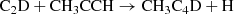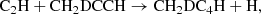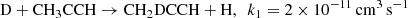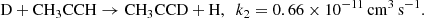| Issue |
A&A
Volume 657, January 2022
|
|
|---|---|---|
| Article Number | L5 | |
| Number of page(s) | 5 | |
| Section | Letters to the Editor | |
| DOI | https://doi.org/10.1051/0004-6361/202142814 | |
| Published online | 10 January 2022 | |
Letter to the Editor
New deuterated species in TMC-1: Detection of CH2DC4H with the QUIJOTE line survey⋆
1
Grupo de Astrofísica Molecular, Instituto de Física Fundamental (IFF-CSIC), C/ Serrano 121, 28006 Madrid, Spain
e-mail: carlos.cabezas@csic.es, jose.cernicharo@csic.es
2
LERMA, Observatoire de Paris, PSL Research University, CNRS, Sorbonne Universités, 92190 Meudon, France
3
Observatorio Astronómico Nacional (IGN), C/ Alfonso XII, 3, 28014 Madrid, Spain
4
Centro de Desarrollos Tecnológicos, Observatorio de Yebes (IGN), 19141 Yebes, Guadalajara, Spain
Received:
2
December
2021
Accepted:
19
December
2021
We report the first detection in space of the single deuterated isotopologue of methyldiacetylene, CH2DC4H. A total of 12 rotational transitions, with J = 8–12 and Ka = 0 and 1, were identified for this species in TMC-1 in the 31.0–50.4 GHz range using the Yebes 40m radio telescope. The observed frequencies allowed us to obtain, for the first time, the spectroscopic parameters of this deuterated isotopologue. We derived a column density of (5.5 ± 0.2) × 1011 cm−2. The abundance ratio between CH3C4H and CH2DC4H is 24 ± 2. This ratio is similar to that found for the CH3C3N/CH2DC3N analogue system, which is 22 ± 2. We did not detect the deuterated species CH3C4D, which has already been observed in laboratory experiments. The detection of deuterated CH3C4H allows us to extend the discussion on the chemical mechanisms of deuterium fractionation at work in TMC-1 using a new gas-phase chemical model with multiply deuterated molecules. Introducing a possible deuterium exchange reaction between CH3CCH and atomic deuterium allows us to account for the CH3C4H/CH2DC4H abundance ratio.
Key words: astrochemistry / ISM: molecules / ISM: individual objects: TMC-1 / line: identification / molecular data
© ESO 2022
1. Introduction
Studies of deuterium fractionation provide insight into the chemical reaction mechanisms that occur both in the gas phase and on the surfaces of dust particles. Deuterium fractionation also may provide us with sensitive probes for the early stages of protostellar evolution. It is therefore important not only to discover the degree of molecular deuteration possible in specific interstellar species, but also to determine this as a function of the environment. Deuterium fractionation is understood to be a very efficient process responsible for the extremely high D/H ratios found for interstellar molecules such as HDCS (Marcelino et al. 2005) and CH2DOH (Parise et al. 2006). Moreover, it allows multiple deuteration for abundant molecules containing more than one hydrogen atom (Agúndez et al. 2021a and references therein).
Very sensitive broadband line surveys of astronomical sources have helped to increase the number of new molecular identifications in the last years, because weak lines arising from low-abundance species and from low-dipole moment species can now be easily detected (Agúndez et al. 2021b; Cernicharo et al. 2021a; Cabezas et al. 2021a,b). The downside of this high sensitivity is the large number of new lines that appear in the survey. Deuterated isotopologues of abundant interstellar molecules significantly contribute to these new spectral features. Considering all the information mentioned above, the astronomical identification of these isotopologues is of the utmost importance, not only to gain knowledge on their molecular formation pathways or on how deuterium fractionation works, but also to assign unidentified features in line surveys.
Several single deuterated isotopologues of species such as H3CN, CH3CCH, c-C3H2, C4H, H2C4, H2CCN, HC3N, and HC5N (Cabezas et al. 2021c) have been detected in TMC-1 using the on going QUIJOTE1 line survey (Cernicharo et al. 2021b). As part of the QUIJOTE line survey, we also identified the single deuterated isotopologues HDCCN (Cabezas et al. 2021c) and CH2DC3N (Cabezas et al. 2021d) in TMC-1. The identification of HDCCN in TMC-1 is supported by the observation of the rotational spectrum of that species, while that for CH2DC3N is based on quantum chemical calculations.
In our previous work on the CH2DC3N observation (Cabezas et al. 2021d), we searched for the spectral signatures of the deuterated species of methyldiacetylene (CH3C4H). This molecule was discovered in TMC-1 by MacLeod et al. (1984), Walmsley et al. (1984) and it has been found to be 7.5 times more abundant than CH3C3N (Marcelino et al. 2021; Cernicharo et al. 2021c). We followed the same strategy used for CH2DC3N to predict the transition frequencies of CH2DC4H. We found only two lines at the predicted transition frequencies, but no other lines predicted in the frequency range of the line survey were observed. We derived a 3σ upper limit to its column density of 3.7 × 1011 cm−2. Here we report the identification of spectral lines of the deuterated species CH2DC4H in TMC-1, based on our previous ab initio calculations and on a wider spectral search. The observed deuterium fractions are compared to an extended chemical model including the related deuterated compounds.
2. Observations
The data presented in this work are part of the QUIJOTE spectral line survey in the Q band towards TMC-1 (αJ2000 = 4h41m41.9s and δJ2000 = +25° 41′27.0″), which was performed at the Yebes 40m radio telescope during various observing sessions between November 2019 and April 2021. A detailed description of the QUIJOTE line survey is provided in Cernicharo et al. (2021b). All observations were carried out using the frequency switching technique, with a frequency throw of 10 MHz during the two first observing runs and of 8 MHz in the later ones. This observing mode provides a noise level 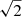 times lower than the unfolded data, but on the other hand it produces negative spectral features at ±10 MHz or ±8 MHz of each rotational transition. These negative features can be easily identified because of their symmetric displacement by exactly the frequency throw. The selected temperature scale is
times lower than the unfolded data, but on the other hand it produces negative spectral features at ±10 MHz or ±8 MHz of each rotational transition. These negative features can be easily identified because of their symmetric displacement by exactly the frequency throw. The selected temperature scale is 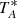 . The TMB can be easily obtained by dividing the observed
. The TMB can be easily obtained by dividing the observed 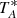 by the beam efficiency. Values of ηMB have been provided by Tercero et al. (2021). Furthermore,
by the beam efficiency. Values of ηMB have been provided by Tercero et al. (2021). Furthermore, 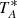 was calibrated using two absorbers at different temperatures and the atmospheric transmission model (ATM; Cernicharo 1985; Pardo et al. 2001).
was calibrated using two absorbers at different temperatures and the atmospheric transmission model (ATM; Cernicharo 1985; Pardo et al. 2001).
Different frequency coverages were observed, 31.08–49.52 GHz and 31.98–50.42 GHz, which permitted us to check that no spurious ghosts were produced in the down-conversion chain in which the signal coming from the receiver was downconverted to 1–19.5 GHz, and then split into eight bands with a coverage of 2.5 GHz, each of which were analysed by the Fast Fourier Transform spectrometers. Calibration uncertainties were adopted to be 10% based on the observed repeatability of the line intensities between different observing runs. All data were analysed using the GILDAS package2.
3. Results
In a previous work, we reported the identification of the CH2DC3N species (Cabezas et al. 2021d). Among the forest of the unidentified lines in our QUIJOTE line survey, we found a series of five lines with a harmonic relation between them and two additional series of lines at higher and lower frequencies from those lines. After discarding other candidates, we assigned those lines to CH2DC3N. The assignment was based on the change in the rotational parameters of CH3C3N produced by the H–D exchange, which were obtained by ab initio calculations. As mentioned above, we already considered that the rotational transitions of CH2DC4H should also be observed in our line survey. As for the CH3C3N/CH2DC3N system, we performed geometry optimisation calculations for CH3C4H and CH2DC4H in order to estimate the isotopic shift on the rotational constants for the CH3C4H/CH2DC4H species. We employed the CCSD/cc-pVTZ level of theory (Cížek 1969; Dunning 1989) which reproduces the B rotational constant well for CH3C4H, 2027.4 MHz versus the experimental value of 2035.74 MHz. The theoretical values for the rotational constants of CH2DC4H were then scaled using the experimental/theoretical ratio obtained for CH3C4H, and the results are shown in Table 1. Using these rotational constants, we predicted the transition frequencies for CH2DC4H and we observed only two lines at those frequencies with intensities of ∼1 mK in 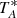 . We searched for other lines predicted in the frequency range of the line survey, but we could not observed them. We assumed that the lines for CH2DC4H species could not be detected with the sensitivity achieved at that moment.
. We searched for other lines predicted in the frequency range of the line survey, but we could not observed them. We assumed that the lines for CH2DC4H species could not be detected with the sensitivity achieved at that moment.
Observationally derived and theoretical spectroscopic parameters (in MHz) for CH2DC4H.
A deeper inspection of the frequency regions where the CH2DC4H lines are predicted reveals the presence of a series of lines that we did not consider in our first analysis. In our previous search, we focussed on two lines with intensities of ∼1 mK at 31 382.4 and 35 304.7 MHz that agree well with the predicted frequencies. We incorrectly assigned them to the 80, 8–70, 7 and 90, 9–80, 8 transitions of CH2DC4H since the deviation from predictions were similar to that found for the lines of CH2DC3N. The new detected lines corresponding to these transitions appear at higher frequencies, 31 391.2 and 35 315.0 MHz. Using these two lines as a guide, we also observed another two lines for the J + 10, J + 1 ← J0, J series and eight transitions for the J + 11, J + 1 ← J1, J and J + 11, J ← J1, J − 1 progressions. The rotational transitions 100, 10–90, 9 and 121, 11–111, 10 were not included in the fit because they were detected below the 3σ noise level. On the other hand, Ka = 2 transitions are too weak to be detected with the current sensitivity (see Fig. 1). All the observed lines, shown in Table 2 and Fig. 1, were analysed using an asymmetric rotor Hamiltonian in the FITWAT code (Cernicharo et al. 2018) to derive the rotational and centrifugal distortion constants shown in Table 1. As it can be seen, the predicted values for CH2DC4H perfectly agree with those derived from our fit, which allows us to conclude that the spectral carrier of our lines is CH2DC4H. It should be noted that the calculations provide the equilibrium values for the rotational constants (Ae, Be, and Ce), while the experimental values are the ground state rotational constants (A0, B0, and C0). Despite the equilibrium rotational constants slightly differing from the ground state constants, we can assume similar discrepancies for CH3C4H and CH2DC4H and, thus, the estimated constants for CH2DC4H are essentially unaffected by this fact. Using experimental/theoretical ratios is the most common method to predict the expected experimental rotational constants for an isotopic species of a given molecule when the rotational constants for its parent species are known. The (B + C) calculated value shows a relative error from the experimental value of 0.01%, similar to what was found in the case of CH2DC3N. With the available data for CH2DC4H, we cannot determine the experimental value for the A rotational constant, which was kept fixed to the ab initio value, as it was done for CH2DC3N.
 |
Fig. 1. Observed lines of CH2DC4H in TMC-1 in the 31.0–50.4 GHz range. Frequencies and line parameters are given in Table 2. Quantum numbers for the observed transitions are indicated in each panel. The red line shows the synthetic spectrum computed for a rotational temperature of 8 K and a column density of (5.5 ± 0.2) × 1011 cm−2 (see text); also, the dashed blue line indicates the 3σ noise level. Blank channels correspond to negative features created during the folding of the frequency switching data. The asterisk indicates Ka = 2 transitions and the label U corresponds to features above 4σ. |
Observed line parameters for CH2DC4H in TMC-1.
The synthetic spectrum of CH2DC4H was computed assuming a dipole moment value identical to that of the main isotopologue CH3C4H (1.207 D; Bester et al. 1984) and the partition function shown in Table 3. The column density of CH2DC4H was derived from a rotational diagram analysis of the observed intensities. We considered a source of uniform brightness with a radius of 40″ (Fossé et al. 2001). We derived Tr = 8.0 ± 0.5 K and N(CH2DC4H) = (5.5 ± 0.2) × 1011 cm−2. This value is not far from the 3σ upper limit to the CH2DC4H column density of 3.7 × 1011 cm−2 that we provided in our previous work (Cabezas et al. 2021d). As shown in Fig. 1, the agreement between the synthetic spectrum and the observations is excellent. The column density is not very sensitive to the adopted value of the rotational temperature between 6 and 10 K. The column density for the parent isotopologue CH3C4H was derived by Cernicharo et al. (2021c) to be (1.30 ± 0.04) × 1013 cm−2. Therefore, the CH3C4H/CH2DC4H abundance ratio is 24 ± 2. This ratio is similar to that of 22 ± 2 found for the CH3C3N/CH2DC3N analogue system. For the deuterated species CH3C4D, for which laboratory spectroscopy is available (Heath et al. 1955), we derived a 3σ upper limit to its column density of 9 × 1010 cm−2. Hence, N(CH3C4H)/N(CH3C4D) ≥ 144.
Rotational partition function for CH2DC4H at different temperatures calculated at a maximum value of J = 70.
4. Chemical modelling
This new detection of deuterated CH3C4H allows us to extend the discussion on the chemical mechanisms of deuterium fractionation at work in TMC-1. We would like to point out that our results have been obtained with the same assumed physical conditions for the TMC-1 environment as in our previous studies (Cabezas et al. 2021c,d). In Table 4, we display the different deuterium fractions found in TMC-1 so far, as well as the previous steady state results corresponding to models A and B of Cabezas et al. (2021d). We recall that a full scrambling scenario of the reactions between C2D and CH3CCH (model B) had predicted a CH3C4H/CH2DC4H ratio of 20, which is close to the value derived with the present detection. In model A, these reactions were assumed to proceed directly, that is
Deuteration fractions in TMC-1 compared to our gas phase chemical model.
and
resulting in a much larger CH3C4H/CH2DC4H ratio. The relevant chemical processes principally involve the reactions of C2D with methylacetylene CH3CCH and the allene isomer CH2CCH2 as well as the reactions between C2H with their deuterated substitutes CH3CCD, CH2DCCH, and CHDCCH2. Dissociative recombination reactions of CH2DC4H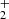 and CH3C4HD+ may also contribute to deuterated CH3C4H as ion-molecule reactions between H2D+ and CH3C4H are leading to these complex molecular ions. This is in contrast with CH3CCH or CH2CCH2 and H2D+ reactions, which result in the ejection of two hydrogen molecules, and the formation of the linear and cyclic C3H
and CH3C4HD+ may also contribute to deuterated CH3C4H as ion-molecule reactions between H2D+ and CH3C4H are leading to these complex molecular ions. This is in contrast with CH3CCH or CH2CCH2 and H2D+ reactions, which result in the ejection of two hydrogen molecules, and the formation of the linear and cyclic C3H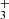 ions as reported in the experiments of Abeysekera et al. (2015).
ions as reported in the experiments of Abeysekera et al. (2015).
We would like to point out that deuterated methylacetyne is underproduced compared to the hydrogenated compound in all chemical scenarios considered so far. We thus introduced a possible deuterium exchange reaction between CH3CCH and atomic deuterium:
and
We used a modest value for the corresponding reaction rate coefficients consistent with values expected for neutral-neutral reactions without an activation barrier and assumed that the channel to CH3CCD is one-third of that of CH2DCCH, following statistical arguments. The computed deuterium fractions are reported in column An and Bn of Table 4. This single change, introduced in the previous reported models, allowed us to obtain good agreement for the deuterium fractions of both isotopologues of methyl acetylene. The ratio for methylcyanoacetylene, CH3C3N/CH2DC3N, was reduced as well, allowing for a satisfactory comparison with observations. We see that the values of the CH3C4H/CH2DC4H ratio are also consequently somewhat reduced, as expected. The values corresponding to A and B models bracket the observational values now. We shall also point out that both models predict a smaller than observed ratio for CH3C4H/CH3C4D. We feel that the level of agreement between observations and models An and Bn is nevertheless quite supportive of a reasonable understanding of the deuterium enhancement in the TMC-1 environment. Further experimental and theoretical studies of the CH3CCH exchange reaction with atomic deuterium would be very useful in order to probe the relevance of our hypothesis.
We acknowledge the remaining uncertainties on the chemical network. We did not introduce allenyl acetylene, H2CCCHCCH, a new C5H4 isomer detected recently by Cernicharo et al. (2021c) in TMC-1, but we focussed our efforts on understanding the possible deuterium enhancement scenarios. This particular attempt did not allow to discriminate if the reactions between C2H (C2D) with CH3CCH (CH3CCD, CH2DCCH) proceed directly or involve the formation of a temporary intermediate complex redistributing the different stable deuterated compounds. The significant deuterium enhancement in methylacetylene obtained with the assumed deuterium exchange reaction allowed us to account for CH3C4H/CH2DC4H. We also found that ion-molecule reactions involving H2D+, which were introduced in the present chemical model, do not play a significant role for both the deuteration of CH3CCH and CH3C4H, as reactions with C2H (C2D) are more efficient. An additional test of our assumptions would be the observational derivation of the C2H/C2D ratio which is predicted to be about 7 in our models. Transition frequencies of CCD have been recently updated by Cabezas et al. (2021e), and CCD has been detected in TMC-1 using IRAM 30m data. A derivation and discussion of the CCH/CCD ratio in TMC-1 is currently in process and will be presented in the near future in a separate article.
5. Conclusions
We have presented the first detection in space of a new single deuterated compound derived from methyldiacetylene, CH2DC4H, towards the dark cloud TMC-1. Using the Yebes 40m radio telescope, we observed a total of 12 rotational transitions, with J = 8–12 and Ka = 0 and 1, in the 31.0–50.4 GHz range. These transitions were assigned to CH2DC4H based on the excellent agreement found between the ab initio molecular constants and those derived from the frequency transitions fit. We derived a column density of (5.5 ± 0.2) × 1011 cm−2 and a CH3C4H/CH2DC4H abundance ratio of 24 ± 2. The ratio is similar to that of 22 ± 2 found for the CH3C3N/CH2DC3N analogue system. We have constructed a new gas-phase chemical model, including multiply deuterated molecules and introducing a possible deuterium exchange reaction between CH3CCH and atomic deuterium. This new model is able to reproduce the abundance ratio for CH3C4H/CH2DC4H and also that for CH3C3N/CH2DC3N.
Acknowledgments
We thank ERC for funding through grant ERC-2013-Syg-610256-NANOCOSMOS. The Spanish authors thank Ministerio de Ciencia e Innovación for funding support through projects PID2019-106235GB-I00 and PID2019-107115GB-C21/AEI/10.13039/501100011033. M. A. thanks Ministerio de Ciencia e Innovación for grant RyC-2014-16277. E. R. acknowledges the support of the Programme National ‘Physique et Chimie du Milieu Interstellaire’ (PCMI) of CNRS/INSU with INC/INP co-funded by CEA and CNES. Several kinetic data we used have been taken from the online databases KIDA Wakelam et al. (2012), https://kida.astrochem-tools.org/) and UMIST2012 McElroy et al. (2013), http://udfa.ajmarkwick.net).
References
- Abeysekera, C., Joalland, B., Ariyasingha, N., et al. 2015, J. Phys. Chem. Lett., 6, 1599 [Google Scholar]
- Agúndez, M., Roueff, E., Cabezas, C., et al. 2021a, A&A, 649, A171 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Agúndez, M., Cabezas, C., Tercero, B., et al. 2021b, A&A, 647, L10 [EDP Sciences] [Google Scholar]
- Bester, M., Yamada, K. M. T., Winnewisser, G., et al. 1984, A&A, 137, L20 [NASA ADS] [Google Scholar]
- Cabezas, C., Tercero, B., Agúndez, M., et al. 2021a, A&A, 650, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2021b, A&A, 654, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Endo, Y., Roueff, E., et al. 2021c, A&A, 646, L1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Roueff, E., Tercero, B., et al. 2021d, A&A, 650, L15 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cabezas, C., Agúndez, M., Marcelino, N., et al. 2021e, A&A, 654, A45 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J. 1985, Internal IRAM report (Granada: IRAM) [Google Scholar]
- Cernicharo, J., Guélin, M., Agúndez, M., et al. 2018, A&A, 618, A4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Marcelino, N., Agúndez, M., et al. 2020, A&A, 642, L17 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Cabezas, C., et al. 2021a, A&A, 649, L15 [EDP Sciences] [Google Scholar]
- Cernicharo, J., Agúndez, M., Kaiser, R., et al. 2021b, A&A, 652, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Cernicharo, J., Cabezas, C., Agúndez, M., et al. 2021c, A&A, 647, L3 [EDP Sciences] [Google Scholar]
- Cížek, J. 1969, in Advances in Chemical Physics, ed. P. C. Hariharan (New York: Wiley Interscience), 14, 35 [Google Scholar]
- Dunning, T. H. 1989, J. Chem. Phys., 90, 1007 [Google Scholar]
- Fossé, D., Cernicharo, J., Gerin, M., & Cox, P. 2001, ApJ, 552, 168 [Google Scholar]
- Heath, G. A., Thomas, L. F., Sherrard, E. I., & Sheridan, J. 1955, Faraday Soc. Disc., 19, 38 [Google Scholar]
- MacLeod, J.M., Avery, L. W., & Broten, N. W. 1984, ApJ, 282, L89 [NASA ADS] [CrossRef] [Google Scholar]
- Marcelino, N., Cernicharo, J., Roueff, E., et al. 2005, ApJ, 620, 308 [Google Scholar]
- Marcelino, N., Tercero, B. N., Agúndez, M., & Cernicharo, J. 2021, A&A, 646, L9 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- McElroy, D., Walsh, C., Markwick, A. J., et al. 2013, A&A, 550, A36 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pardo, J. R., Cernicharo, J., & Serabyn, E. 2001, IEEE Trans. Antennas Propag., 49, 12 [Google Scholar]
- Parise, B., Ceccarelli, C., Tielens, A. G. G. M., et al. 2006, A&A, 453, 949 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Tercero, F., López-Pérez, J. A., Gallego, J. D., et al. 2021, A&A, 645, A37 [EDP Sciences] [Google Scholar]
- Wakelam, V., Loison, J.-C., Herbst, E., et al. 2012, ApJS, 199, 21 [Google Scholar]
- Walmsley, C. M., Jewell, P. R., Snyder, L. E., & Winnewisser, G. 1984, A&A, 134, L11 [NASA ADS] [Google Scholar]
All Tables
Observationally derived and theoretical spectroscopic parameters (in MHz) for CH2DC4H.
Rotational partition function for CH2DC4H at different temperatures calculated at a maximum value of J = 70.
All Figures
 |
Fig. 1. Observed lines of CH2DC4H in TMC-1 in the 31.0–50.4 GHz range. Frequencies and line parameters are given in Table 2. Quantum numbers for the observed transitions are indicated in each panel. The red line shows the synthetic spectrum computed for a rotational temperature of 8 K and a column density of (5.5 ± 0.2) × 1011 cm−2 (see text); also, the dashed blue line indicates the 3σ noise level. Blank channels correspond to negative features created during the folding of the frequency switching data. The asterisk indicates Ka = 2 transitions and the label U corresponds to features above 4σ. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.