Free Access
Table 1
Designation of, and formulæ for, the major eRCN bulk material structural groups.
| Carbon atom | Bonding | Atomic | Atomic fraction | |
| hybridisation | structure | fraction | expression | Notes |
|
|
||||
| Atoms | ||||
| H | — | XH | = XH | |
| sp2 C | — | Xs p2 | = Xsp2 | |
| sp3 C | — | Xs p3 | = Xsp3 | |
| Aromatic C-H bonds | ||||
| s p2 | CH aromatic |
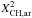
|
= η mar fXsp2 | η = XH/(1 − XH), the [H]/[C] atom ratio |
| mar = (aromatic cluster coord. no.)/(no. of C atoms) | ||||
| Olefinic and aliphatic C-H bonds – for XH ≤ 0.5 | ||||
| s p2 | CH olefinic |
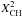
|
= u η (1 − f)Xsp2 | u = { 1 + ((1 − mar)/ [(1 − XH)/(fXsp2) − 1] ) } a |
| s p2 | CH2 olefinic |
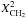
|
= 0 | |
| s p3 | CH aliphatic |
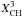
|
= u η Xsp3 | |
| s p3 | CH2 aliphatic |
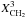
|
= 0 | |
| s p3 | CH3 aliphatic |
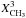
|
= 0 | |
| Olefinic and aliphatic C-H bonds – for XH > 0.5 | ||||
| s p2 | CH olefinic |
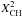
|
= u (2 − η) (1 − f) Xsp2 | |
| s p2 | CH2 olefinic |
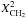
|
= 2 u (η − 1) (1 − f) Xsp2 | |
| s p3 | CH aliphatic |
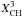
|
= u (2 − η) Xsp3/(1 + φ) | φ = 30(XH − 0.5)2 |
| s p3 | CH2 aliphatic |
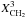
|
= 2 u (η − 1) Xsp3/(1 + φ) | |
| s p3 | CH3 aliphatic |
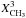
|
= 3 u φ Xsp3 /(1 + φ) | (1 + φ) re-normalises for CH3 |
| C-C bondsb | ||||
| s p2 | C ≃ C aromatic | XC ≃ C | = 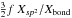 |
f = 0.5ex/(1 + ex) |
| s p2 | C=C olefinic | XC = C | = 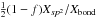 |
x = (XH − X0)/δ , X0 = 0.35, δ = 0.085 |
| s p3 | C−C aliphatic | XC−C | = Xsp3/Xbond |
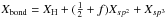
|
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


