Fig. 3
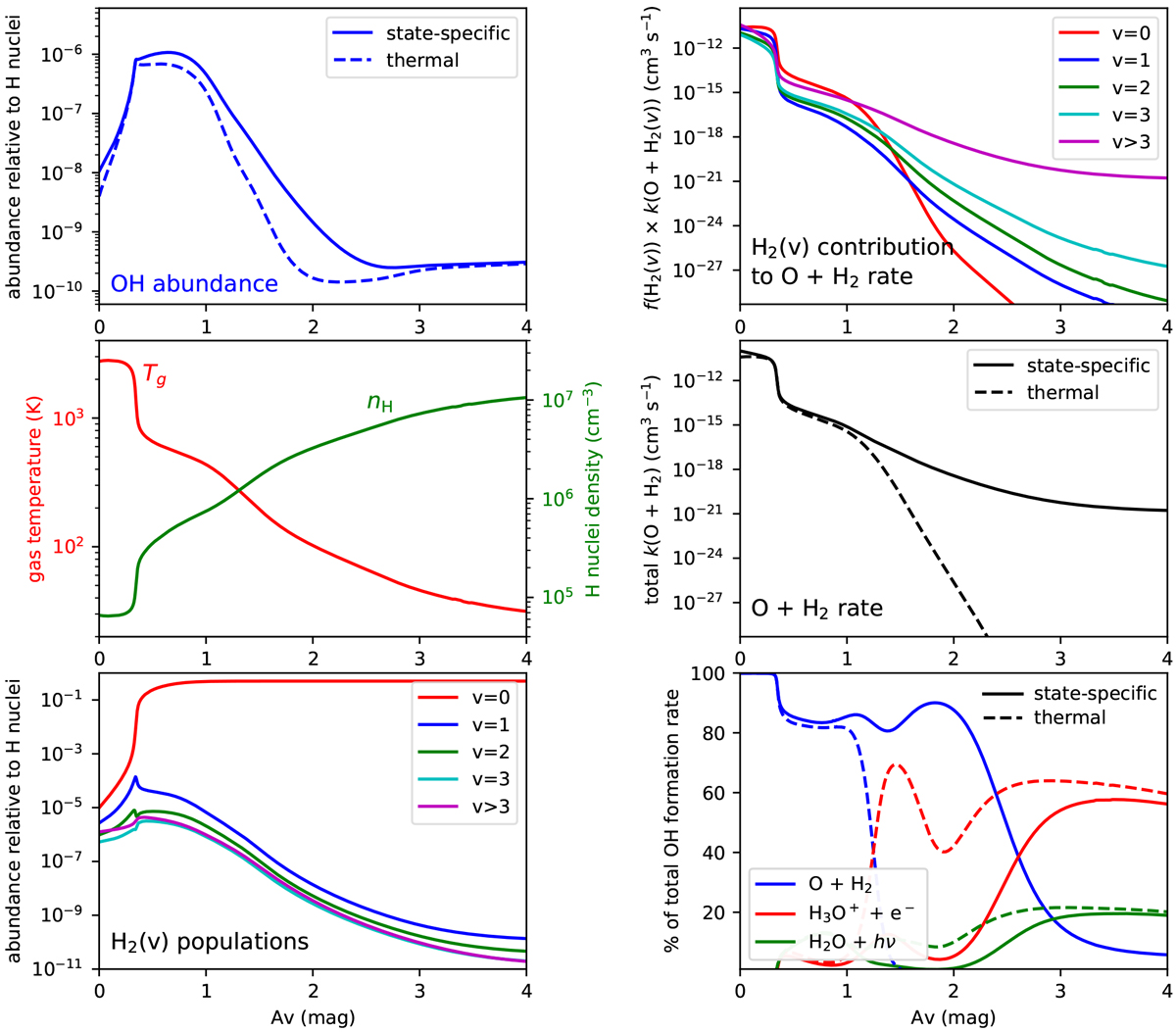
Assorted quantities calculated in the PDR model of the Orion Bar plotted as a function of AV. The solid lines refer to the model including H2 state-specific rate coefficients for reaction (1), and the dashed lines to the reference model in which a thermal rate coefficient is used for reaction (1). Top right panel: contribution of each vibrational state of H2 to the total rate coefficient of the O + H2 reaction, expressed as f(H2(v)) × k(O + H2 (v)), where f(H2(v)) is the fractional population of H2 in the vibrational state v. Bottom right panel: contribution of the three main gas-phase reactions of formation of OH, expressed as a percentage of the total OH formation rate.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


