Fig. 13
Download original image
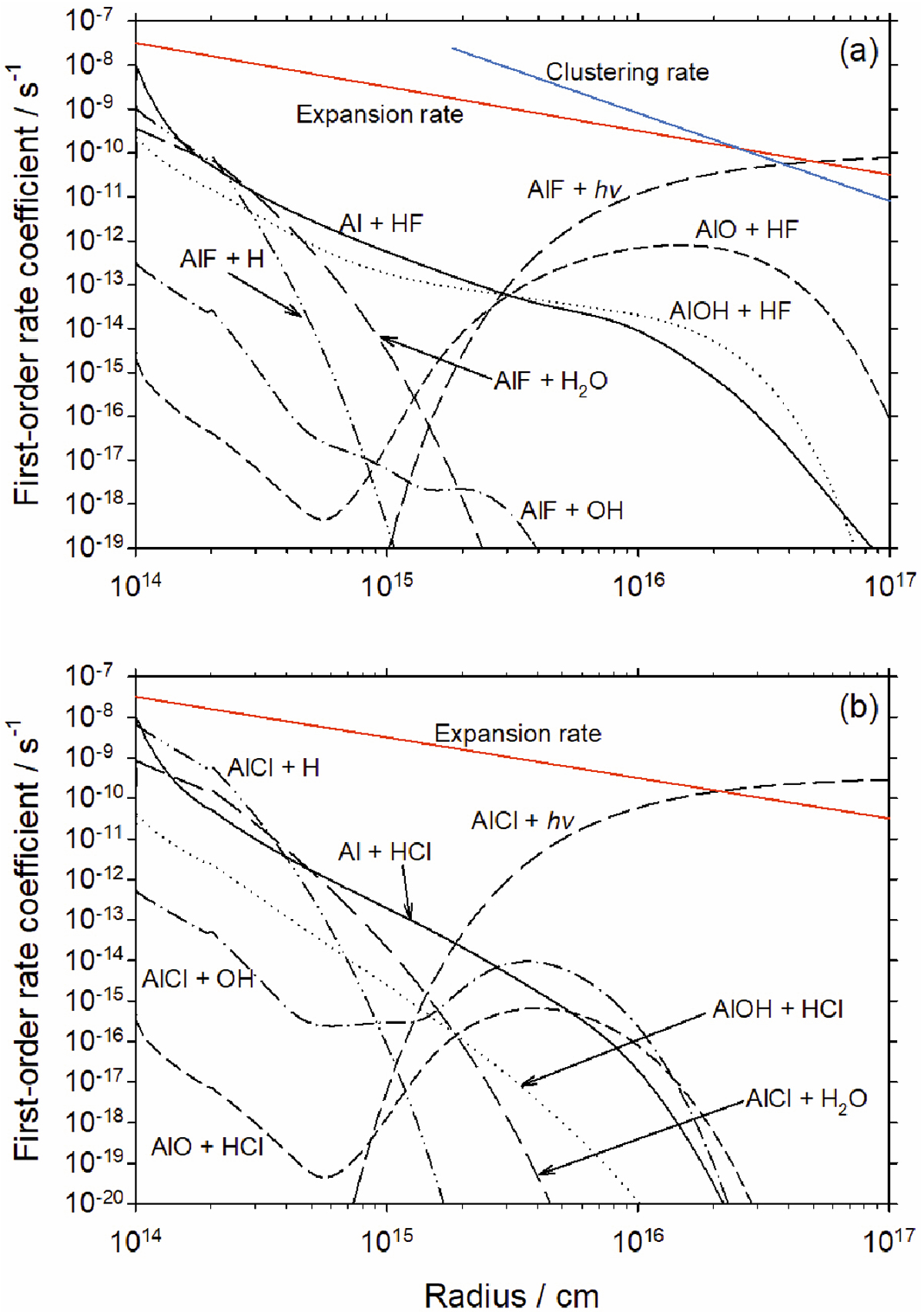
(a) Calculated first-order rates for conversion of HF to AlF by reaction with Al, AlOH and AlO; and loss of AlF by photolysis and reaction with H, H2O and OH. The clustering rate with metallic compounds (blue line, see text for further details) is shown in the region where the temperature is below 500 K. (b) Calculated first-order rates for conversion of HCl to AlCl by reaction with Al, AlOH and AlO; and loss of AlCl by photolysis and reaction with H, H2O and OH. The red lines show the molecular expansion rate of the outflow at a constant velocity of 16.5 km s−1.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


