Fig. A.1
Download original image
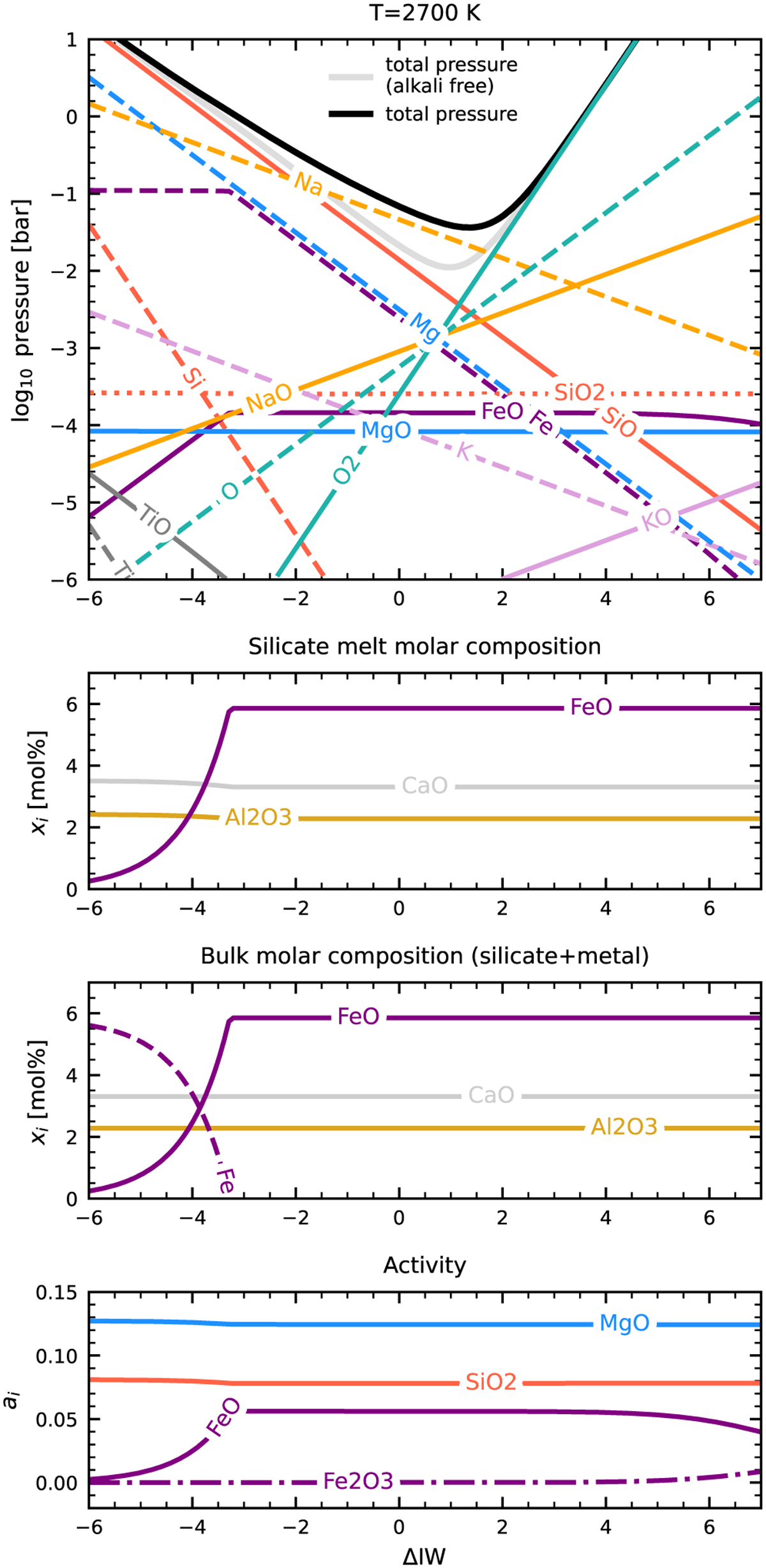
Vapour pressures and thermodynamics of a silicate melt of BSE composition. Upper: Similar to Fig. 3, but for a melt of BSE composition (including Na2O). The dominance of Na(g) at intermediate redox states (ΔIW-3 to ΔIW+2) is evident, which is consistent with the findings of other studies (Schaefer & Fegley 2004; Miguel et al. 2011; Wolf et al. 2023; van Buchem et al. 2023). Upper center: Concentration of oxides in the silicate melt. FeO concentration deplets under reducing conditions, as Fe is formed. This is evident in Lower center: here, the bulk system composition is shown, highlighting the conversion of FeO to Fe. Lower: Activity of Fe and Fe2O3 in the melt, compared to the far more dominant SiO2 and MgO.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


