| Issue |
A&A
Volume 677, September 2023
|
|
|---|---|---|
| Article Number | A166 | |
| Number of page(s) | 8 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/202347023 | |
| Published online | 22 September 2023 | |
Detection of ethynylbenzene in TMC-1 and the interstellar search for 1,2-diethynylbenzene★
1
Deutsches Elektronen-Synchrotron DESY,
Notkestr. 85,
22607
Hamburg,
Germany
e-mail: melanie.schnell@desy.de
2
Grupo de Astrofísica Molecular, Instituto de Física Fundamental (IFF-CSIC),
C/ Serrano 121,
28006
Madrid,
Spain
3
Departamento de Química Física y Química Inorgánica, Facultad de Ciencias-I.U. CINQUIMA, Universidad de Valladolid,
47011
Valladolid,
Spain
e-mail: amanda.steber@uva.es
4
Christian-Albrechts-Universität zu Kiel, Institute of Physical Chemistry,
24118
Kiel,
Germany
Received:
26
May
2023
Accepted:
17
July
2023
Aims. We investigate the outcome of an electrical discharge of naphthalene and search for the resulting products in the Taurus Molecular Cloud (TMC-1).
Methods. Using chirped pulse Fourier transform microwave spectroscopy paired with an electric discharge source, we investigated products resulting from the naphthalene discharge. Quantum chemical calculations were used to help assign species and investigate potential reaction pathways relevant to the interstellar medium. These products were searched for in TMC-1 using the QUIJOTE line survey, covering 31.0-50.3 GHz.
Results. We confirm the detection of ethynylbenzene in TMC-1, and we also present a new molecular species, 1,2-diethynylbenzene, which could play an important role in the formation of naphthalene. Over ten products have been identified as resulting from the discharge, with only one of these species found in a previous IR-UV discharge study of naphthalene.
Conclusions. Ethynylbenzene has definitively been detected in TMC-1, and while we have identified a potentially important species for the formation of naphthalene and an exothermic reaction pathway, there is no current indication of its presence in TMC-1.
Key words: astrochemistry / ISM: molecules / ISM: individual objects: TMC-1 / methods: laboratory: molecular / molecular processes
© The Authors 2023
 Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Open Access article, published by EDP Sciences, under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article is published in open access under the Subscribe to Open model. Subscribe to A&A to support open access publication.
1 Introduction
Ring-containing species have been of great interest to various areas in chemistry, including combustion, climate, biochemistry, and astrochemistry. This is due to their impact on biological processes, atmospheric degradation, and polycyclic aromatic hydrocarbon (PAH) formation and growth. With respect to PAHs, smaller ring-containing species, such as substituted benzenes, can act as the blueprint for PAH formation. These ring-containing species can then continue to grow to contain hundreds of carbon atoms. In the interstellar medium (ISM), the identity of individual PAH species is not well cataloged, even though the family of PAHs is thought to contain a significant total of the galactic carbon budget, given it is at the heart of a rich carbon-dominated chemistry in the ISM. With the recent detections of the PAH indene (Cernicharo et al. 2021a; Burkhardt et al. 2021), along with two isomers of the substituted PAH, 1- and 2-cyanonaphthalene (McGuire et al. 2021), the substituted PAH, 2-cyanoindene (Sita et al. 2022), several smaller ring-containing species in the Taurus Molecular Cloud (TMC-1; Cernicharo et al. 2021a,b,c, 2022; Cabezas et al. 2022), such as (tentatively) ethynylbenzene (Cernicharo et al. 2021c), and the purported contributions of PAHs to unidentified infrared (UIR) bands, there is mounting evidence of the significance of PAHs and ring-containing species.
While the majority of PAHs is difficult to unambiguously assign among the ISM with radio astronomy due to their lack of a permanent electric dipole moment and their increasing partition function, indirect detections can be achieved via the detection of chemically related molecules. Their formation pathways can also be inferred based on intermediate species that have been identified in astronomical sources. However, this requires a better mastery of the potential intermediate species and possible reaction steps that would generate PAHs and PAH derivatives. In a recent study aimed at doing just that, Lemmens et al. (2020) investigated the species generated using an electric discharge followed by a supersonic expansion of naphthalene as the precursor molecule by means of gas-phase infrared-ultraviolet (IR-UV) spectroscopy. From this study, these authors found that the experiment triggered ring growth, that is, the formation of the three-ring PAH phenanthrene from the two-ring naphthalene precursor, and they were able to detect key resonance-stabilized radical intermediates and intermediates containing di-acetylenic side groups.
In a similar study using chirped-pulse Fourier transform microwave (CP-FTMW) spectroscopy, the resulting products of three benzene discharges under different conditions were investigated, with more than 150 distinct species identified in an exhaustive product analysis (McCarthy et al. 2020). In that study, as in the IR-UV work on the discharge of naphthalene, species heavier than the precursor benzene were observed. Many of these species were ring-chain molecules that could prove to be instrumental in understanding the chemistry occurring in astronomical environments, such as the isomers of cyanocyclopentadiene (McCarthy et al. 2021; Lee et al. 2021). While both studies worked to provide an exhaustive analysis of the species that were formed, differences in technique and precursors can elucidate various intermediate species and potential reaction pathways.
As CP-FTMW and IR-UV spectroscopy are complementary techniques, they can be used in tandem to target different molecular species. CP-FTMW spectroscopy is sensitive to molecular structures, the magnitude of the molecule’s permanent electric dipole moment, as well as its partition function. In the case of IR-UV ion dip spectroscopy, based on the detection of ions generated using resonant UV radiation, the presence of chromophores is required. Thus, it would not be surprising if studies using the two techniques revealed the presence of different species and, consequently, a comparison between the generated products could further shed light into the complex chemistry of PAHs. In the present work, we report a CP-FTMW spectroscopic study that re-investigates the species formed from an electric discharge of the precursor naphthalene in an effort to better understand intermediate species that could play a role in PAH growth mechanisms. We pair our study with an astronomical search for the products in TMC-1, confirming the tentative detection of ethynylbenzene (Cernicharo et al. 2021c) and providing upper limits on the column density of diethynylbenzene.
2 Experimental
We investigated the discharge products of naphthalene across the 2–8 GHz and 8–12GHz frequency ranges using the CP-FTMW spectrometer COMPACT (compact-passage acquired coherence technique) coupled with an electrical discharge source. Thorough descriptions of the instrument (Schmitz et al. 2012; Pérez et al. 2013; Fatima et al. 2020) and the electric discharge nozzle, which resembles the design of McCarthy et al. (2000), have been previously reported, so only a brief overview is provided here. The sample of naphthalene (99% purity) was purchased from Sigma Aldrich and used without any further purification. At room temperature, naphthalene is a solid, with a tabulated melting point of 80 °C. To transfer the molecules into the gas phase, a small quantity of sample was placed into a heat-able reservoir situated at the orifice of a modified pulsed valve, which was heated to a temperature of 115 °C. A pulsed supersonic molecular jet was created by using a commercial pulsed valve (General Valve 9) and by seeding the molecules into an inert gas (Ne or Ar) at a backing pressure of 2.5 bar. At the exit of the pulsed valve, the molecular jet flowed through the discharge nozzle installed directly at the throat of the nozzle. The discharge nozzle consists of a 14 mm teflon spacer situated directly after the nozzle and two annular oxygen-free copper electrodes separated by a 10 mm teflon insulator. There were no subsequent spacers used after the second electrode. A current of approximately 50 mA was used in these experiments, which corresponds to a voltage of 1.2–1.4kV and 0.8–0.9kV when using Ne and Ar as the carrier gas, respectively. At the exit of the discharge nozzle, the molecular beam containing a mixture of the precursor and discharge products in the carrier gas was supersonically expanded into the vacuum chamber, where it interacted with the microwave radiation. The ensemble was found to have a rotational temperature of 3 K.
As mentioned above, the discharge products of naphthalene were investigated in two frequency ranges: 2–8 GHz and 8–12GHz. In both measurements, chirped pulses of 4 μs duration, created by the arbitrary waveform generator (AWG), were used to excite the molecules. In our experimental setup, a chirped pulse spanning a 2–8 GHz frequency range is directly generated by the AWG and amplified by a 300 W travelling wave tube amplifier before being transmitted into the vacuum chamber. For the 8–12 GHz excitation chirped pulse, an initial chirp spanning 4–6 GHz is generated by the AWG. The bandwidth of this pulse is then up-converted with a doubler to create a 4 GHz bandwidth pulse (8–12GHz), which is amplified using a 50 W solid state amplifier before being broadcast into the vacuum chamber using a horn antenna. After interaction of the molecules with the microwave excitation radiation resulting in a macroscopic dipole moment, the molecular free induction decay (FID) of this macroscopic dipole moment is collected by a receiving horn antenna, amplified using a low noise amplifier, and digitized in the time domain using an oscilloscope. The resulting spectrum is subsequently Fourier transformed into the frequency domain. The experiment was conducted with a repetition rate of 4 Hz. However, we used eight chirped pulses to probe each molecular pulse, which resulted in an effective repetition rate of 32 Hz. For both ranges, final spectra were obtained by averaging together 1 million FIDs. A preliminary fit of the experimental rotational spectrum of the newly identified molecule, 1,2-diethynylbenzene, was performed using the program PGOPHER (Western 2017). The final experimental spectroscopic parameters were obtained using the SPFIT/SPCAT program by Pickett (Pickett 1991).
Theoretical calculations of potential molecular candidates from the discharge were optimized at the B3LYP/aug-cc-pVTZ level of theory with a correction for dispersion (GD3BJ), using the Orca 4 program package (Neese 2018). The centrifugal distortion constants for 1,2-diethynylbenzene (DEB) were then calculated at the same level of theory using the Gaussian16 software (Frisch et al. 2016). Calculations were also performed to identify the potential reaction pathways that could lead to the formation of 1,2-DEB from naphthalene. Saddle points were identified by performing nudge elastic band (NEB; Ásgeirsson et al. 2021) calculations. The identified structures were then optimized at the PBEh-3c level of theory (Grimme et al. 2015), using the Orca 4 program package (Neese 2018).
3 Results and discussion
The 2–12 GHz experimental rotational spectrum of the electrical discharge of naphthalene is shown in Fig. 1. In a first analysis, ten molecular species, all arising from fragmentation processes of naphthalene, were identified in the spectrum: 1,2-didehydrobenzene (o-benzyne), ethynylbenzene, diacetylenebenzene, methyldiacetylene, methyltriacetylene, ethynylallene, ethynylbutatriene, vinyldiacetylene, vinyltriacetylene, and allenyldiacetylene (Fig. 2). These species have previously been observed in the microwave spectroscopy discharge experiments of benzene performed by McCarthy et al. (2020). In conjunction with these studies, diacetylenebenzene was also identified as a fragmentation product in the electrical discharge of naphthalene in the IR-UV mass-selective ion dip spectroscopy study (Lemmens et al. 2020). For our initial identifications, we used the identified products from these two works to guide our search and for the assignment of these species in our experimental spectrum. Despite the plethora of other discharge products that were reported in these studies, we did not find other common species within our spectrum.
While these ten species were found in the electric discharge of both benzene and naphthalene, we expect that their formation mechanisms follow different pathways due to the variation in precursors. In the example of o-benzyne, chemical processes involving hydrogen loss are needed if benzene is the precursor, whereas one of the two aromatic rings needs to be fragmented in the case where naphthalene is the precursor. In the electrical discharge from benzene, vinyldiacetylene and ethynylbutatriene can be formed upon C–C cleavage; however, in the electrical discharge of naphthalene, a whole aromatic ring needs to undergo fragmentation before these species can be formed. Phenylacetylene and diacetylenebenzene feature more carbon atoms than benzene but less than naphthalene, thus suggesting that these species are presumably formed upon recombination chemistry and fragmentation chemistry in the electric discharge of benzene and naphthalene, respectively. The story of vinyltriacetylene and methyltriacetylene is similar. In the electric discharge of benzene, these molecules are expected to result from an interplay between fragmentation and recombination chemistry, whereas in the electric discharge of naphthalene, fragmentation processes are likely to play the main role.
After the identification of these “known” species, their rotational transitions were removed from the experimental spectrum to ease the identification of new species. While there were some lines still present in our spectrum, the line density of unassigned lines was not high. An investigation of these remaining lines led to the identification of a characteristic spectral pattern of a-type transitions. A good match of the experimental rotational constants was found with those calculated at the B3LYP-D3BJ/def2-TZVP level of theory for one of the isomers of diacetylenebenzene: 1,2-diethynylbenzene (1,2-DEB). A comparison between the experimental and theoretical spectroscopic parameters of 1,2-DEB and the theoretical parameters for the other isomers of diethynylbenzene are reported in Table 1. Overall, 25 rotational transitions of this species were assigned in the 2–12 GHz frequency range and the measured transition frequencies are provided in Table 2. Unfortunately, at the measured frequencies, the distortion constants could not be determined. This could have impacted the frequencies that were predicted for astronomical search between 30 and 50 GHz by several MHz. Also, 1,2-DEB has three pairs of equivalent hydrogens, making the rotational transitions subjected to spin statistics. For even and odd Ka transitions, respectively, these statistical weights are 9:7. While this did not impact the assignment of any of the transitions, it did play a role in the astronomical searches reported below. The weaker lines remaining in the spectrum have yet to be assigned.
For all of the observed species, we can identify two qualitatively different classes of molecules: ring-containing species and highly unsaturated carbon chains featuring ethenyl and ethynyl units. Furthermore, they either contain the same number of carbon atoms or are smaller than the precursor naphthalene, suggesting that under our experimental conditions, fragmentation processes are more favored than carbon insertion reactions. This is in contrast to what was observed in previous discharge experiments based on benzene and naphthalene, where a significant fraction of the identified molecules contained more carbon atoms than the precursors. There are several reasons that could contribute to this difference. The first is the differences in the conditions between the experiments, mainly in terms of voltages applied to the electrodes and length of the recombination zone. The latter is particularly crucial in defining the duration of the reaction time and, therefore, it influences the number of collisions happening before the molecular beam leaves the discharge nozzle. In this study, there was effectively no recombination zone; whereas in the discharge study based on benzene (McCarthy et al. 2020), a recombination zone of 20 mm was used, and in the discharge study based on naphthalene (Lemmens et al. 2020), a recombination zone of 6 mm was used. Another factor that may influence the product species resulting from the electrical discharge is the carrier gas. While the benzene studies were carried out with Ne as a carrier gas (McCarthy et al. 2020), the IR-UV naphthalene experiment by Lemmens et al. (2020) was performed with Ar as their carrier gas.
To analyze the influence of the carrier gas on our discharge chemistry and to be able to compare our results with those of both experiments, we investigated the electrical discharge products coming from naphthalene in both Ne and Ar. When using Ar, we observed an overall decrease in the intensity of the experimental rotational spectrum compared to our experiment in Ne. Despite this trend, while specific transitions originating from 1,2-DEB as well as diacetylenebenzene, such as the ones shown in the lower panels of Fig. 3, became weaker in the Ar spectrum, others exhibit a comparable intensity in both carrier gases (upper panels in Fig. 3). This result demonstrates that 1,2-DEB forms no matter what carrier gas is used. The final difference that plays a role in determining if we would observe the same species present in the previous naphthalene discharge experiments is the fact that species identified by IR-UV ion dip spectroscopy are not required to have a permanent electric dipole moment in order to observe them (as is the case with rotational spectroscopy); instead, they require a UV chromophore. Some of the species presented by Lemmens et al. (2020) have a low or no dipole moment, making them non-detectable in our study. These species include those such as the pure PAHs phenanthrene and pyrene.
Despite having a chromophore which would enable its detection by IR-UV ion dip spectroscopy, 1,2-DEB was not observed in the previous discharge study using naphthalene as the precursor carried out by Lemmens et al. (2020); thus, its formation pathway within the discharge has not been previously investigated. To do so, we have performed quantum-chemical calculations, which will provide some insight not only into the potential reaction pathways that could lead to its formation within the discharge, but also its relevance with respect to the formation of naphthalene from smaller ring species. Based on chemical intuition, we identified two potential initiation processes for the formation of 1,2-DEB from naphthalene: C–C bond cleavage and dehydrogenation. The proposed mechanisms, labelled as I, II, and III, are displayed in Fig. 4. Both mechanisms I and II are initiated with the rupture of the C–C bond in one of the aromatic rings of naphthalene, which results in the formation of a doublet radical intermediate. This intermediate can subsequently undergo consecutive hydrogen loss to form the final product (II) or undergo isomerization via intramolecular proton transfer (I). The latter leads to the formation of 1-ethenyl-2-ethynylbenzene, after which it undergoes consecutive hydrogen loss similar to route II. A search for 1-ethenyl-2-ethynylbenzene based on the theoretical rotational constants in the experimental rotational spectrum revealed no evidence of this species. However, due to its rather low dipole moment (µa = 0.4 D), we cannot fully exclude that this reaction pathway is also taking place in our discharge experiment. Mechanism III involves the consecutive loss of two adjacent hydrogen atoms to form 2,3-didehydronaphthalene, which can undergo an intramolecular rearrangement and loss of two additional hydrogen atoms to form 1,2-DEB. However, none of the proposed reaction intermediates have been identified in our experimental spectrum, meaning the formation mechanism of 1,2-DEB in the electrical discharge of naphthalene remains speculative.
Furthermore, we cannot exclude a formation pathway of 1,2-DEB via the addition of two acetylene moieties to benzene. Acetylene is known to be produced in discharge experiments involving aromatic carbonaceous molecules, that is, PAHS, as is o-benzyne which is present in our molecular beam and known to be a reactive intermediate with a high tendency to undergo addition reactions. The fact that 1,2-DEB is not reported as a product of the discharge of benzene and that only molecules arising from fragmentation processes of naphthalene have been identified in our discharge spectrum triggers the hypothesis that 1,2-DEB is produced upon fragmentation of naphthalene, for example, via reaction pathways discussed in Fig. 4. We will investigate this further by performing additional discharge experiments involving isotopic tagging as well as discharge experiments of molecules which could be potential precursors of 1,2-DEB, such as benzene and acetylene.
Even if we cannot identify how 1,2-DEB was formed within the discharge, Fig. 4 points to the fact that it could be considered as a potential observational proxy for naphthalene in the ISM. If we consider reaction pathway II, we can see that 1,2-DEB can undergo two subsequent exothermic bimolecular reactions to eventually form naphthalene.
 |
Fig. 1 Experimental discharge spectrum of naphthalene recorded in the 2–12GHz frequency range. The spectrum was acquired across two frequency ranges: 2–8GHz and 8–12GHz. This required the use of different power amplifiers as described in the experimental section. The black trace shows the experimental spectrum. The colored traces are the simulated spectra of the identified species based on the experimental rotational constants and at a rotational temperature of 3 K. The zoom-in at the bottom-left of the spectrum shows the assignment of the newly identified species: 1,2-diethynylbenzene. |
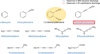 |
Fig. 2 Molecular species identified in the microwave spectrum obtained with an electric discharge of naphthalene recorded in the 2–12 GHz frequency range. The blue text indicates the products found in the microwave spectroscopic study of the discharge products coming from benzene (McCarthy et al. 2020) and the red box indicates species found in the IR-UV spectroscopic study of the discharge products coming from naphthalene (Lemmens et al. 2020). |
Experimental parameters of 1,2-diethynylbenzene and theoretical parameters (B3LYP-D3BJ/def2-TZVPP) of 1,2-diethynylbenzene, 1,3-diethynylbenzene, and 1,4-diethynylbenzene.
Measured frequencies and residuals (νobs − νcalc) in MHz for the rotational transitions of 1,2-diethynylbenzene.
 |
Fig. 3 Comparison of rotational transitions of 1,2-DEB (left two panels) and diacetylenebenzene (right two panels) for the two different carrier gases Ar and Ne. |
4 Astronomical search
As this is the case, searches for ethynylbenzene and 1,2-DEB were performed in TMC-1, where other pure cyclic and aromatic hydrocarbons have been recently found, such as o-benzyne (Cernicharo et al. 2021b), cyclopentadiene and its derivatives (Cernicharo et al. 2021c, 2022), and indene (Cernicharo et al. 2021a; Burkhardt et al. 2021). The spectral data employed in this work are part of the ongoing QUIJOTE1 line survey (Cernicharo et al. 2021b) performed toward TMC-1 (αJ2000 = 4h41m41.9s and δJ2000 = +25°41′27.0″) in the Q band (30–50 GHz) using the Yebes 40 m radiotelescope.
The QUIJOTE line survey uses a 7 mm receiver covering the Q band (31.0–50.3 GHz) with horizontal and vertical polarizations. Receiver temperatures during 2019 and 2020 varied from 22 K at 32 GHz to 42 K at 50 GHz. In 2021, some power adaptation carried out in the down-conversion chains allowed the receiver temperatures to drop to 16 K at 32 GHz and 25 K at 50 GHz. The backends are fast Fourier transform spectrometers which provide a bandwidth of 8 × 2.5 GHz in each polarization, thereby practically covering the whole Q band, with a spectral resolution of 38.15 kHz. The system is described in detail by Tercero et al. (2021).
The observations, carried out during different observing runs, were performed using the frequency-switching mode with a frequency throw of 10 MHz in the very first observing runs, during November 2019 and February 2020, 8 MHz during the observations of January to November 2021, and then alternating these frequency throws in the last observing runs between October 2021 and February 2023. The total on-source telescope time is 850 h in each polarization (twice this value after averaging the two polarizations), which can be split into 385 and 465 h for the 8 MHz and 10 MHz frequency throws, respectively. The sensitivity of the QUIJOTE line survey varies between 0.17 and 0.25 mK in the 31–50.3 GHz domain. The telescope beam size varies from 56.7″ at 31 GHz to 35.6″ at 49.5 GHz. The intensity scale used in this work, antenna temperature (Tļ), was calibrated using two absorbers at different temperatures and the atmospheric transmission model (ATM; Cernicharo 1985; Pardo et al. 2001). Calibration uncertainties have been adopted to be 10%. The beam efficiency of the Yebes 40 m telescope in the Q band is given as a function of frequency by Beff = 0.797 exp[−(υ(GHz)/71.1)2]. The forward telescope efficiency is 0.97.
A few lines of ethynylbenzene (c-C6H5CCH) have been reported by Cernicharo et al. (2021c), who claimed a tentative detection. We have used the spectroscopic information obtained by Kisiel & Kraśnicki (2010) for this molecule and adopted a dipole moment of µa = 0.65 D (Cox et al. 1975) to search for additional lines of c-C6H5CCH in the last product of the QUIJOTE line survey (Cernicharo, J. et al. 2023). The spin statistics of 5 and 3 for Ka odd and even, respectively, have been taken into account. We used the MADEX code (Cernicharo 2012) to predict the synthetic spectrum of ethynylbenzene, and we adopted a rotational temperature identical to that derived from the observation of hundred lines of benzonitrile in this source [Trot = 9 K] (Cernicharo et al. 2021c). The lines are expected to be significantly weaker than those of benzonitrile due to the smaller dipole moment of ethynylbenzene, 0.65 D versus 4.5 D for the cyanide derivative of benzene. The search for ethynylbenzene leads to the detection of 25 of its rotational lines, which are shown in Fig. 5. The corresponding line parameters are given in Table 3. In order to derive a column density, we assumed a source of uniform brightness temperature with a radius of 40″ (Fossé et al. 2001) and a linewidth of 0.6 km s−1 for all lines in the band. The best fit to the observed lines provides a column density of (3.0 ± 0.5) × 1012 cm−2. From the column density derived by Cernicharo et al. (2021c) for c-C6H5CN in TMC-1, we derived an abundance ratio between the CCH and CN derivatives of benzene of ~2.5. This value is similar to the one obtained for the CCH and CN derivatives of C3H4 (Marcelino et al. 2021; Cernicharo et al. 2021c) and a factor of two smaller than the same ratio for the derivatives of cyclopentadiene (Cernicharo et al. 2021c). These ratios depend on the CCH/CN abundance ratio and on the reaction rates of CCH and CN with benzene and cyclopentadiene and have been discussed in detail by Cernicharo et al. (2021c). The present work definitively confirms the detection of ethynylbenzene in TMC-1.
From the spectroscopic parameters presented in Table 1, we generated frequency predictions to guide the astronomical search for 1,2-DEB toward TMC-1. The frequency predictions were implemented in the MADEX code (Cernicharo 2012) to compute synthetic spectra assuming a rotational temperature of 9 K, namely, identical to that of ethynylbenzene and benzonitrile (Cernicharo et al. 2021c). We used the dipole moment components from Table 1. The velocity of the cloud was fixed to υlSR = 5.83 km s−1. Taken into account that the dipole moment is not so different from that of ethynylbenzene we could expect very weak lines for 1,2-DEB. By adopting the observed upper limits to the intensity of the strongest components of 1,2-DEB, we derived a 3σ upper limit to the column density of this species in TMC-1 of 2.0 × 1012 cm−2. This upper limit is similar to the value derived above for the column density of ethynylbenzene. Improved future data from the QUIJOTE line survey could make the detection of this species possible.
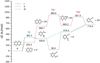 |
Fig. 4 Possible initial decomposition steps of naphthalene for the formation of 1,2-diethynylbenzene. Likewise, if viewed from right to left, the pathways can be inferred as naphthalene formation steps from 1,2-DEB. |
 |
Fig. 5 Observed lines of ethynylbenzene in the 31–50.3 GHz frequency range towards TMC-1. Line parameters for the complete list of detected lines of ethynylbenzene are given in Table 3. |
Line parameters of the observed transitions of ethynylbenzene in TMC-1.
5 Conclusion
The discharge spectrum of naphthalene has yielded products that closely mirror those seen in the rotational spectroscopy discharge study of benzene. Of the eleven species identified, ten of those species were previously seen in the work on benzene and only one of them, diacetylenebenzene, was observed in the IR-UV ion dip spectroscopic work on the discharge of naphthalene. The similarities between the naphthalene discharges end there. This is partly due to the fact that some of the species that the IR-UV spectroscopic study observed have very low or no permanent dipole moments, but it also indicates that the chemistry that is taking place within the discharge is different. Another indication of this is the lack of observation of 1,2-DEB in the previous experiment. Conducting these experiments with different carrier gases did not significantly change the products of our discharge. For the first time, we present the rotational spectrum of 1,2-DEB, and we have presented potential pathways for its formation within the discharge. The formation of 1,2-DEB from naphthalene is endothermic; however, the reverse pathway is exothermic and based on this information, we performed a search for 1,2-DEB in TMC-1. Although it was not detected, we did manage to derive an upper limit to its column density of 2.0 × 1012 cm−2. However, with the increased sensitivity of the QUIJOTE survey, we have been able to provide a definitive detection of ethynylbenzene, for which we derived a column density of (3.0 ± 0.5) × 1012 cm−2 and an abundance ratio of 2.5, which lies between the ethynyl and cyano derivatives of benzene.
Acknowledgements
D.L. acknowledges the support of an Alexander von Humboldt postdoctoral fellowship. C.C. and J.C. thank the ERC for the grant ERC-2013-Syg-610256-NANOCOSMOS and Ministerio de Ciencia e Innovación of Spain for the project PID2019-107115GB-C21/AEI/ 10.13039/501100011033. A.L.S. acknowledges the MSCA fellowship 894433 – AstroSsearch.
References
- Asgeirsson, V., Birgisson, B.O., Bjornsson, R., et al. 2021, J. Chem. Theory Comput., 17, 4929 [CrossRef] [Google Scholar]
- Burkhardt, A.M., Lee, K.L.K., Changala, P.B., et al. 2021, ApJ, 913, L18 [NASA ADS] [CrossRef] [Google Scholar]
- Cabezas, C., Agundez, M., Fuentetaja, R., et al. 2022, A&A, 663, A2 [Google Scholar]
- Cernicharo, J. 1985, IRAM internal report [Google Scholar]
- Cernicharo, J. 2012, Laboratory Astrophysics and Astrochemistry in the Herschel/ALMA era, eds. C. Stehle, C. Joblin, & L. d’Hendecourt, EAS Publications Series, 58, 251 [NASA ADS] [Google Scholar]
- Cernicharo, J., Agundez, M., Cabezas, C., et al. 2021a, A&A, 649, A15 [Google Scholar]
- Cernicharo, J., Agundez, M., Kaiser, R.I., et al. 2021b, A&A, 652, A9 [Google Scholar]
- Cernicharo, J., Agundez, M., Kaiser, R.I., et al. 2021c, A&A, 655, A1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Fuentetaja, R., Agundez, M., et al. 2022, A&A, 663, A9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Pardo, J.R., Cabezas, C., et al. 2023, A&A, 670, A19 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cox, A.P., Ewart, I.C., & Stigliani, W.M. 1975, J. Chem. Soc., Faraday Trans. 2, 71, 504 [CrossRef] [Google Scholar]
- Fatima, M., Perez, C., Arenas, B.E., Schnell, M., & Steber, A.L. 2020, Phys. Chem. Chem. Phys., 22, 17042 [CrossRef] [Google Scholar]
- Fossé, D., Cernicharo, J., Gerin, M., & Cox, P. 2001, ApJ, 552, 168 [Google Scholar]
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., et al. 2016, Gaussian~16 Revision C.01 (Wallingford, CT: Gaussian Inc.) [Google Scholar]
- Grimme, S., Brandenburg, J.G., Bannwarth, C., & Hansen, A. 2015, J. Chem. Phys., 143, 054107 [NASA ADS] [CrossRef] [Google Scholar]
- Kisiel, Z., & Krásnicki, A. 2010, J. Mol. Spectrosc., 262, 82 [NASA ADS] [CrossRef] [Google Scholar]
- Lee, K.L.K., Changala, P.B., Loomis, R.A., et al. 2021, ApJ, 910, L2 [NASA ADS] [CrossRef] [Google Scholar]
- Lemmens, A.K., Rap, D.B., Thunnissen, J.M.M., Willemsen, B., & Rijs, A.M. 2020, Nat. Commun., 11, 269 [CrossRef] [Google Scholar]
- Marcelino, N., Tercero, B., Agundez, M., & Cernicharo, J. 2021, A&A, 646, A9 [Google Scholar]
- McCarthy, M.C., Chen, W., Travers, M.J., & Thaddeus, P. 2000, ApJS, 129, 611 [NASA ADS] [CrossRef] [Google Scholar]
- McCarthy, M.C., Lee, K.L.K., Carroll, P.B., et al. 2020, J. Phys. Chem. A, 124, 5170 [NASA ADS] [CrossRef] [Google Scholar]
- McCarthy, M.C., Lee, K.L.K., Loomis, R.A., et al. 2021, Nat. Astron., 5, 176 [NASA ADS] [CrossRef] [Google Scholar]
- McGuire, B.A., Loomis, R.A., Burkhardt, A.M., et al. 2021, Science, 371, 1265 [NASA ADS] [CrossRef] [Google Scholar]
- Neese, F. 2018, WIREs Comput. Mol. Sci., 8, e1327 [CrossRef] [Google Scholar]
- Pardo, J., Cernicharo, J., & Serabyn, E. 2001, IEEE Trans. Antennas Propag., 49, 1683 [Google Scholar]
- Perez, C., Lobsiger, S., Seifert, N.A., et al. 2013, Chem. Phys. Lett., 571, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Pickett, H.M. 1991, J. Mol. Spectr., 148, 371 [NASA ADS] [CrossRef] [Google Scholar]
- Schmitz, D., Shubert, V.A., Betz, T., & Schnell, M. 2012, J. Mol. Spectr., 280, 77 [NASA ADS] [CrossRef] [Google Scholar]
- Sita, M.L., Changala, P.B., Xue, C., et al. 2022, ApJ, 938, L12 [NASA ADS] [CrossRef] [Google Scholar]
- Tercero, F., Lopez-Perez, J.A., Gallego, J.D., et al. 2021, A&A, 645, A37 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Western, C.M. 2017, Spectr. Rad. Transf., 186, 221 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Experimental parameters of 1,2-diethynylbenzene and theoretical parameters (B3LYP-D3BJ/def2-TZVPP) of 1,2-diethynylbenzene, 1,3-diethynylbenzene, and 1,4-diethynylbenzene.
Measured frequencies and residuals (νobs − νcalc) in MHz for the rotational transitions of 1,2-diethynylbenzene.
All Figures
 |
Fig. 1 Experimental discharge spectrum of naphthalene recorded in the 2–12GHz frequency range. The spectrum was acquired across two frequency ranges: 2–8GHz and 8–12GHz. This required the use of different power amplifiers as described in the experimental section. The black trace shows the experimental spectrum. The colored traces are the simulated spectra of the identified species based on the experimental rotational constants and at a rotational temperature of 3 K. The zoom-in at the bottom-left of the spectrum shows the assignment of the newly identified species: 1,2-diethynylbenzene. |
| In the text | |
 |
Fig. 2 Molecular species identified in the microwave spectrum obtained with an electric discharge of naphthalene recorded in the 2–12 GHz frequency range. The blue text indicates the products found in the microwave spectroscopic study of the discharge products coming from benzene (McCarthy et al. 2020) and the red box indicates species found in the IR-UV spectroscopic study of the discharge products coming from naphthalene (Lemmens et al. 2020). |
| In the text | |
 |
Fig. 3 Comparison of rotational transitions of 1,2-DEB (left two panels) and diacetylenebenzene (right two panels) for the two different carrier gases Ar and Ne. |
| In the text | |
 |
Fig. 4 Possible initial decomposition steps of naphthalene for the formation of 1,2-diethynylbenzene. Likewise, if viewed from right to left, the pathways can be inferred as naphthalene formation steps from 1,2-DEB. |
| In the text | |
 |
Fig. 5 Observed lines of ethynylbenzene in the 31–50.3 GHz frequency range towards TMC-1. Line parameters for the complete list of detected lines of ethynylbenzene are given in Table 3. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


