| Issue |
A&A
Volume 656, December 2021
|
|
|---|---|---|
| Article Number | A84 | |
| Number of page(s) | 7 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361/202140744 | |
| Published online | 07 December 2021 | |
Gas-phase formation of interstellar nucleobases from dehydrogenated formamide and vinyl cyanide
1
Laboratory for Relativistic Astrophysics, Department of Physics, Guangxi University,
530004,
Nanning, PR China
e-mail: zw@gxu.edu.cn
2
School of Chemistry and Chemical Engineering, Guangxi University,
530004,
Nanning, PR China
e-mail: xiep@gxu.edu.cn
Received:
8
March
2021
Accepted:
10
September
2021
Context. Cytosine, thymine, and uracil are three of the five primary nucleobases that function as the fundamental units of the genetic code in nucleic acids. In searching the extraterrestrial origins of microscopic life, previous studies have reported formation routes of nucleobases in interstellar ice analogs. The present work explores the possibility that nucleobases could form from small molecules through gas-phase reactions in the interstellar medium (ISM).
Aims. We aim to search energetically favorable synthetic routes toward the formation of cytosine, thymine, and uracil via gas-phase reactions, using first principles calculations. Based on the computation of a reaction energy barrier and reactant formation energy, we tried to identify the specific interstellar environments favorable to the formation of the nucleobases, with respect to the previously reported detection of relevant reactants in the ISM.
Methods. Density functional theory calculations were carried out to investigate the chemical reaction pathways using the M06 functional with 6-31+G(d,p)/6-311++G(d,p) basis sets. An ab initio Møller-Plesset perturbation theory in the second order (MP2) was also used to corroborate the results.
Results. We report synthetic routes toward the formation of cytosine, thymine, and uracil through gas-phase reactions between partially dehydrogenated formamide (H2NCHO) and vinyl cyanide (H2CCHCN). The most energetically favorable pathway to the formation of 1H-pyrimidin-2-one (C4H4N2O), a direct precursor of nucleobases, was found in a molecule-radical reaction between HNCHO and H2CCHCN, with an energy barrier of 19.3 kcal mol−1. The energy barriers for the optimal reaction pathways between C4H4N2O and amino, methyl, or hydroxyl to finally produce cytosine, thymine, or uracil are about 11.3, 18.6, or 19.9 kcal mol−1, respectively.
Conclusions. The optimal energy barriers of 19.3 and 23.8 kcal mol−1 roughly correspond to a reaction rate coefficient of 10−11 cm3 s−1 at 180 and 220 K, respectively. This indicates that the reaction could be thermally feasible through a gas-phase reaction in hot molecular cores or in the inner part of the protoplanetary disks. In contrast, the energy barriers for the reactions between other dehydrogenated radicals and molecules are relatively high, which corresponds to the extinction energy of far-ultraviolet photons in photo-dissociation regions. Furthermore, the computed pathways suggest that prior H migration in the reactants could be the key rate-determining process for the synthesis of the primary nucleobases.
Key words: astrochemistry / astrobiology / ISM: molecules / molecular processes / molecular data
© ESO 2021
1 Introduction
Previous analyses of meteorites and materials ejected from comets have detected the presence of biologically relevant organic compounds including uracil, amino acids, hydroxy acids, nitrogen heterocycles, and glycine (Stoks & Schwartz 1979; Cronin & Chang 1993; Elsila et al. 2009). Those findings support the hypothesis of extraterrestrial origins of the fundamental building blocks of life (Quirico & Bonal 2019; Ehrenfreund & Charnley 2000). So far, more than 200 species of molecules have been detected in the interstellar medium (ISM), a major part of which are organic (Herbst 2014). The chemical evolution of those interstellar organic compounds is thought to be crucial for the formation of biologically relevant compounds (Herbst 2014). Many efforts were therefore devoted to understanding the possible reaction pathways that could lead to the formation of biomolecules in interstellar conditions (Sandford et al. 2020). A focus of attention in this regard is the formation of the primary nucleobases, namely adenine, cytosine, guanine, thymine, and uracil, which are nitrogen-containing compounds that function as the fundamental units of the genetic code in ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) (Zamirri et al. 2019).
Most studies on this topic to date consider a formation route through reactions of pyrimidine (C4H4N2), due to its direct structural relevance to the nucleobase. In the astronomical context, C4H4N2 is understood to be a primary precursor of nucleobases in interstellar ice analogs. For example, using laboratory simulations of interstellar environments, Nuevo et al. (2009) detected uracil in the residue formed from the ultraviolet (UV) photolysis of pyrimidine in pure H2O ice. Oba et al. (2019) reported the presence of pyrimidine and purine nucleobases in interstellar ice analogs composed of H2O, CO, NH3 and CH3OH after exposure to UV irradiation followed by thermal processes. Laboratory experiments show that UV photoprocessing of pyrimidine and purine in simple ices of astrophysical interest can result in the production of all five primary nucleobases (Materese et al. 2018).
In the gas phase, however, pyrimidine is proven to be unstable under UV radiation (Peeters et al. 2005), which regulates the heating and the chemistry of the molecular gas in galaxies, particularly in photo-dissociation regions (PDRs). It is hence rational to explore the possibility that nucleobases could form in these regions from more elementary gas molecules than pyrimidine. In this context, formamide (H2NCHO) has been proposed as an essential reactant for the gas-phase formation of nucleobases (Saladino et al. 2016; Rotelli et al. 2016). It has been shown that the polymerization of the dehydration products of H2NCHO in water can lead to nucleobase formation, although with high energy barriers (130 kJ/mol at a minimum) (Nguyen et al. 2013, 2015; Jeilani et al. 2016). Quantum chemical calculations suggested that the reactions of free radicals (e.g. CCCNH, CCCO, CCCH and HCCCN) in the gas phase may lead to the formation of nucleobases (Wang & Bowie 2012; Gupta et al. 2013). However, these highly-dehydrogenated free radicals might be very unstable in the gas phase due to their high chemical reactivity.
Photodehydrogenation in PDRs is proposed as one of the main mechanisms leading to the formation of new molecular species in the ISM (Castellanos et al. 2018; Mackie et al. 2015; Piani et al. 2017; Choe 2020). Partially dehydrogenated products of H2NCHO and H2CCHCN, including isocyanic acid (HNCO) and cyanoacetylene (HCCCN), were detected in the ISM (Snyder & Buhl 1972; Gardner & Winnewisser 1975a). Based on those observations, we here propose new routes for the gas-phase formation of nucleobases, through reactions between partially dehydrogenated H2NCHO and vinyl cyanide (H2CCHCN). Using density functional theory (DFT) calculations, we investigated the possible reactions leading to the formation of 1H-pyrimidin-2-one (C4H4N2O), a direct intermediate precursor of nucleobases, and its final reaction to form three primary nucleobases: cytosine, thymine, and uracil.
2 Methods
DFT calculations are conducted to investigate the chemical reaction pathways between individual pairs of molecules in single collision events. The resulting transitional and final molecular structures in the collision were optimized using the M06 functional (Zhao & Truhlar 2008) with 6-31+G(d,p)/6-311++G(d,p) basis sets as implemented in Gaussian 16 B.01 (Frisch et al. 2016). Vibrational frequency calculations were performed to confirm the intermediate (IM) or the transition state (TS). Intrinsic reaction coordinate (IRC) calculations were carried out to ensure that the TS and the IM reasonably connect the reactant (RC) and product (PD). Furthermore, calculations based on the Møller-Plesset perturbation theory in the second order (MP2) were performed to corroborate the results, and an agreement is achieved as shown in the supporting information online1, in which we also provide the atomic coordinate data of the optimized molecular structures (including RC, IM, TS, PD) and their formation energy.
Multiple possible collision trajectories were simulated for each pair of reactant molecules. Two scenarios are considered here: direct collision and collision after H migration, a process in which a H moves from the vicinity of its originally bonded atom to that of another atom in the molecule. The energy cost of the H migration is taken into account in the calculation of the final energy barrier.
We searched for the optimal (i.e., most energetically favorable) pathways in the simulated reactions. As illustrated in the next section, most of the computed optimal pathways follow a common sequence. First, H migration takes place inside the reactants; then, two reaction sites on different reactants collide and lead to the formation of an intermediate transitional structure with a closed aromatic ring; finally, the intermediate structure transforms into the final product by means of another H migration. In such a process, hydrogen may separate in the form of H2 molecules (the so-called H roaming; Suits 2008).
We computed the Gibbs free energies (G, in units of kcal mol−1) of all RC, IM, TS, and PD molecular structures. The thermodynamically representative temperature is set to 100 K under the assumption of noninteracting particles (Ochterski 2000), which roughly corresponds to the reported average temperature of Sgr B2 (N3-N5) and the surrounding star forming region Sgr B2 (N) (Bonfand et al. 2019; de Vicente et al. 1996), where relevant reactant molecules used in this work were detected (Halfen et al. 2011; Gardner & Winnewisser 1975b). According to our tests, a change of 100 or 200 K in this representative temperature only results in a slight difference (≲ 2.0 kcal mol−1) in G.
As many collision directions were considered for each combination of the reactants, we selected one or two pathways with the lowest energy barrier to discuss in this paper. For each combination of the reactants, the energy barrier of the most likely pathway is denoted by ΔG, while that of the second most likely one is denoted by ΔG2. Given the condition of astrochemistry, numerous steps (even of large relative heights) will be overcome if the heights to be crossed are lower than the energy of the starting materials. We assume that this energy does not leave the system by irradiation on a timescales typical of the atomic rearrangements, which is faster than the required vibrational cascade. ΔG is therefore defined as the difference in G between the state of the highest energy and the RC.
We tested the reaction between pristine formamide (H2NCHO) and vinyl cyanide (H2CCHCN) to form 1H-pyrimidin-2-one (C4H4N2O), as shown in Fig. 1. We find that the rearrangement of the C-bonded H atom in H2NCHO is energetically favorable for reacting with H2CCHCN to produce C4H4N2O. Without this H migration, the reaction is constrained by a high energy barrier of about 71.6 kcal mol−1, which roughly corresponds to an activation temperature above 650 K for exhibiting rate coefficients greater than 10−11 cm3 s−1, at which speed the most important reactions occur in the ISM. This reaction could therefore hardly be thermally feasible.
In light of the weak feasibility of the molecule-molecule reaction, we focused on the reactions leading from the products of the partial dehydrogenation of H2NCHO and H2CCHCN toward the formation of C4H4N2O. Considering four hydrogen atoms are needed to construct C4H4N2O, we calculated all the possible combinations of dehydrogenated H2NCHO and H2CCHCN with one or two hydrogen atoms removed but keeping at least four H atoms in total. This results in a total of 12 combinations that are classified into three categories, namely the reactions A1-4, B1-4, or C1-4, according to the total number of hydrogen atoms in the reactants as indicated by the lines of different colors in Fig. 2a. We note that, among these reactants, H2NCHO, isocyanic acid (HNCO), H2CCHCN, and cyanoacetylene (HCCCN) have been reported to be present in the ISM (Halfen et al. 2011; Gardner & Winnewisser 1975b; Snyder & Buhl 1972; Gardner & Winnewisser 1975a).
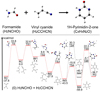 |
Fig. 1 Potential energy diagram for the two most likely reactions between H2NCHO and H2CCHCN in single collision events (Reaction 0). The most energetically favorable reaction pathway is highlighted in red, with the second one in black. |
3 Results and discussions
3.1 Energy barriers for forming 1H-pyrimidin-2-one (C4H4N2O) from dehydrogenated reactants
Figure 2b shows the energy barrier for the two most energetically favorable pathways of the reactions between H2NCHO and H2CCHCN at different dehydrogenation levels (Fig. 2b). The most energetically favorable reaction is A3, which occurs between H2CCHCN and HNCHO, that is with only one H removed from the pristine molecule H2NCHO. Its lowest energy barrier is 19.3 kcal mol−1, which roughly corresponds to a reaction rate coefficient of 10−11 cm3 s−1 at 180 K, indicating a thermal feasibility in certain interstellar environments as discussed in detail in Sect. 3.4. The second energetically favorable reaction is B4, which exhibits an optimal energy barrier of 23.8 kcal mol−1. The lowest energy barriers of the other considered reactions are all higher than 30.0 kcal mol−1.
3.2 Reaction pathways
Figure 3 shows the reaction pathways of H2NCHO and H2CCHCN with one H atom removed in one of the initial molecules (Reactions A1-4). The reaction route of A1 (between H2NCHO and HCCHCN) indicates a simple synthesis pathway of C4H4N2O common for all these reactions. The pathway of A2 (between H2NCHO and H2CCCN) is almost consistent with A1, except an additional step at the onset, in which β-cyanovinyl radical (H2CCCN) is convertedinto HCCHCN through H-migration overcoming a relatively high energy barrier of 51.7 kcal mol−1. This step is a rate-determining step and its energy diagram is shown in the supporting information online2. The remaining processes of the most energetically favorable pathways of A1 and A2 are the following steps. The NH2 group of H2NCHO and the vinyl group of HCCHCN collided to form IM1. One proton is transferred from the NH2 group to the CN group, and then the H in the aldehyde group is transferred to CN through a TS3 to form IM3. Consequently, IM3 is converted toIM4 by a typical intramolecular cyclization reaction, in which the CNH group attacked the CO group via TS4. Finally, C4H4N2O (PD) is formed by the dehydrogenated IM4.
The optimal pathway of A3 (between HNCHO and H2CCHCN) is more complicated than A1, but having a lower energy barrier of only 19.3 kcal mol−1 (RC-TS3). A4 (between H2NCO and H2CCHCN) includes an additional step, in which H2NCO transforms into HNCHO via H migration that incurs an energy cost of 50.9 kcal mol−1, the highest activation energy barrier for this reaction. The remaining steps of A4 coincide with those of A3 and are described in the following, as indicated by the red lines in the bottom panel of Fig. 3. The NH group of HNCHO directly connects with the vinyl group of H2CCHCN to produce IM1 via a transition state TS1 with 3.7 kcal mol−1, right before IM2 is generated by a 3,4-H shift. The H in the CH2 moves to the CN through a TS3 leads to IM3 with a local energy barrier of 48.9 kcal mol−1. One proton in the aldehyde then moves to the N overcoming 10.7 kcal mol−1 to form IM4. Finally, C4H4N2O (PD) comes into being after an intramolecular cyclization (IM5) and a dehydrogenation step overcoming a local barrier of 54.4 kcal mol−1.
The pathways of B1-4 consist of more steps than those of A1-4, which are in general disadvantageous for the synthesis. However, B4 is a reaction with a barrierless entrance, and its overall energy barrier is only 23.8 kcal mol−1 (RC-TS4). In the most energetically favorable pathways of B1-3, HNCHO and HCCHC are firstly converted into H2NCO and H2CCCN via H-migration, as shown in the upper panel of Fig. 4. H2NCO and H2CCCN bonded to each other to form IM1 in a barrierless way. In the subsequent step, the O on the carbonyl group bonded with an H from the NH2 group of IM1, this costs energy of 47.6 kcal mol−1 to form IM2. The head-end C bonded with tail-end N forming a ring (IM3), which underwent a series of H-shift and a double bond rearrangement processes to finally produce C4H4N2O (PD).
The mechanisms of the most possible pathways of C1-4 between H2NCHO and H2CCHCN with two H atoms removed are illustrated in Fig. 5. We observe that the pathway of C1 (between NCHO and H2CCHCN) coincides with that of C2 (between HNCO and H2CCHCN), except for the first step, in which NCHO is readily converted into HNCO by a 1,2-H shift (as shown in the supporting information online3). Then, as indicated by the red line in the middle panel of Fig. 5, HNCO and H2CCHCN undergo a cyclization reaction to form IM1. Subsequently, C4H4N2O (PD) is finally produced after a series of H rearrangements with a very high energy cost of 101.8 kcal mol−1 (RC-TS2).
As illustrated in the bottom panel of Fig. 5, at the onset of C3 (between H2NCHO and CCHCN) and C4 (between H2NCHO and HCCCN), H2NCHO first needs to be converted into H2NCOH with an energy barrier of 71.6 kcal mol−1. This is the rate-determining step. Meanwhile, CCHCN transforms into HCCCN through H migration in C3. In a key intermediate step, the C of H2NCOH attacks the CN group of HCCCN, and leads to the formation of IM1. IM1 then undergoes a cyclization process to produce the ring-shaped IM2. Sequential steps involving H migration finally lead to the formation of the target molecule C4H4N2O (PD), with an energy barrier reaching 71.6 kcal mol−1 (RC’-TS’). Overall, the reactions in the C family exhibit high energy barriers and thus have low astronomical significance.
 |
Fig. 2 (a) Reactions between dehydrogenated H2NCHO and H2CCHCN. The dashed lines in different colors indicate reactions in various categories with different amounts of hydrogen atoms in the reactants. (b) Energy barriers for the two most possible pathways of the reactions between the dehydrogenated H2NCHO and H2CCHCN molecules. |
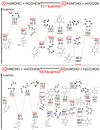 |
Fig. 3 Potential energy diagrams for the two most energetically favorable pathways for A1-4 in single collision events. |
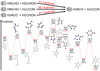 |
Fig. 4 Potential energy diagrams for the two most energetically favorable pathways for reactions B1-4 in single collision events. |
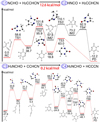 |
Fig. 5 Potential energy diagrams for the two most energetically favorable pathways for the reactions C1-4 in single collision events. |
3.3 Formation of nucleobases from C4H4N2O
Finally, we simulated the reactions between C4H4N2O and three functional groups: amino, methyl, and hydroxyl (Feuchtgruber et al. 2000; van Dishoeck & Jansen 1993; Wyrowski et al. 2010). These reactions lead to the formation of the three nucleobases, cytosine, thymine, and uracil, through the nucleophilic substitution mechanism (Gupta et al. 2013). The most energetically favorable pathways of those reactions are illustrated in Fig. 6. It can be seen that the energy barriers are fairly low, at 11.3, 19.9, and 18.6 kcal mol−1 for cytosine,uracil, and thymine, respectively. The values of the minimum energy barriers are comparable to those previously reported in the literature for the formation of cytosine, thymine, uracil under abiotic conditions (Wang & Bowie 2012; Gupta et al. 2013; Nguyen et al. 2015; Jeilani et al. 2016; Choe 2020). Wang & Bowie (2012) proposed feasible reaction pathways for the formation ofcytosine and uracil by using CCCNH, CCCO, and monosolvated urea, and reported a primary reaction energy barrier of about 27.0 kcal mol−1. Jeilani et al. (2016) studied the forming mechanisms of uracil, adenine, purine, cytosine, and thymine from H2NCHO and 1,2-diaminomaleonitrile, and found that all reactions pointed to the same precursor with a maximum barrier of 27.8 kcal mol−1. Those reported energy barriers are comparable to the results shown in Fig. 6.
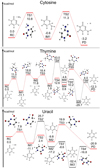 |
Fig. 6 Potential energy diagrams for the one or two most energetically favorable pathways for the reactions between C4H4N2O and NH2, CH3, OH, which eventually leads to the formation of nucleobases including (a) cytosine, (b) thymine, and (c) uracil. |
 |
Fig. 7 Reaction rate coefficient (in logarithmic scale) versus temperature for the most favorable pathways of the studied reactions. |
3.4 Feasibility of the reactions in the ISM
In laboratory experiments, a reaction with a barrier < 25.0 kcal mol−1 is commonly considered to readily occur at room temperature. In the ISM, the gas-phase reaction has a very low molecular density, but the reaction time is extremely long. The possibility of a reaction is measured by the rate coefficient, which rapidly increases with rising temperature and can be determined from the energy barrier by using the transition state theory; for example, the classical Eyring equation (Eyring 1935). In general, most important reactions in the ISM proceed with rate coefficients of 10−11 cm3 s−1 or larger (as highlighted by the orange reference line in Fig. 7). In the simulated reactions, the lowest activation energy is found to be 19.3 kcal mol−1 for A3 to form C4H4N2O in a molecule-radical reaction. That barrier roughly corresponds to a reaction rate coefficient of about 10−11 cm3 s−1 at 180 K, or about 0.05 cm3 s−1 at 300 K, as shown in Fig. 7.
In the following step, the energy barriers of C4H4N2O reacting with free radicals to form cytosine, uracil, and thymine are 11.3, 19.9, and 18.6 kcal mol−1, respectively. The corresponding reaction rates of these energy barriers are 0.07, 2.6 × 10−12, and 9.9 × 10−11 cm3 s−1 at 180 K, or 3.6 × 104, 0.02, and 1.7 × 10−1 cm3 s−1 at 300 K, respectively. This indicates that the reactions of A3 toward the formation of cytosine and uracil could be thermally feasible in interstellar regions with relatively high temperatures such as hot molecular cores or the inner part of the protoplanetary disks. The temperature in these regions ranges from 100 to several hundred kelvins. For example, high-mass young stellar objects in giant clouds and the inner envelope of the protostar have been observed to be associated with warm ambient gas at average temperatures of 100–300 K (the so-called hot cores) (Herbst & Van Dishoeck 2009). The middle and the outer parts of the protoplanetary disk exhibit temperatures as low as 10 K, while the temperature gradually increases to several thousand kelvins when approaching the central star (Akimkin et al. 2013). Those regions often show a rich organic chemistry in the gas phase, since the environmental temperature could be high enough to evaporate the ice mantles.
Among the reactants considered here, H2NCHO and H2CCHCN have been detected in various regions of the ISM and were classified as “hot” molecules (with a characteristic rotation temperature > 100 K) (Bisschop et al. 2007; Agúndez et al. 2008; Turner 1971). Their co-existence was reported by a radio telescope survey of Suzuki et al. (2018) in high-mass star forming regions (including Orion KL, NGC 6334F and W51 e1/e2) (Tercero et al. 2013; Hollis et al. 2006). Two other reactants, HNCO, and HCCCN have also been detected in the ISM (Snyder & Buhl 1972; Gardner & Winnewisser 1975a). Evolving stars and young massive stars provide UV radiations that exhibit an important effect on the molecular clouds, as observed in the envelope of Sgr B2, where several species of our reactants were detected (Martín-Pintado et al. 1999; Goicoechea & Cernicharo 2002). The studied reactions, including A3, are likely to be triggered by radiation in low-temperature (10–20 K) interstellar molecular clouds. For A3, despite the reported existence of interstellar vinyl cyanide formamide, HNCHO has not yet been detected in the ISM.
There should be two different ways to produce the dehydrogenated reactants. The first way would be the photodehydrogenation of pristine molecules by cosmic radiation (Castellanos et al. 2018); for example, by far-UV photons from massive stars with energies typically ranging from 6.0 to 13.6 eV (i.e., 138.3 to 313.6 kcal mol−1) (Hollenbach & Tielens 1999). This energy range is comparable to our computed atomization enthalpy of the reactants (140.2 to 282.5 kcal mol−1) for producing the reactants via dehydrogenation of the pristine molecules (H2NCHO and H2CCHCN), as shown in Table 1 (the zero point is set at the atomization entropy of the pristine molecule). These results indicate that the production of the partially dehydrogenated molecules in this way would be strongly correlated with the energy of the excitation photons, which must fall within an appropriate spectral range. For example, to prepare A3, the synthesis of HNCHO from H2NCHO requires an extinction energy of about 165.5 kcal mol−1. A too high or a too low energy may instead lead to the formation of H2NCO, HNCO, or even NCHO. Therefore, there could be specific regions where the reactants are preferentially present, as the radiation level of PDRs specifically depends upon the gas density and the structure of the molecular clouds (Goicoechea et al. 2003; Haid et al. 2018; Kovács et al. 2020). Besides, UV radiation also plays a similar role in the protoplanetary disks around young stars, an environment in which the formation and chemistry of pre-biotic molecules is directly relevant to planet formation (Henning & Semenov 2013).
Another route to the formation of the reactant is through the bottom-up reactions between interstellar elementary molecules and ions (Herbst & Van Dishoeck 2009; Hanine et al. 2020; Qi et al. 2018). There are many references for reactions of this type. For example, it has been reported that HNCO, H2CCCN, HCCCN, H2NCO and HNCHO could be formed through the reactions of NH3+CO2, CN+C2H2, CN+C2H2, H+HNCO and NH+HCO, respectively (Schuurman et al. 2004; da Silva et al. 2016; Balucani et al. 2000; Nguyen et al. 1996; Garrod et al. 2008). The energy barriers of these reactions were estimated to range from 9.2 to 42.1 kcal mol−1, indicating that these reactions could be thermally feasible. This is supported by the fact that all the listed elementary reactants were detected in the ISM (Nguyen et al. 1984; Whittet & Walker 1991; Adams 1941; Ridgway et al. 1976; Gralewicz et al. 1997; Johansson et al. 1984; Meyer & Roth 1991; Schenewerk et al. 1986). Moreover, barrierless reactions between NH and HCO have been reported to lead to the formation of HNCHO (Hrušák et al. 1993). We note that HCCCN can form as an intermediate product of the reaction between SiN and C2H2 (Parker et al. 2012), both of which have been detected in the ISM (Ziurys 2006; Ridgway et al. 1976).
We studied the reactions of amino, methyl, and hydroxyl with C4H4N2O toward the formation of cytosine, thymine, and uracil, respectively. Among those, the amino radical was detected in Sgr B2 (van Dishoeck & Jansen 1993), where H2CCHCN and HCCCN have also been detected (Turner 1971; Gardner & Winnewisser 1975a). The methyl radical was first detected by the observations of the Infrared Space Observatory in Sgr A* (Feuchtgruber et al. 2000). The hydroxyl was detected in stellar outflow (Tappe et al. 2008; Goicoechea et al. 2006), supernova remnants (Reach & Rho 1998), low-mass young stellar objects SAS (Wampfler et al. 2010), local molecular clouds (Harju et al. 2000), and a translucent high-latitude cloud (Cotten et al. 2012).
Enthalpy ΔH (in kcal mol−1) for producing the reactants.
4 Conclusion
Using first principles calculations, we investigated the gas phase reaction between partially dehydrogenated formamide (H2NCHO) and vinyl cyanide (H2CCHCN) toward the formation of 1H-pyrimidin-2-one (C4H4N2O), which is a direct precursor of the primary nucleobases. Thirteen combinations of ten different reactant species were tested. To produce C4H4N2O, the most energetically favorable pathway is found in the molecule-radical reaction between HNCHO and H2CCHCN with an energy barrier of 19.3 kcal mol−1. Energetically favorable pathways are calculated for the synthesis of nucleobases based on C4H4N2O interacting with amino, methyl, or hydroxyl. The energy barrier is computed to be 11.3, 18.6, or 19.9 kcal mol−1 to produce cytosine, thymine, or uracil from C4H4N2O, respectively.Given the previously reported detection of relevant reactants in the ISM, these energy barriers would suggest that the gas-phase synthesis of a nucleobase (cytosine in particular) could be thermally feasible in hot cores or in the inner part of protoplanetary disks. The second energetically favorable reaction is found to occur between H2NCO and H2CCCN, with an energy barrier of 23.8 kcal mol−1. Meanwhile, the minimum energy barrier of the other simulated reactions are all higher than 30.0 kcal mol−1, indicating that the reactions are unlikely to be thermally activated but can instead be triggered by radiation in the typically low-temperature ISM environments.
The computed optimal pathways indicate that H migration is the rate-determining process in most of the studied reactions. This is in line with the recent works of Castellanos et al. (2018) and Zhen et al. (2018), which reported that the photodehydrogenation and the photodissociation could be the key process in the photoinduced formation of polycyclic aromatic hydrocarbon molecules. The optimal reaction (A3) is found to be a molecule-radical reaction (instead of an expected radical-radical reaction), in which HNCHO readily reacts with H2CCHCN by a low energy barrier entrance reaction. Moreover, the possible formation mechanism in the ISM is discussed in terms of the previous detection of the partially dehydrogenated molecules. The computed atomization enthalpy of the reactants gives the referential energy that would be needed for generating the reactants from pristine molecules in the ISM, and is comparable to the reported ionization potential carried by far-UV photons from massive stars.
Acknowledgements
Partial financial supports from the National Natural Science Foundation of China (11964002, 12133003), the Guangxi Science Foundation (2020GXNSFAA159119, 2018GXNSFAA138179), the Special Funding for Guangxi Distinguished Professors (2017AD22006) and the Scientific Research Foundation of Guangxi University (XTZ160532) are acknowledged.
References
- Adams, W. S. 1941, ApJ, 93, 11 [NASA ADS] [CrossRef] [Google Scholar]
- Agúndez, M., Fonfría, J., Cernicharo, J., Pardo, J. R., & Guélin, M. 2008, A&A, 479, 493 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Akimkin, V., Zhukovska, S., Wiebe, D., et al. 2013, ApJ, 766, 8 [NASA ADS] [CrossRef] [Google Scholar]
- Balucani, N., Asvany, O., Huang, L. C. L., et al. 2000, ApJ, 545, 892 [Google Scholar]
- Bisschop, S. E., Jørgensen, J. K., van Dishoeck, E. F., & de Wachter, E. B. M. 2007, A&A, 465, 913 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bonfand, M., Belloche, A., Garrod, R. T., et al. 2019, A&A, 628, A27 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Castellanos, P., Candian, A., Zhen, J., Linnartz, H., & Tielens, A. G. G. M. 2018, A&A, 616, A166 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Choe, J. C. 2020, ApJ, 898, 13 [CrossRef] [Google Scholar]
- Cotten, D. L., Magnani, L., Wennerstrom, E. A., Douglas, K. A., & Onello, J. S. 2012, ApJ, 144, 163 [CrossRef] [Google Scholar]
- Cronin, J. R., & Chang, S. 1993, Biosignatures for Astrobiology, eds. J. M. Greenberg, C. X. Mendoza-Gömez, & V. Pirronello, 416 (Springer), 209 [NASA ADS] [Google Scholar]
- da Silva, W. B., Gargano, R., Silva, G. M., & Albernaz, A. F. 2016, Rev. Virtual Quim., 8, 515 [NASA ADS] [CrossRef] [Google Scholar]
- de Vicente, P., Martín-Pintado, J., & Wilson, T. L. 1996, A&A, 102, 64 [Google Scholar]
- Ehrenfreund, P., & Charnley, S. B. 2000, ARA&A, 38, 427 [Google Scholar]
- Elsila, J. E., Glavin, D. P., & Dworkin, J. P. 2009, Meteor. Planet. Sci., 44, 1323 [NASA ADS] [CrossRef] [Google Scholar]
- Eyring, H. 1935, J. Chem. Phys., 3, 107 [CrossRef] [Google Scholar]
- Feuchtgruber, H., Helmich, F. P., van Dishoeck, E. F., & Wright, C. M. 2000, ApJ, 535, L111 [NASA ADS] [CrossRef] [Google Scholar]
- Frisch, M. J., Trucks, G. W., Schlegel, H. B., et al. 2016, Gaussian 16 vB.01 (Wallingford, CT: Gaussian Inc.) [Google Scholar]
- Gardner, F. F., & Winnewisser, G. 1975a, ApJ, 197, L73 [NASA ADS] [CrossRef] [Google Scholar]
- Gardner, F. F., & Winnewisser, G. 1975b, ApJ, 195, L127 [NASA ADS] [CrossRef] [Google Scholar]
- Garrod, R. T., Weaver, S. L., & Herbst, E. 2008, ApJ, 682, 283 [NASA ADS] [CrossRef] [Google Scholar]
- Goicoechea, J. R., & Cernicharo, J. 2002, ApJ, 576, L77 [NASA ADS] [CrossRef] [Google Scholar]
- Goicoechea, J. R., Rodríguez-Fernández, N. J., & Cernicharo, J. 2003, Astron. Nachr. Suppl., 324, 139 [NASA ADS] [CrossRef] [Google Scholar]
- Goicoechea, J. R., Cernicharo, J., Lerate, M. R., et al. 2006, ApJ, 641, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Gralewicz, P., Wdowczyk, J., Wolfendale, A. W., & Zhang, L. 1997, A&A, 318, 925 [NASA ADS] [Google Scholar]
- Gupta, V. P., Tandon, P., & Mishra, P. 2013, Adv. Space Res., 51, 797 [NASA ADS] [CrossRef] [Google Scholar]
- Haid, S., Walch, S., Seifried, D., et al. 2018, MNRAS, 478, 4799 [Google Scholar]
- Halfen, D. T., Ilyushin, V., & Ziurys, L. M. 2011, ApJ, 743, 60 [Google Scholar]
- Hanine, M., Meng, Z., Lu, S., et al. 2020, ApJ, 900, 188 [NASA ADS] [CrossRef] [Google Scholar]
- Harju, J., Winnberg, A., & Wouterloot, J. G. A. 2000, A&A, 353, 1065 [NASA ADS] [Google Scholar]
- Henning, T., & Semenov, D. 2013, Chem. Rev., 113, 9016 [Google Scholar]
- Herbst, E. 2014, PCCP, 16, 3344 [NASA ADS] [CrossRef] [Google Scholar]
- Herbst, E., & Van Dishoeck, E. F. 2009, ARA&A, 47, 427 [NASA ADS] [CrossRef] [Google Scholar]
- Hollenbach, D. J., & Tielens, A. G. G. M. 1999, RvMP, 71, 173 [NASA ADS] [Google Scholar]
- Hollis, J. M., Lovas, F. J., Remijan, A. J., et al. 2006, ApJ, 643, L25 [Google Scholar]
- Hrušák, J., Holthausen, M. C., Goldberg, N., et al. 1993, Isr. J. Chem., 33, 277 [CrossRef] [Google Scholar]
- Jeilani, Y. A., Williams, P. N., Walton, S., & Nguyen, M. T. 2016, PCCP, 18, 20177 [NASA ADS] [CrossRef] [Google Scholar]
- Johansson, L. E. B., Andersson, C., Elldér, J., et al. 1984, A&A, 130, 227 [Google Scholar]
- Kovács, P., Zhu, X., Carrete, J., Madsen, G. K. H., & Wang, Z. 2020, ApJ, 902, 100 [CrossRef] [Google Scholar]
- Mackie, C. J., Peeters, E., Bauschlicher Jr, C. W., & Cami, J. 2015, ApJ, 799, 131 [NASA ADS] [CrossRef] [Google Scholar]
- Martín-Pintado, J., Gaume, R. A., Rodríguez-Fernández, N., de Vicente, P., & Wilson, T. L. 1999, ApJ, 519, 667 [Google Scholar]
- Materese, C. K., Nuevo, M., McDowell, B. L., Buffo, C. E., & Sandford, S. A. 2018, ApJ, 864, 44 [NASA ADS] [CrossRef] [Google Scholar]
- Meyer, D. M.,& Roth, K. C. 1991, ApJ, 376, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Nguyen, Q.-R., Graham, D., & Bujarrabl, V. 1984, A&A, 138, L5 [NASA ADS] [Google Scholar]
- Nguyen, M. T., Sengupta, D., Vereecken, L., Peeters, J., & Vanquickenborne, L. G. 1996, J. Phys. Chem., 100, 1615 [CrossRef] [Google Scholar]
- Nguyen, V. S., Orlando, T. M., Leszczynski, J., & Nguyen, M. T. 2013, J. Phys. Chem., 117, 2543 [NASA ADS] [CrossRef] [Google Scholar]
- Nguyen, H. T., Jeilani, Y. A., Leszczynski, J., & Nguyen, M. T. 2015, J. Phys. Chem. A, 119, 8871 [CrossRef] [Google Scholar]
- Nuevo, M., Milam, S. N., Sandford, S. A., Elsila, J. E., & Dworkin, J. P. 2009, Astrobiology, 9, 683 [NASA ADS] [CrossRef] [Google Scholar]
- Oba, Y., Takano, Y., Naraoka, H., Watanabe, N., & Kouchi, A. 2019, Nat. Commun., 10, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Ochterski, J. W. 2000, Gaussian Inc., 1, 19 [Google Scholar]
- Parker, D. S. N., Wilson, A. V., Kaiser, R. I., Labrador, T., & Mebel, A. M. 2012, J. Am. Chem. Soc., 134, 13896 [CrossRef] [Google Scholar]
- Peeters, Z., Botta, O., Charnley, S. B., et al. 2005, A&A, 433, 583 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Piani, L., Tachibana, S., Hama, T., et al. 2017, ApJ, 837, 35 [Google Scholar]
- Qi, H., Picaud, S., Devel, M., Liang, E., & Wang, Z. 2018, ApJ, 867, 133 [NASA ADS] [CrossRef] [Google Scholar]
- Quirico, E., & Bonal, L. 2019, Biosignatures for Astrobiology, eds. B. Cavalazzi, & F. Westall (Springer), 23 [CrossRef] [Google Scholar]
- Reach, W. T., & Rho, J. 1998, ApJ, 507, L93 [NASA ADS] [CrossRef] [Google Scholar]
- Ridgway, S. T., Hall, D. N. B., Kleinmann, S. G., Weinberger, D. A., & Wojslaw, R. S. 1976, Nature, 264, 345 [Google Scholar]
- Rotelli, L., Trigo-Rodríguez, J. M., Moyano-Cambero, C. E., et al. 2016, Sci. Rep., 6, 38888 [CrossRef] [Google Scholar]
- Saladino, R., Botta, G., Bizzarri, B. M., Di Mauro, E., & Garcia Ruiz, J. M. 2016, Biochem., 55, 2806 [Google Scholar]
- Sandford, S. A., Nuevo, M., Bera, P. P., & Lee, T. J. 2020, Chem. Rev., 160, 4616 [NASA ADS] [CrossRef] [Google Scholar]
- Schenewerk, M. S., Snyder, L. E., & Hjalmarson, A. 1986, ApJ, 303, L71 [NASA ADS] [CrossRef] [Google Scholar]
- Schuurman, M. S., Muir, S. R., Allen, W. D., & Schaefer III, H. F. 2004, J. Chem. Phys., 120, 11586 [NASA ADS] [CrossRef] [Google Scholar]
- Snyder, L.E., & Buhl, D. 1972, ApJ, 177, 619 [NASA ADS] [CrossRef] [Google Scholar]
- Stoks, P. G., & Schwartz, A. W. 1979, Nature, 282, 709 [NASA ADS] [CrossRef] [Google Scholar]
- Suits, A. G. 2008, Acc. Chem. Res., 41, 873 [CrossRef] [Google Scholar]
- Suzuki, T., Ohishi, M., Saito, M., et al. 2018, ApJS, 237, 3 [NASA ADS] [CrossRef] [Google Scholar]
- Tappe, A., Lada, C. J., Black, J. H., & Muench, A. A. 2008, ApJ, 680, L117 [Google Scholar]
- Tercero, B., Kleiner, I., Cernicharo, J., et al. 2013, ApJ, 770, L13 [NASA ADS] [CrossRef] [Google Scholar]
- Turner, B. E. 1971, ApJ, 163, L35 [NASA ADS] [CrossRef] [Google Scholar]
- van Dishoeck, E. F., & Jansen, D. J. 1993, ApJ, 416, L83 [NASA ADS] [CrossRef] [Google Scholar]
- Wampfler, S. F., Herczeg, G. J., Bruderer, S., et al. 2010, A&A, 521, L36 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wang, T., & Bowie, J. H. 2012, Org. Biomol. Chem., 10, 652 [CrossRef] [Google Scholar]
- Whittet, D. C. B., & Walker, H. J. 1991, MNRAS, 252, 63 [NASA ADS] [CrossRef] [Google Scholar]
- Wyrowski, F., Menten, K. M., Güsten, R., & Belloche, A. 2010, A&A, 518, A26 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Zamirri, L., Ugliengo, P., Ceccarelli, C., & Rimola, A. 2019, ESC, 3, 1499 [Google Scholar]
- Zhao, Y., & Truhlar, D. G. 2008, Theor. Chem. Acc., 120, 215 [CrossRef] [Google Scholar]
- Zhen, J., Chen, T., & Tielens, A. G. G. M. 2018, ApJ, 863, 128 [NASA ADS] [CrossRef] [Google Scholar]
- Ziurys, L. M. 2006, PNAS, 103, 12274 [CrossRef] [Google Scholar]
All Tables
All Figures
 |
Fig. 1 Potential energy diagram for the two most likely reactions between H2NCHO and H2CCHCN in single collision events (Reaction 0). The most energetically favorable reaction pathway is highlighted in red, with the second one in black. |
| In the text | |
 |
Fig. 2 (a) Reactions between dehydrogenated H2NCHO and H2CCHCN. The dashed lines in different colors indicate reactions in various categories with different amounts of hydrogen atoms in the reactants. (b) Energy barriers for the two most possible pathways of the reactions between the dehydrogenated H2NCHO and H2CCHCN molecules. |
| In the text | |
 |
Fig. 3 Potential energy diagrams for the two most energetically favorable pathways for A1-4 in single collision events. |
| In the text | |
 |
Fig. 4 Potential energy diagrams for the two most energetically favorable pathways for reactions B1-4 in single collision events. |
| In the text | |
 |
Fig. 5 Potential energy diagrams for the two most energetically favorable pathways for the reactions C1-4 in single collision events. |
| In the text | |
 |
Fig. 6 Potential energy diagrams for the one or two most energetically favorable pathways for the reactions between C4H4N2O and NH2, CH3, OH, which eventually leads to the formation of nucleobases including (a) cytosine, (b) thymine, and (c) uracil. |
| In the text | |
 |
Fig. 7 Reaction rate coefficient (in logarithmic scale) versus temperature for the most favorable pathways of the studied reactions. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


