2
(top-right panel). Other examples include C2H3 (produced from
recombination of C H
H ), which then reacts with N atoms to
produce a corresponding enhancement in the CH2CN abundance.
), which then reacts with N atoms to
produce a corresponding enhancement in the CH2CN abundance.
Associative detachment reactions of polyyne anions with H atoms boost the abundances of
the polyacetylenes (HCnH) due to the reaction,
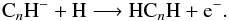 (14)However, these reactions are only efficient
in the outer envelope where the H-atom abundance becomes sufficiently large. In the outer
envelope the density is relatively low, so the resulting contributions to the total
polyacetylene column densities are small.
(14)However, these reactions are only efficient
in the outer envelope where the H-atom abundance becomes sufficiently large. In the outer
envelope the density is relatively low, so the resulting contributions to the total
polyacetylene column densities are small.
6. Summary
In this paper, we have presented the new release of the UMIST database for Astrochemistry, Rate12, describing, in detail, the updates and new additions made. We have presented results from a dark cloud model and a circumstellar envelope model using the Rate12 network and shown that these models give resonable agreement with observations, even in the absence of grain-surface chemistry.
These codes, along with sample output files and instructions on their usage, are available for download from our website7. Also available for download, are the Rate12 reaction network and files listing surface binding energies of species, a state-specific deuterated reaction network, a singly-deuterated version of Rate12 and a list of the important deuterium exchange reactions, so that users can construct their own deuterated networks.
We have updated the search facility on the website, which now displays all available information pertaining to a particular reaction or species. The website includes considerably more information than previously, including plots of molecule abundances in each model. Users can explore the effect that changing each rate coefficient has on the overall results of each model.
For more information about InChIs see http://www.inchi-trust.org
For more information about VAMDC and its associated standards, see http://www.vamdc.eu
Acknowledgments
We are grateful to E. van Dishoeck, E. Vigren, M. Röllig and R. Garrod for sending data and giving advice. We would also like to thank the referee for providing useful comments, which helped improve the paper. Research in molecular astrophysics at QUB, and in particular that of DMcE, is supported by a grant from the STFC. VAMDC is funded under the “Combination of Collaborative Projects and Coordination and Support Actions” Funding Scheme of The Seventh Framework Program. Call topic: INFRA-2008-1.2.2 Scientific Data Infrastructure. Grant Agreement number: 239108.
References
- Adams, N., & Smith, D. 1977, Chem. Phys. Lett., 47, 383 [NASA ADS] [CrossRef] [Google Scholar]
- Adams, N. G., Smith, D., & Paulson, J. F. 1980, J. Chem. Phys., 72, 288 [Google Scholar]
- Adams, N. G., Smith, D., & Millar, T. J. 1984, MNRAS, 211, 857 [NASA ADS] [Google Scholar]
- Adusei, G. Y., & Fontijn, A. 1994, Symposium (International) on Combustion, 25, 801 [CrossRef] [Google Scholar]
- Agúndez, M., Fonfría, J. P., Cernicharo, J., Pardo, J. R., & Guélin, M. 2008, A&A, 479, 493 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Cernicharo, J., Guélin, M., et al. 2010, A&A, 517, L2 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Fonfría, J. P., Cernicharo, J., et al. 2012, A&A, 543, A48 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Allen, M., & Robinson, G. W. 1977, ApJ, 212, 396 [NASA ADS] [CrossRef] [Google Scholar]
- Andreazza, C. M., & Singh, P. D. 1997, MNRAS, 287, 287 [NASA ADS] [CrossRef] [Google Scholar]
- Andreazza, C. M., Singh, P. D., & Sanzovo, G. C. 1995, ApJ, 451, 889 [NASA ADS] [CrossRef] [Google Scholar]
- Anicich, V. G. 1993, J. Phys. Chem. Ref. Data, 22, 1469 [NASA ADS] [CrossRef] [Google Scholar]
- Anicich, V. G., Laudenslager, J. B., Huntress, W. T., & Futrell, J. H. 1977, J. Chem. Phys., 67, 4340 [NASA ADS] [CrossRef] [Google Scholar]
- Anicich, V. G., Blake, G. A., Kim, J. K., McEwan, M. J., & Huntress, W. T. 1984, J. Phys. Chem., 88, 4608 [CrossRef] [Google Scholar]
- Anicich, V. G., Sen, A. D., Huntress, W. T., & McEwan, M. J. 1990, J. Chem. Phys., 93, 7163 [NASA ADS] [CrossRef] [Google Scholar]
- Anicich, V., Aeronautics, U. S. N., Administration, S., & (U.S.), J. P. L. 2003a, An Index of the Literature for Bimolecular Gas Phase Cation-Molecule Reaction Kinetics, JPL Publication (Jet Propulsion Laboratory, National Aeronautics and Space Administration) [Google Scholar]
- Anicich, V. G., Wilson, P., & McEwan, M. J. 2003b, J. Am. Soc. Mass Spectrom., 14, 900 [CrossRef] [Google Scholar]
- Apponi, A. J., McCarthy, M. C., Gottlieb, C. A., & Thaddeus, P. 1999, ApJ, 516, L103 [NASA ADS] [CrossRef] [Google Scholar]
- Bakker, E. J., van Dishoeck, E. F., Waters, L. B. F. M., & Schoenmaker, T. 1997, A&A, 323, 469 [NASA ADS] [Google Scholar]
- Barckholtz, C., Snow, T. P., & Bierbaum, V. M. 2001, ApJ, 547, L171 [NASA ADS] [CrossRef] [Google Scholar]
- Barlow, S. G. 1984, Ph.D. Thesis, University of Colorado [Google Scholar]
- Barlow, S., & Dunn, G. 1987, Int. J. Mass Spectrom. Ion Proc., 80, 227 [Google Scholar]
- Baulch, D. L. 2005, J. Phys. Chem. Ref. Data, 34, 757 [NASA ADS] [CrossRef] [Google Scholar]
- Baulch, D. L., Cobos, C. J., Cox, R. A., et al. 1992, J. Phys. Chem. Ref. Data, 21, 411 [NASA ADS] [CrossRef] [Google Scholar]
- Bell, M. B., Avery, L. W., & Feldman, P. A. 1993, ApJ, 417, L37 [NASA ADS] [CrossRef] [Google Scholar]
- Bell, M. B., Feldman, P. A., Watson, J. K. G., et al. 1999, ApJ, 518, 740 [Google Scholar]
- Berteloite, C., Le Picard, S. D., Balucani, N., Canosa, A., & Sims, I. R. 2010, Phys. Chem. Chem. Phys., 12, 3666 [CrossRef] [Google Scholar]
- Best, T., Otto, R., Trippel, S., et al. 2011, ApJ, 742, 63 [NASA ADS] [CrossRef] [Google Scholar]
- Bettens, R. P. A., Hansen, T. A., & Collins, M. A. 1999, J. Chem. Phys., 111, 6322 [NASA ADS] [CrossRef] [Google Scholar]
- Bieging, J. H., & Rieu, N.-Q. 1988, ApJ, 329, L107 [NASA ADS] [CrossRef] [Google Scholar]
- Biham, O., Furman, I., Pirronello, V., & Vidali, G. 2001, ApJ, 553, 595 [NASA ADS] [CrossRef] [Google Scholar]
- Black, J. H. 1975, Ph.D. Thesis, Harvard University [Google Scholar]
- Blake, G. A., Anicich, V. G., & Huntress, W. T. J. 1986, ApJ, 300, 415 [NASA ADS] [CrossRef] [Google Scholar]
- Bocherel, P., Herbert, L. B., Rowe, B. R., et al. 1996, J. Phys. Chem., 100, 3063 [CrossRef] [Google Scholar]
- Bohlin, R. C., Savage, B. D., & Drake, J. F. 1978, ApJ, 224, 132 [NASA ADS] [CrossRef] [Google Scholar]
- Bohme, D., Rakshit, A., & Schiff, H. 1982, Chem. Phys. Lett., 93, 592 [NASA ADS] [CrossRef] [Google Scholar]
- Brown, W. A., & Bolina, A. S. 2007, MNRAS, 374, 1006 [NASA ADS] [CrossRef] [Google Scholar]
- Bruhns, H., Kreckel, H., Miller, K., Urbain, X., & Savin, D. 2010, Phys. Rev. A, 82 [Google Scholar]
- Brunetti, B., & Liuti, G. 1975, Z. Phys. Chem., 94, 19 [CrossRef] [Google Scholar]
- Brünken, S., Gupta, H., Gottlieb, C. A., McCarthy, M. C., & Thaddeus, P. 2007, ApJ, 664, L43 [NASA ADS] [CrossRef] [Google Scholar]
- Brünken, S., Belloche, A., Martín, S., Verheyen, L., & Menten, K. M. 2010, A&A, 516, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Canosa, A., Sims, I. R., Travers, D., Smith, I. W. M., & Rowe, B. R. 1997, A&A, 323, 644 [NASA ADS] [Google Scholar]
- Canosa, A., Páramo, A., Le Picard, S. D., & Sims, I. R. 2007, Icarus, 187, 558 [NASA ADS] [CrossRef] [Google Scholar]
- Carty, D., Le Page, V., Sims, I. R., & Smith, I. W. 2001, Chem. Phys. Lett., 344, 310 [NASA ADS] [CrossRef] [Google Scholar]
- Caselli, P., Hasegawa, T. I., & Herbst, E. 1998, ApJ, 495, 309 [NASA ADS] [CrossRef] [Google Scholar]
- Cazaux, S., & Tielens, A. G. G. M. 2002, ApJ, 575, L29 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Gottlieb, C. A., Guelin, M., Thaddeus, P., & Vrtilek, J. M. 1989, ApJ, 341, L25 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Gottlieb, C. A., Guelin, M., et al. 1991, ApJ, 368, L39 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Kahane, C., Guelin, M., & Hein, H. 1987, A&A, 181, L9 [NASA ADS] [Google Scholar]
- Cernicharo, J., Guélin, M., & Kahane, C. 2000, A&AS, 142, 181 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Guélin, M., Agúndez, M., et al. 2007, A&A, 467, L37 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Guélin, M., Agúndez, M., McCarthy, M. C., & Thaddeus, P. 2008, ApJ, 688, L83 [NASA ADS] [CrossRef] [Google Scholar]
- Charnley, S. B. 1998, ApJ, 509, L121 [NASA ADS] [CrossRef] [Google Scholar]
- Chastaing, D., James, P. L., Sims, I. R., & Smith, I. W. M. 1998, Faraday Discuss., 109, 165 [NASA ADS] [CrossRef] [Google Scholar]
- Chastaing, D., James, P. L., Sims, I. R., & Smith, I. W. M. 1999, Phys. Chem. Chem. Phys., 1, 2247 [Google Scholar]
- Chastaing, D., Le Picard, S. D., & Sims, I. R. 2000, J. Chem. Phys., 112, 8466 [Google Scholar]
- Cohen, N., & Westberg, K. R. 1991, J. Phys. Chem. Ref. Data, 20, 1211 [NASA ADS] [CrossRef] [Google Scholar]
- Collings, M. P., Anderson, M. A., Chen, R., et al. 2004, MNRAS, 354, 1133 [NASA ADS] [CrossRef] [Google Scholar]
- Cordiner, M. A., & Charnley, S. B. 2012, ApJ, 749, 120 [NASA ADS] [CrossRef] [Google Scholar]
- Cordiner, M. A., & Millar, T. J. 2009, ApJ, 697, 68 [NASA ADS] [CrossRef] [Google Scholar]
- Cordiner, M. A., Charnley, S. B., Buckle, J. V., Walsh, C., & Millar, T. J. 2011, ApJ, 730, L18 [NASA ADS] [CrossRef] [Google Scholar]
- Crosas, M., & Menten, K. M. 1997, ApJ, 483, 913 [NASA ADS] [CrossRef] [Google Scholar]
- Cuppen, H. M., & Herbst, E. 2005, MNRAS, 361, 565 [NASA ADS] [CrossRef] [Google Scholar]
- Dalgarno, A., Du, M. L., & You, J. H. 1990, ApJ, 349, 675 [NASA ADS] [CrossRef] [Google Scholar]
- Danielsson, M., Hamberg, M., Zhaunerchyk, V., et al. 2008, Int. J. Mass Spectrom., 273, 111 [NASA ADS] [CrossRef] [Google Scholar]
- Daugey, N., Caubet, P., Retail, B., et al. 2005, Phys. Chem. Chem. Phys., 7, 2921 [CrossRef] [Google Scholar]
- Daugey, N., Caubet, P., Bergeat, A., Costes, M., & Hickson, K. M. 2008, Phys. Chem. Chem. Phys., 10, 729 [CrossRef] [Google Scholar]
- De Beck, E., Lombaert, R., Agúndez, M., et al. 2012, A&A, 539, A108 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Decker, B. K., Adams, N. G., & Babcock, L. M. 2000, Int. J. Mass Spectrom., 195, 185 [NASA ADS] [CrossRef] [Google Scholar]
- Draine, B. T. 1978, ApJS, 36, 595 [NASA ADS] [CrossRef] [Google Scholar]
- Drdla, K., Knapp, G. R., & van Dishoeck, E. F. 1989, ApJ, 345, 815 [NASA ADS] [CrossRef] [Google Scholar]
- Dubernet, M. L., Boudon, V., Culhane, J. L., et al. 2010, J. Quant. Spec. Radiat. Transf., 111, 2151 [NASA ADS] [CrossRef] [Google Scholar]
- Edridge, J. L. 2010, Ph.D. Thesis, UCL (University College London) [Google Scholar]
- Eichelberger, B., Snow, T. P., Barckholtz, C., & Bierbaum, V. M. 2007, ApJ, 667, 1283 [NASA ADS] [CrossRef] [Google Scholar]
- Elitzur, M., & de Jong, T. 1978, A&A, 67, 323 [NASA ADS] [Google Scholar]
- Ercolano, B., & Storey, P. J. 2006, MNRAS, 372, 1875 [NASA ADS] [CrossRef] [Google Scholar]
- Ferguson, E. E. 1973, At. Data Nucl. Data Tab., 12, 159 [NASA ADS] [CrossRef] [Google Scholar]
- Field, D., Adams, N. G., & Smith, D. 1980, MNRAS, 192, 1 [NASA ADS] [Google Scholar]
- Flower, D. R., Pineau des Forêts, G., & Walmsley, C. M. 2004, A&A, 427, 887 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ford, K. E. S., Neufeld, D. A., Schilke, P., & Melnick, G. J. 2004, ApJ, 614, 990 [NASA ADS] [CrossRef] [Google Scholar]
- Forte, L., Lien, M. H., Hopkinson, A. C., & Bohme, D. K. 1989, Can. J. Chem., 67, 1576 [CrossRef] [Google Scholar]
- Fosse, D., Cernicharo, J., Gerin, M., & Cox, P. 2001, ApJ, 552, 168 [NASA ADS] [CrossRef] [Google Scholar]
- Freeman, C. G., Harland, P. W., & McEwan, M. J. 1979, MNRAS, 187, 441 [NASA ADS] [Google Scholar]
- Frost, M. J., Sharkey, P., & Smith, I. W. M. 1993, J. Phys. Chem., 97, 12254 [CrossRef] [Google Scholar]
- Fukuzawa, K., & Osamura, Y. 1997, ApJ, 489, 113 [NASA ADS] [CrossRef] [Google Scholar]
- Gannon, K. L., Glowacki, D. R., Blitz, M. A., et al. 2007, J. Phys. Chem. A, 111, 6679 [CrossRef] [Google Scholar]
- Garrod, R. T., & Herbst, E. 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garrod, R. T., Weaver, S. L. W., & Herbst, E. 2008, ApJ, 682, 283 [NASA ADS] [CrossRef] [Google Scholar]
- Graedel, T. E., Langer, W. D., & Frerking, M. A. 1982, ApJS, 48, 321 [NASA ADS] [CrossRef] [Google Scholar]
- Gredel, R., Lepp, S., Dalgarno, A., & Herbst, E. 1989, ApJ, 347, 289 [NASA ADS] [CrossRef] [Google Scholar]
- Gu, X., Kaiser, R. I., Mebel, A. M., et al. 2009, ApJ, 701, 1797 [NASA ADS] [CrossRef] [Google Scholar]
- Guélin, M., Neininger, N., & Cernicharo, J. 1998, A&A, 335, L1 [NASA ADS] [Google Scholar]
- Guélin, M., Neininger, N., Lucas, R., & Cernicharo, J. 1999, in The Physics and Chemistry of the Interstellar Medium, eds. V. Ossenkopf, J. Stutzki, & G. Winnewisser, 326 [Google Scholar]
- Guélin, M., Muller, S., Cernicharo, J., McCarthy, M. C., & Thaddeus, P. 2004, A&A, 426, L49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Halfen, D. T., Clouthier, D. J., & Ziurys, L. M. 2008, ApJ, 677, L101 [NASA ADS] [CrossRef] [Google Scholar]
- Hamberg, M., Geppert, W. D., Thomas, R. D., et al. 2007, Mol. Phys., 105, 899 [NASA ADS] [CrossRef] [Google Scholar]
- Hamberg, M., Österdahl, F., Thomas, R. D., et al. 2010, A&A, 514, A83 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Harada, N., & Herbst, E. 2008, ApJ, 685, 272 [NASA ADS] [CrossRef] [Google Scholar]
- Harrison, J., Whyte, A., & Phillips, L. 1986, Chem. Phys. Lett., 129, 346 [NASA ADS] [CrossRef] [Google Scholar]
- Hasegawa, T. I., & Herbst, E. 1993, MNRAS, 261, 83 [NASA ADS] [CrossRef] [Google Scholar]
- Hasegawa, T. I., Herbst, E., & Leukng, C. M. 1992, ApJS, 82, 167 [NASA ADS] [CrossRef] [Google Scholar]
- Hawley, M., Mazely, T., Randeniya, L., et al. 1990, Int. J. Mass Spectrom. Ion Proc., 97, 55 [NASA ADS] [CrossRef] [Google Scholar]
- Hemsworth, R., Payzant, J., Schiff, H., & Bohme, D. 1974, Chem. Phys. Lett., 26, 417 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E. 1983, ApJS, 53, 41 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & Leung, C. M. 1986, ApJ, 310, 378 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & Leung, C. M. 1989, ApJS, 69, 271 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Herbst, E., & Leung, C. M. 1990, A&A, 233, 177 [NASA ADS] [Google Scholar]
- Herbst, E., & Osamura, Y. 2008, ApJ, 679, 1670 [Google Scholar]
- Herbst, E., Smith, D., & Adams, N. G. 1984, A&A, 138, L13 [NASA ADS] [Google Scholar]
- Herbst, E., Defrees, D. J., & Koch, W. 1989a, MNRAS, 237, 1057 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., Millar, T. J., Wlodek, S., & Bohme, D. K. 1989b, A&A, 222, 205 [NASA ADS] [Google Scholar]
- Herbst, E., Smith, D., Adams, N. G., & McIntosh, B. J. 1989c, J. Chem. Soc., Faraday Trans. 2, 85, 1655 [CrossRef] [Google Scholar]
- Herbst, E., Terzieva, R., & Talbi, D. 2000, MNRAS, 311, 869 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Herbst, E., Roueff, E., & Talbi, D. 2010, Mol. Phys., 108, 2171 [NASA ADS] [CrossRef] [Google Scholar]
- Herrero, V. J., Galvez, O., Mate, B., & Escribano, R. 2010, Phys. Chem. Chem. Phys., 12, 3164 [Google Scholar]
- Hoobler, R. J., & Leone, S. R. 1997, J. Geophys. Res., 102, 28717 [NASA ADS] [CrossRef] [Google Scholar]
- Hugo, E., Asvany, O., & Schlemmer, S. 2009, J. Chem. Phys., 130, 164302 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Huo, R.-P., Zhang, X., Huang, X.-R., Li, J.-L., & Sun, C.-C. 2011, J. Phys. Chem. A, 115, 3576 [CrossRef] [Google Scholar]
- Iglesias, E. 1977, ApJ, 218, 697 [NASA ADS] [CrossRef] [Google Scholar]
- Inomata, S., & Washida, N. 1999, J. Phys. Chem. A, 103, 5023 [CrossRef] [Google Scholar]
- Johnson, D. G., Blitz, M. A., & Seakins, P. W. 2000, Phys. Chem. Chem. Phys., 2, 2549 [CrossRef] [Google Scholar]
- Kaiser, R. I., & Gu, X. 2009, J. Chem. Phys., 131, 104311 [NASA ADS] [CrossRef] [Google Scholar]
- Kalenskii, S. V., Slysh, V. I., Goldsmith, P. F., & Johansson, L. E. B. 2004, ApJ, 610, 329 [NASA ADS] [CrossRef] [Google Scholar]
- Kalhori, S., Viggiano, A. A., Arnold, S. T., et al. 2002, A&A, 391, 1159 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Kamińska, M., Vigren, E., Zhaunerchyk, V., et al. 2008, ApJ, 681, 1717 [NASA ADS] [CrossRef] [Google Scholar]
- Kern, R. D., Singh, H. J., & Wu, C. H. 1988, Int. J. Chem. Kinet., 20, 731 [CrossRef] [Google Scholar]
- Klippenstein, S. J., Georgievskii, Y., & McCall, B. J. 2010, J. Phys. Chem. A, 114, 278 [CrossRef] [Google Scholar]
- Kuan, W., Jiang, T., & Chung, K. 1999, Phys. Rev. A, 60, 364 [NASA ADS] [CrossRef] [Google Scholar]
- Larsson, M., Ehlerding, A., Geppert, W. D., et al. 2005, J. Chem. Phys., 122, 156101 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Laufer, A. H., & Fahr, A. 2004, Chem. Rev., 104, 2813 [CrossRef] [Google Scholar]
- Lawson, P. A., Osborne, D., & Adams, N. G. 2011, Int. J. Mass Spectrom., 304, 41 [Google Scholar]
- LePicard, S. D., Canosa, A., Reignier, D., & Stoecklin, T. 2002, Phys. Chem. Chem. Phys., 4, 3659 [CrossRef] [Google Scholar]
- LeTeuff, Y. H., Millar, T. J., & Markwick, A. J. 2000, A&AS, 146, 157 [NASA ADS] [CrossRef] [EDP Sciences] [MathSciNet] [PubMed] [Google Scholar]
- Leung, C. M., Herbst, E., & Huebner, W. F. 1984, ApJS, 56, 231 [NASA ADS] [CrossRef] [Google Scholar]
- Lias, S. G., & Ausloos, P. 1987, Int. J. Mass Spectrom. Ion Proc., 81, 165 [NASA ADS] [CrossRef] [Google Scholar]
- Liddy, J. P., Freeman, C. G., & McEwan, M. J. 1975, Astrophys. Lett., 16, 155 [NASA ADS] [Google Scholar]
- Lin, P., & Lucchese, R. R. 2001, J. Chem. Phys., 114, 9350 [NASA ADS] [CrossRef] [Google Scholar]
- Lique, F., Cernicharo, J., & Cox, P. 2006, ApJ, 653, 1342 [NASA ADS] [CrossRef] [Google Scholar]
- Loison, J.-C., & Bergeat, A. 2004, Phys. Chem. Chem. Phys., 6, 5396 [CrossRef] [Google Scholar]
- Loison, J.-C., & Bergeat, A. 2009, Phys. Chem. Chem. Phys., 11, 655 [CrossRef] [Google Scholar]
- Loison, J.-C., Halvick, P., Bergeat, A., Hickson, K. M., & Wakelam, V. 2012, MNRAS, 421, 1476 [NASA ADS] [CrossRef] [Google Scholar]
- Lovas, F. J., Remijan, A. J., Hollis, J. M., Jewell, P. R., & Snyder, L. E. 2006, ApJ, 637, L37 [NASA ADS] [CrossRef] [Google Scholar]
- Luca, A., Voulot, D., & Gerlich, D. 2002, in WDS02 Proc. Contribution Papers, Part II, ed. J. Safrankova, 294 [Google Scholar]
- Marcelino, N., Cernicharo, J., Agúndez, M., et al. 2007, ApJ, 665, L127 [NASA ADS] [CrossRef] [Google Scholar]
- Marcelino, N., Cernicharo, J., Tercero, B., & Roueff, E. 2008, ApJ, 690, L27 [Google Scholar]
- Martinez, Jr., O., Betts, N. B., Villano, S. M., et al. 2008, ApJ, 686, 1486 [NASA ADS] [CrossRef] [Google Scholar]
- Martinez, O., Yang, Z., Demarais, N. J., Snow, T. P., & Bierbaum, V. M. 2010, ApJ, 720, 173 [NASA ADS] [CrossRef] [Google Scholar]
- McCarthy, M. C., Gottlieb, C. A., Gupta, H., & Thaddeus, P. 2006, ApJ, 652, L141 [NASA ADS] [CrossRef] [Google Scholar]
- McEwan, M. J., Anicich, V. G., & Huntress, Jr., W. T. 1980, in Interstellar Molecules, ed. B. H. Andrew, IAU Symp., 87, 299 [Google Scholar]
- McEwan, M. J., Scott, G. B., & Anicich, V. G. 1998, Int. J. Mass Spectrom. Ion Proc., 172, 209 [NASA ADS] [CrossRef] [Google Scholar]
- Midey, A., Dotan, I., & Viggiano, A. A. 2008, J. Phys. Chem. A, 112, 3040 [CrossRef] [Google Scholar]
- Milam, S. N., Halfen, D. T., Tenenbaum, E. D., et al. 2008, ApJ, 684, 618 [NASA ADS] [CrossRef] [Google Scholar]
- Millar, T. J. 1991, A&A, 242, 241 [NASA ADS] [Google Scholar]
- Millar, T. J. 1998, The Molecular Astrophysics of Stars and Galaxies, eds. T. W. Hartquist, & D. A. Williams (Oxford: Clarendon Press), 4, 331 [Google Scholar]
- Millar, T. J., & Herbst, E. 1990, A&A, 231, 466 [NASA ADS] [Google Scholar]
- Millar, T. J., Adams, N. G., Smith, D., & Clary, D. C. 1985, MNRAS, 216, 1025 [NASA ADS] [Google Scholar]
- Millar, T. J., Adams, N. G., Smith, D., Lindinger, W., & Villinger, H. 1986, MNRAS, 221, 673 [Google Scholar]
- Millar, T. J., Bennett, A., & Herbst, E. 1987, MNRAS, 229, 41P [NASA ADS] [CrossRef] [Google Scholar]
- Millar, T. J., Defrees, D. J., McLean, A. D., & Herbst, E. 1988, A&A, 194, 250 [NASA ADS] [PubMed] [Google Scholar]
- Millar, T. J., Bennett, A., Rawlings, J. M. C., Brown, P. D., & Charnley, S. B. 1991, A&AS, 87, 585 [NASA ADS] [Google Scholar]
- Millar, T. J., Farquhar, P. R. A., & Willacy, K. 1997a, A&AS, 121, 139 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Millar, T. J., MacDonald, G. H., & Gibb, A. G. 1997b, A&A, 325, 1163 [NASA ADS] [Google Scholar]
- Millar, T. J., Herbst, E., & Bettens, R. P. A. 2000, MNRAS, 316, 195 [NASA ADS] [CrossRef] [Google Scholar]
- Millar, T. J., Walsh, C., Cordiner, M. A., Ní Chuimín, R., & Herbst, E. 2007, ApJ, 662, L87 [NASA ADS] [CrossRef] [Google Scholar]
- Milligan, D. B., Wilson, P. F., Freeman, C. G., Meot-Ner, M., & McEwan, M. J. 2002, J. Phys. Chem. A, 106, 9745 [CrossRef] [Google Scholar]
- Minh, Y. C., Irvine, W. M., & McGonagle, D. 1993, J. Korean Astron. Soc., 26, 99 [Google Scholar]
- Mitchell, G. F. 1984, ApJS, 54, 81 [NASA ADS] [CrossRef] [Google Scholar]
- Mitchell, G. F., Kuntz, P. J., & Ginsburg, J. L. 1977, ApJ, 212, 71 [NASA ADS] [CrossRef] [Google Scholar]
- Mitchell, G. F., Kuntz, P. J., & Ginsburg, J. L. 1978, ApJS, 38, 39 [NASA ADS] [CrossRef] [Google Scholar]
- Montaigne, H., Geppert, W. D., Semaniak, J., et al. 2005, ApJ, 631, 653 [NASA ADS] [CrossRef] [Google Scholar]
- Moran, T. F., & Hamill, W. H. 1963, J. Chem. Phys., 39, 1413 [NASA ADS] [CrossRef] [Google Scholar]
- Morris, M., & Jura, M. 1983, ApJ, 264, 546 [NASA ADS] [CrossRef] [Google Scholar]
- Nahar, S. N., & Pradhan, A. K. 1997, ApJS, 111, 339 [NASA ADS] [CrossRef] [Google Scholar]
- Nejad, L. A. M., & Millar, T. J. 1988, MNRAS, 230, 79 [NASA ADS] [Google Scholar]
- Neufeld, D. A., & Wolfire, M. G. 2009, ApJ, 706, 1594 [NASA ADS] [CrossRef] [Google Scholar]
- Nguyen, T. L., Peeters, J., & Vereecken, L. 2006, J. Phys. Chem. A, 110, 12166 [CrossRef] [Google Scholar]
- Novotný, O., Buhr, H., Stützel, J., et al. 2010, J. Phys. Chem. A, 114, 4870 [CrossRef] [PubMed] [Google Scholar]
- Öberg, K. I., van Broekhuizen, F., Fraser, H. J., et al. 2005, ApJ, 621, L33 [NASA ADS] [CrossRef] [Google Scholar]
- Öberg, K. I., Garrod, R. T., van Dishoeck, E. F., & Linnartz, H. 2009a, A&A, 504, 891 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Öberg, K. I., Linnartz, H., Visser, R., & van Dishoeck, E. F. 2009b, ApJ, 693, 1209 [NASA ADS] [CrossRef] [Google Scholar]
- Öberg, K. I., van Dishoeck, E. F., & Linnartz, H. 2009c, A&A, 496, 281 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ohishi, M., Kaifu, N., Kawaguchi, K., et al. 1989, ApJ, 345, L83 [NASA ADS] [CrossRef] [Google Scholar]
- Öjekull, J., Andersson, P. U., Någård, M. B., et al. 2004, J. Chem. Phys., 120, 7391 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Osamura, Y., Fukuzawa, K., Terzieva, R., & Herbst, E. 1999, ApJ, 519, 697 [NASA ADS] [CrossRef] [Google Scholar]
- Pequignot, D., & Aldrovandi, S. M. V. 1986, A&A, 161, 169 [NASA ADS] [Google Scholar]
- Petrie, S., & Herbst, E. 1997, ApJ, 491, 210 [NASA ADS] [CrossRef] [Google Scholar]
- Petrie, S., Freeman, C. G., McEwan, M. J., & Ferguson, E. E. 1991, MNRAS, 248, 272 [NASA ADS] [Google Scholar]
- Petrie, S., Millar, T. J., & Markwick, A. J. 2003, MNRAS, 341, 609 [NASA ADS] [CrossRef] [Google Scholar]
- Petuchowski, S. J., Dwek, E., Allen, J. E., J., & Nuth, J. A. I. 1989, ApJ, 342, 406 [NASA ADS] [CrossRef] [Google Scholar]
- Pineau des Forêts, G., Roueff, E., & Flower, D. R. 1986, MNRAS, 223, 743 [NASA ADS] [CrossRef] [Google Scholar]
- Plasil, R., Mehner, T., Dohnal, P., et al. 2011, ApJ, 737, 60 [NASA ADS] [CrossRef] [Google Scholar]
- Pradhan, A., & Dalgarno, A. 1994, Phys. Rev. A, 49, 960 [NASA ADS] [CrossRef] [Google Scholar]
- Prasad, S. S., & Huntress, W. T. J. 1980, ApJS, 43, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Pulliam, R. L., Edwards, J. L., & Ziurys, L. M. 2011, ApJ, 743, 36 [NASA ADS] [CrossRef] [Google Scholar]
- Quan, D., Herbst, E., Osamura, Y., & Roueff, E. 2010, ApJ, 725, 2101 [NASA ADS] [CrossRef] [Google Scholar]
- Rawlings, J. M. C., Drew, J. E., & Barlow, M. J. 1993, MNRAS, 265, 968 [NASA ADS] [Google Scholar]
- Rebrion, C., Marquette, J., Rowe, B., & Clary, D. 1988, Chem. Phys. Lett., 143, 130 [NASA ADS] [CrossRef] [Google Scholar]
- Remijan, A. J., Hollis, J. M., Snyder, L. E., Jewell, P. R., & Lovas, F. J. 2006, ApJ, 643, L37 [NASA ADS] [CrossRef] [Google Scholar]
- Remijan, A. J., Hollis, J. M., Lovas, F. J., et al. 2007, ApJ, 664, L47 [NASA ADS] [CrossRef] [Google Scholar]
- Rixon, G., Dubernet, M. L., Piskunov, N., et al. 2011, in AIP Conf. Ser. 1344, eds. A. Bernotas, R. Karazija, & Z. Rudzikas, 107 [Google Scholar]
- Roberge, W. G., Jones, D., Lepp, S., & Dalgarno, A. 1991, ApJS, 77, 287 [NASA ADS] [CrossRef] [Google Scholar]
- Roberts, H., & Millar, T. J. 2000, A&A, 361, 388 [NASA ADS] [Google Scholar]
- Röhrig, M., & Wagner, H. G. 1994, Symposium (International) on Combustion, 25, 975 [CrossRef] [Google Scholar]
- Röllig, M. 2011, A&A, 530, A9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Ruffle, D. P., Bettens, R. P. A., Terzieva, R., & Herbst, E. 1999, ApJ, 523, 678 [NASA ADS] [CrossRef] [Google Scholar]
- Sakai, N., Saruwatari, O., Sakai, T., Takano, S., & Yamamoto, S. 2010a, A&A, 512, A31 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Sakai, N., Shiino, T., Hirota, T., Sakai, T., & Yamamoto, S. 2010b, ApJ, 718, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Savage, B. D., & Mathis, J. S. 1979, ARA&A, 17, 73 [NASA ADS] [CrossRef] [Google Scholar]
- Sidhu, K. S., Miller, S., & Tennyson, J. 1992, A&A, 255, 453 [NASA ADS] [Google Scholar]
- Sims, I. R., Queffelec, J.-L., Travers, D., et al. 1993, Chem. Phys. Lett., 211, 461 [NASA ADS] [CrossRef] [Google Scholar]
- Sims, I. R., Queffelec, J.-L., Defrance, A., et al. 1994, J. Chem. Phys., 100, 4229 [NASA ADS] [CrossRef] [Google Scholar]
- Singh, P. D., & Andreazza, C. M. 2000, ApJ, 537, 261 [NASA ADS] [CrossRef] [Google Scholar]
- Singh, P. D., Sanzovo, G. C., Borin, A. C., & Ornellas, F. R. 1999, MNRAS, 303, 235 [NASA ADS] [CrossRef] [Google Scholar]
- Singleton, D. L., & Cvetanović, R. J. 1988, J. Phys. Chem. Ref. Data, 17, 1377 [NASA ADS] [CrossRef] [Google Scholar]
- Smith, D., & Adams, N. G. 1985, ApJ, 298, 827 [NASA ADS] [CrossRef] [Google Scholar]
- Smith, D., Adams, N. G., Giles, K., & Herbst, E. 1988, A&A, 200, 191 [NASA ADS] [Google Scholar]
- Smith, D., Spanel, P., & Millar, T. J. 1994, MNRAS, 266, 31 [NASA ADS] [Google Scholar]
- Smith, J. R., Kim, J. B., & Lineberger, W. C. 1997, Phys. Rev. A, 55, 2036 [NASA ADS] [CrossRef] [Google Scholar]
- Smith, I. W. M., Herbst, E., & Chang, Q. 2004, MNRAS, 350, 323 [NASA ADS] [CrossRef] [Google Scholar]
- Snyder, L. E., Hollis, J. M., Jewell, P. R., Lovas, F. J., & Remijan, A. 2006, ApJ, 647, 412 [NASA ADS] [CrossRef] [Google Scholar]
- Stancil, P. C., Lepp, S., & Dalgarno, A. 1998, ApJ, 509, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Stancil, P. C., Schultz, D. R., Kimura, M., et al. 1999, A&AS, 140, 225 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Stoliarov, S. I., Knyazev, V. D., & Slagle, I. R. 2000, J. Phys. Chem. A, 104, 9687 [CrossRef] [Google Scholar]
- Su, T., & Chesnavich, W. J. 1982, J. Chem. Phys., 76, 5183 [NASA ADS] [CrossRef] [Google Scholar]
- Sun, J., Tang, Y., Sun, H., et al. 2008, Chem. Phys. Lett., 463, 315 [NASA ADS] [CrossRef] [Google Scholar]
- Suutarinen, A., Geppert, W. D., Harju, J., et al. 2011, A&A, 531, A121 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Talbi, D., Ellinger, Y., & Herbst, E. 1996, A&A, 314, 688 [NASA ADS] [Google Scholar]
- Tenenbaum, E. D., Apponi, A. J., Ziurys, L. M., et al. 2006, ApJ, 649, L17 [NASA ADS] [CrossRef] [Google Scholar]
- Tenenbaum, E. D., Dodd, J. L., Milam, S. N., Woolf, N. J., & Ziurys, L. M. 2010, ApJ, 720, L102 [NASA ADS] [CrossRef] [Google Scholar]
- Thaddeus, P., Cummins, S. E., & Linke, R. A. 1984, ApJ, 283, L45 [NASA ADS] [CrossRef] [Google Scholar]
- Thaddeus, P., Gottlieb, C. A., Gupta, H., et al. 2008, ApJ, 677, 1132 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. G. G. M., & Hagen, W. 1982, A&A, 114, 245 [NASA ADS] [Google Scholar]
- Tielens, A. G. G. M., & Allamandola, L. J. 1987, in Interstellar Processes, eds. D. J. Hollenbach, & H. A. Thronson, Jr., Astrophysics and Space Science Library, 134, 397 [Google Scholar]
- Troe, J. 1987, J. Chem. Phys., 87, 2773 [NASA ADS] [CrossRef] [Google Scholar]
- Tsang, W., & Hampson, R. F. 1986, J. Phys. Chem. Ref. Data, 15, 1087 [NASA ADS] [CrossRef] [Google Scholar]
- Turner, B. E. 1992, ApJ, 388, L35 [Google Scholar]
- van Dishoeck, E. F. 1988, in Rate Coefficients in Astrochemistry, eds. T. J. Millar, & D. A. Williams, Astrophys. Space Sci. Lib., 146, 49 [Google Scholar]
- van Dishoeck, E. F., & Black, J. H. 1988, ApJ, 334, 771 [NASA ADS] [CrossRef] [Google Scholar]
- van Dishoeck, E. F., Jonkheid, B., & van Hemert, M. C. 2006, Faraday Discuss., 133, 231 [Google Scholar]
- van Hemert, M., & van Dishoeck, E. 2008, Chem. Phys., 343, 292 [NASA ADS] [CrossRef] [Google Scholar]
- Vigren, E., Kamińska, M., Hamberg, M., et al. 2008, Phys. Chem. Chem. Phys., 10, 4014 [Google Scholar]
- Vigren, E., Hamberg, M., Zhaunerchyk, V., et al. 2009, ApJ, 695, 317 [NASA ADS] [CrossRef] [Google Scholar]
- Vigren, E., Hamberg, M., Zhaunerchyk, V., et al. 2010a, ApJ, 709, 1429 [NASA ADS] [CrossRef] [Google Scholar]
- Vigren, E., Hamberg, M., Zhaunerchyk, V., et al. 2010b, ApJ, 722, 847 [NASA ADS] [CrossRef] [Google Scholar]
- Vigren, E., Hamberg, M., Zhaunerchyk, V., et al. 2010c, Phys. Chem. Chem. Phys., 12, 11670 [CrossRef] [Google Scholar]
- Vigren, E., Zhaunerchyk, V., Hamberg, M., et al. 2012, ApJ, 757, 34 [NASA ADS] [CrossRef] [Google Scholar]
- Vikor, L., Al-Khalili, A., Danared, H., et al. 1999, A&A, 344, 1027 [NASA ADS] [Google Scholar]
- Wakelam, V., Herbst, E., & Selsis, F. 2006, A&A, 451, 551 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wakelam, V., Loison, J.-C., Herbst, E., et al. 2009, A&A, 495, 513 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wakelam, V., Smith, I. W. M., Herbst, E., et al. 2010a, Space Sci. Rev., 156, 13 [NASA ADS] [CrossRef] [Google Scholar]
- Wakelam, V., Herbst, E., Le Bourlot, J., et al. 2010b, A&A, 517, A21 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wakelam, V., Herbst, E., Loison, J.-C., et al. 2012, ApJS, 199, 21 [NASA ADS] [CrossRef] [Google Scholar]
- Walmsley, C. M., Flower, D. R., & Pineau des Forêts, G. 2004, A&A, 418, 1035 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Walsh, C., Harada, N., Herbst, E., & Millar, T. J. 2009, ApJ, 700, 752 [NASA ADS] [CrossRef] [Google Scholar]
- Whittet, D. C. B., Gerakines, P. A., Tielens, A. G. G. M., et al. 1998, ApJ, 498, L159 [NASA ADS] [CrossRef] [Google Scholar]
- Whyte, A., & Phillips, L. 1983, Chem. Phys. Lett., 98, 590 [NASA ADS] [CrossRef] [Google Scholar]
- Wilson, P. F., Freeman, C. G., & McEwan, M. J. 1993, Int. J. Mass Spectrom. Ion Proc., 128, 83 [NASA ADS] [CrossRef] [Google Scholar]
- Wlodek, S., Bohme, D. K., & Herbst, E. 1988, MNRAS, 235, 493 [NASA ADS] [Google Scholar]
- Woodall, J., Agúndez, M., Markwick-Kemper, A. J., & Millar, T. J. 2007, A&A, 466, 1197 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Woon, D. E., & Herbst, E. 1996, ApJ, 465, 795 [NASA ADS] [CrossRef] [Google Scholar]
- Woon, D. E., & Herbst, E. 2009, ApJS, 185, 273 [NASA ADS] [CrossRef] [Google Scholar]
- Xu, Z.-F., Li, S.-M., Yu, Y.-X., Li, Z.-S., & Sun, C.-C. 1999, J. Phys. Chem. A, 103, 4910 [CrossRef] [Google Scholar]
- Yamamoto, T., Nakagawa, N., & Fukui, Y. 1983, A&A, 122, 171 [NASA ADS] [Google Scholar]
- Yang, Z., Eichelberger, B., Carpenter, M. Y., et al. 2010, ApJ, 723, 1325 [NASA ADS] [CrossRef] [Google Scholar]
- Yang, Z., Cole, C. A., Martinez, Jr., O., et al. 2011, ApJ, 739, 19 [NASA ADS] [CrossRef] [Google Scholar]
- Zachariah, M. R., & Tsang, W. 1995, J. Phys. Chem., 99, 5308 [CrossRef] [Google Scholar]
- Zhang, F., Kim, S., Kaiser, R. I., & Mebel, A. M. 2009, J. Phys. Chem. A, 113, 1210 [CrossRef] [Google Scholar]
- Zhaunerchyk, V., Hellberg, F., Ehlerding, A., et al. 2005, Mol. Phys., 103, 2735 [NASA ADS] [CrossRef] [Google Scholar]
Appendix A: RATE12 references
Original measurements, calculations and estimates are taken from the following sources: Adams & Smith (1977), Adams et al. (1980), Adams et al. (1984), Adusei & Fontijn (1994), Allen & Robinson (1977), Andreazza et al. (1995), Andreazza & Singh (1997), Anicich et al. (2003b), Anicich et al. (2003a), Anicich et al. (1977), Anicich et al. (1984), Anicich et al. (1990), Anicich (1993), Barckholtz et al. (2001), Barlow (1984), Barlow & Dunn (1987), Baulch (2005), Baulch et al. (1992), Berteloite et al. (2010), Berteloite et al. (2010), Best et al. (2011), Bettens et al. (1999), Black (1975), Blake et al. (1986), Bocherel et al. (1996), Bohme et al. (1982), Brown & Bolina (2007). Bruhns et al. (2010), Brunetti & Liuti (1975). Canosa et al. (1997), Canosa et al. (2007), Carty et al. (2001), Cazaux & Tielens (2002), Chastaing et al. (2000), Chastaing et al. (1998), Chastaing et al. (1999), Chastaing et al. (1999), Cohen & Westberg (1991), Collings et al. (2004), Cordiner & Millar (2009), Dalgarno et al. (1990), Danielsson et al. (2008), Daugey et al. (2005), Daugey et al. (2008), Decker et al. (2000), Drdla et al. (1989), Edridge (2010), Eichelberger et al. (2007), Elitzur & de Jong (1978), Ercolano & Storey (2006), Ferguson (1973), Field et al. (1980), Forte et al. (1989), Freeman et al. (1979), Frost et al. (1993), Fukuzawa & Osamura (1997), Gannon et al. (2007), Garrod & Herbst (2006), Gredel et al. (1989), Gu et al. (2009), Hamberg et al. (2007), Hamberg et al. (2010), Hamberg et al. (2010), Harada & Herbst (2008), Harrison et al. (1986), Hasegawa & Herbst (1993), Hawley et al. (1990), Hemsworth et al. (1974), Herbst (1983), Herbst et al. (1984), Herbst & Leung (1986), Herbst et al. (1989a), Herbst & Leung (1989), Herbst et al. (1989b), Herbst et al. (1989c), Herbst & Leung (1990), Herbst et al. (2000), Herbst & Osamura (2008), Herbst et al. (2010), Herrero et al. (2010), Hoobler & Leone (1997), Huo et al. (2011), Iglesias (1977), Inomata & Washida (1999), Johnson et al. (2000), Kaiser & Gu (2009), Kalhori et al. (2002), Kamińska et al. (2008), Kern et al. (1988), Wakelam et al. (2012), Klippenstein et al. (2010), Kuanet al. (1999), Larsson et al. (2005), Laufer & Fahr (2004), Lawson et al. (2011), Le Picard et al. (2002), Leung et al. (1984), Lias & Ausloos (1987), Liddy et al. (1975), Lin & Lucchese (2001), Loison & Bergeat (2004), Loison & Bergeat (2009), Loison et al. (2012), Luca et al. (2002), Martinez et al. (2008), Martinez et al. (2010), McEwan et al. (1980), McEwan et al. (1998), Midey et al. (2008), Millar et al. (1985), Millar et al. (1986), Millar et al. (1987), Millar et al. (1988), Millar & Herbst (1990), Millar (1991), Millar et al. (1991), Millar et al. (1997b), Millar et al. (2000), Millar et al. (2007), Milligan et al. (2002), Mitchell et al. (1977), Mitchell et al. (1978), Mitchell (1984), Montaigne et al. (2005), Moran & Hamill (1963), Nahar & Pradhan (1997), Nejad & Millar (1988), Neufeld & Wolfire (2009), Nguyen et al. (2006), Novotný et al. (2010), Öberg et al. (2005), Öberg et al. (2009a), Öjekull et al. (2004), Osamura et al. (1999), Pequignot & Aldrovandi (1986), Petrie et al. (1991), Petrie et al. (2003), Petrie & Herbst (1997), Petuchowski et al. (1989), Pineau des Forêts et al. (1986), Plasil et al. (2011), Pradhan & Dalgarno (1994), Prasad & Huntress (1980), Quan et al. (2010), Rawlings et al. (1993), Rebrion et al. (1988), Roberge et al. (1991), Röllig (2011), Röhrig & Wagner (1994), Ruffle et al. (1999), Sidhu et al. (1992), Sims et al. (1993), Sims et al. (1994), Singh et al. (1999), Singh & Andreazza (2000), Singleton & Cvetanović (1988), Smith & Adams (1985), Smith et al. (1988), Smith et al. (1994), Smith et al. (1997), Smith et al. (2004), Stancil et al. (1998), Stancil et al. (1998), Stancil et al. (1999), Stoliarov et al. (2000), Sun et al. (2008), Talbi et al. (1996), Tielens & Hagen (1982), Tielens & Allamandola (1987), Tsang & Hampson (1986), van Dishoeck et al. (2006), van Dishoeck & Black (1988), van Hemert & van Dishoeck (2008), van Dishoeck (1988), Vigren et al. (2008), Vigren et al. (2009), Vigren et al. (2010a), Vigren et al. (2010b), Vigren et al. (2010c), Vigren et al. (2012), Vikor et al. (1999), Wakelam et al. (2009), Wakelamet al. (2010a), Walsh et al. (2009), Whyte & Phillips (1983), Wilson et al. (1993), Wlodek et al. (1988), Woon & Herbst (1996), Xu et al. (1999), Yamamoto et al. (1983), Yang et al. (2010), Yang et al. (2011), Zachariah & Tsang (1995), Zhang et al. (2009), Zhaunerchyk et al. (2005).
All Tables
Observed fractional abundances, relative to H2, in TMC-1 (CP) and corresponding modelled “best fit” values.
List of species whose observed abundances are matched by only one of Rate12 or kida.uva.2011.
All Figures
 |
Fig. 1 Plot of C2H fractional abundance, relative to H2 abundance, as a function of time using different values of the rate coefficient for the reaction, C2H + O → CO + CH. The shaded region corresponds to an order of magnitude error on the observed abundance in TMC-1 (CP). |
| In the text | |
 |
Fig. 2 Top left: plot of the fractional abundances, relative to H2, of cyanopolyynes as a function of envelope radius using the Rate12 model (solid lines) compared with the results from CM09 (dotted lines). Top right: plot of fractional abundances of polyynes as a function of envelope radius for the Rate12 model including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). Bottom left: plot of fractional abundances of polyyne anions as a function of envelope radius. Bottom right: comparison of the fractional abundances of various cations and electrons, including anion chemistry (solid lines) and excluding anion chemistry (dashed lines). The C6H− fractional abundance for the “anions included” model is shown, for reference, with a dot-dashed line. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


