Fig. B.2
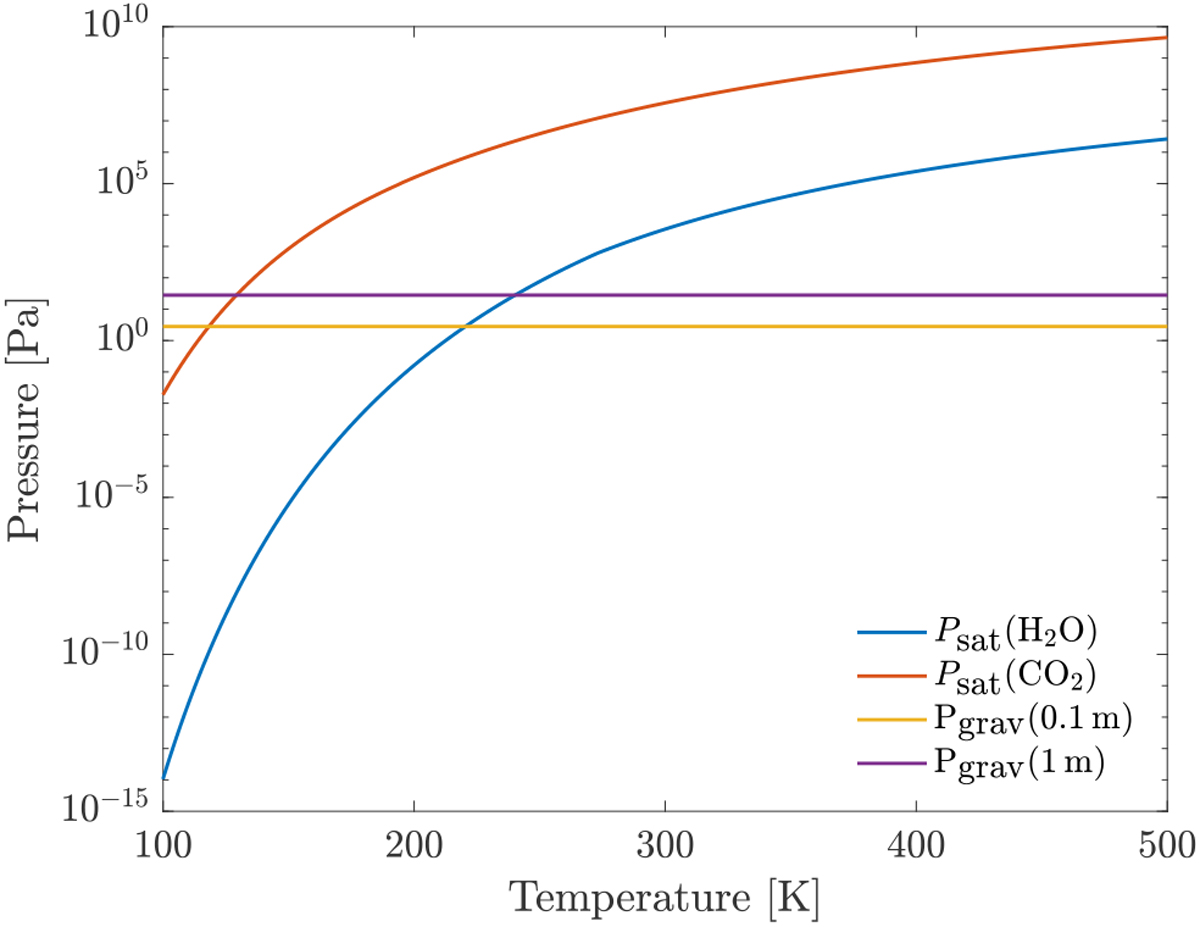
Comparison between the saturated vapor pressure and the gravitational pressure at 0.1 m and 1 m below the surface of a 100 km-sized planetesimal at different temperatures and for different molecules. The temperature down to 1 m below the surface is assumed to be the same as on the surface. The temperature needs to be about twice as high when considering H2O molecules than when considering CO2 molecules inorder for the vapor pressure to win over the gravitational pressure.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


