Fig. 1.
Download original image
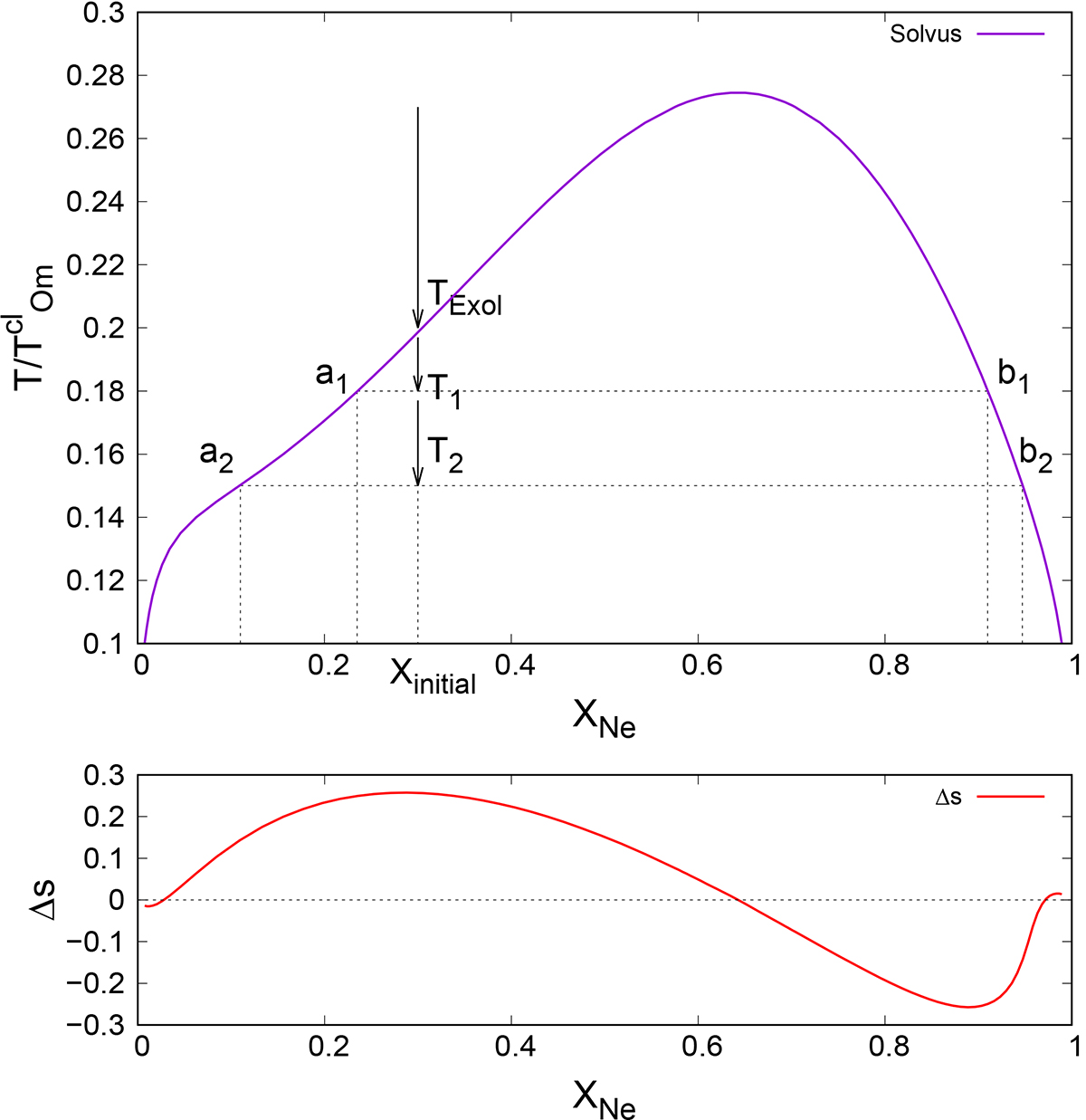
Phase diagram (upper panel) and entropy excess (lower panel) for the exsolution process in a binary ONe mixture taken from Baiko (2023). Upper panel: the purple line is the solvus and the region below it is the miscibility gap. A solid solution with an initial Ne numerical abundance Xinitial will begin exsolution at a temperature TExol determined by the solvus. By the time the temperature drops to the value T1, the solid will be decomposed into two mixtures, one depleted in Ne, with a Ne abundance XNe = a1, and the other enriched in Ne, with a Ne abundance XNe = b1. By the time the temperature drops to the value T2, the two solids will have respective abundances of a2 and b2. The exsolution process is completed at temperature T = 0, where the solid is separated into two components, pure O and pure Ne. Lower panel: excess entropy of exsolution as a function of Ne numerical abundance in a solid undergoing decomposition.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


