Fig. 3
Download original image
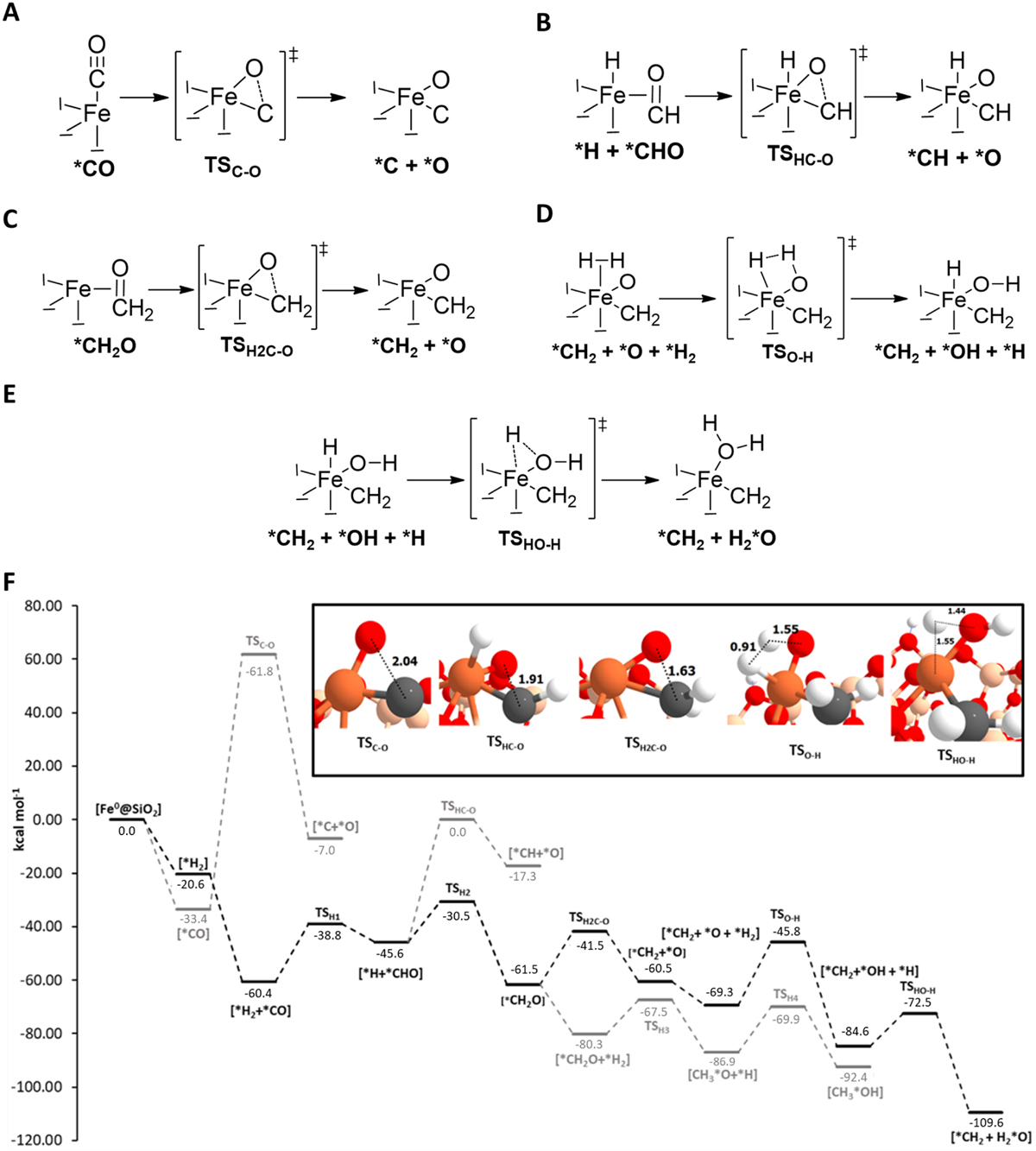
(A) Reaction mechanism for the CO dissociation into C + O. (B) Reaction mechanism for the HCO dissociation into HC + O. (C) Reaction mechanism for the H2CO dissociation into H2C and O. (D) Reaction mechanism for the first oxygen hydrogenation to OH. (E) Reaction mechanism for the second oxygen hydrogenation to H2O. (F) ZPE-corrected PESs (in kcal mol–1) for the different proposed CO dissociation mechanisms, as well as of H2O and CH3OH formation, using as the 0th reference energy the Fe0@SiO2 + H2 + CO asymptote. The optimized geometries of the five transition state structures involved in the three different C-O dissociations and in the H2O formation are also shown (bond distances in Å). The asterisk symbol (*) denotes from which atom the relevant molecule is coordinated with Fe. Color-coding: H atoms are represented in white, C atoms in gray, O atoms in red, Si atoms in beige, and Fe atoms in orange.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


