Table 5.
Parameters derived from the rotational diagrams of the QSO2s and from ALMA CO(2–1) observations.
| QSO2 | Fit | χ2 | β | Tw | 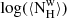 |
Mw | Th | 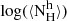 |
Mh | 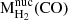 |
Warm-to-cold |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (cm−2) | (106 M⊙) | (K) | (cm−2) | (106 M⊙) | (109 M⊙) | ratio | ||||
| J1010 | PL | 7.33 | 5.24 | … | 20.60 | 10.5 | … | … | … | 0.78 | 0.013 |
| 2T | 0.12 | … | 241 | 20.85 | 18.6 | 1017 | 18.38 | 0.06 | … | 0.024 | |
| J1100 | PL | 2.48 | 4.45 | … | 20.64 | 12.0 | … | … | … | 0.86 | 0.014 |
| 2T | 3.54 | … | 288 | 20.71 | 14.2 | 1010 | 18.85 | 0.20 | … | 0.017 | |
| J1356 | PL | 7.01 | 4.33 | … | 20.66 | 18.0 | … | … | … | 2.05 | 0.009 |
| 2T | 15.7 | … | 294 | 20.58 | 15.0 | 904 | 19.09 | 0.49 | … | 0.008 | |
| J1430 | PL | 6.03 | 5.20 | … | 21.27 | 38.2 | … | … | … | 2.21 | 0.017 |
| 2T | 10.5 | … | 258 | 21.30 | 41.0 | 742 | 19.57 | 0.76 | … | 0.019 | |
| J1509 | PL | 13.0 | 4.53 | … | 20.58 | 12.6 | … | … | … | 2.40 | 0.005 |
| 2T | 6.50 | … | 256 | 20.78 | 20.0 | 1107 | 18.68 | 0.16 | … | 0.008 |
Notes. Results from the fit with the single power law temperature distribution (PL), which represents both the hot (h) and warm (w) molecular gas and assumes that the column density follows a power law (dNH∼TβdT), are shown in the same columns as those corresponding to the warm component of the two-temperature (2T) fits. The column densities correspond to average values within the PSF radius and they should be considered as lower limits. The last two columns correspond to the mass of molecular gas measured in the central kiloparsec of the QSO2s (r = 0.5 kpc for J1100, J1356, J1430, and J1509 and r = 0.7 kpc for J1010), calculated from the CO(2–1) ALMA observations presented in Ramos Almeida et al. (2022) and assuming R21 = 1 and αCO = 4.35 M⊙(K km s−1 pc2)−1, and the corresponding warm-to-cold gas mass ratio.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


