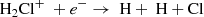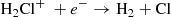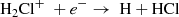| Issue |
A&A
Volume 629, September 2019
|
|
|---|---|---|
| Article Number | A128 | |
| Number of page(s) | 9 | |
| Section | Extragalactic astronomy | |
| DOI | https://doi.org/10.1051/0004-6361/201935860 | |
| Published online | 16 September 2019 | |
Chlorine-bearing molecules in molecular absorbers at intermediate redshifts⋆
1
Institute of Astronomy, KU Leuven, Celestijnenlaan 200D bus 2401, 3001 Leuven, Belgium
e-mail: shj.wallstrom@gmail.com
2
Institute of Astronomy and Astrophysics, Academia Sinica, 11F of Astronomy-Mathematics Building, No.1, Sec. 4, Roosevelt Rd., 10617 Taipei, Taiwan
3
Department of Space, Earth and Environment, Chalmers University of Technology, Onsala Space Observatory, 43992 Onsala, Sweden
4
LERMA/LRA, Ecole Normale Supérieure, Observatoire de Paris, CNRS UMR 8112, PSL Research University, Sorbonne Universités, UPMC Université Paris, 24 Rue Lhomond, 75005 Paris, France
5
Harvard-Smithsonian Center for Astrophysics, 60 Garden Street, Cambridge, MA 02138, USA
Received:
9
May
2019
Accepted:
30
July
2019
We use observations of chlorine-bearing species in molecular absorbers at intermediate redshifts to investigate chemical properties and 35Cl/37Cl isotopic ratios in the absorbing sightlines. Chloronium (H2Cl+) is detected along three independent lines of sight in the z = 0.89 and z = 0.68 molecular absorbers located in front of the lensed quasars PKS 1830−211 and B 0218+357, respectively. Hydrogen chloride (HCl) was observed only toward PKS 1830−211, and is found to behave differently from H2Cl+. It is detected in one line of sight with an abundance ratio [H2Cl+] / [HCl] ∼1, but remains undetected in the other, more diffuse, line of sight, with a ratio [H2Cl+] / [HCl] > 17. The absorption profiles of these two chlorine-bearing species are compared to other species and discussed in terms of the physical properties of the absorbing gas. Our findings are consistent with the picture emerging from chemical models where different species trace gas with different molecular hydrogen fraction. The 35Cl/37Cl isotopic ratios are measured in the different lines of sight and are discussed in terms of stellar nucleosynthesis.
Key words: quasars: absorption lines / quasars: individual: PKS 1830-211 / quasars: individual: B 0218+357 / galaxies: ISM / galaxies: abundances / radio lines: galaxies
The spectra in Fig. 1 are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/cat/J/A+A/629/A128
© ESO 2019
1. Introduction
Chlorine is a minor constituent of the Universe, with a solar abundance of only ∼3 × 10−7 relative to hydrogen (Asplund et al. 2009), that is orders of magnitude smaller than the elemental abundances of carbon, nitrogen, and oxygen. Nevertheless, chlorine has a strong chemical tendency to form hydrides (e.g., H2Cl+, HCl) whose abundances can be as high as those of CH and H2O, for example, in diffuse molecular clouds (Neufeld et al. 2010; Sonnentrucker et al. 2010; Lis et al. 2010). This is because of its unique thermo-chemistry properties: first, chlorine has an ionization potential of 12.97 eV, which is lower than that of hydrogen, such that it is predominantly in its ionized stage, Cl+, in the atomic phase of the interstellar medium (ISM), and second, Cl+ can react exothermically with H2 triggering an active chemistry.
In fact, the interstellar chemical network of chlorine is thought to be relatively simple, dominated by a handful of hydrides (Neufeld & Wolfire 2009), though the vast majority of interstellar chlorine will be in its neutral form in the presence of even small amounts of H2. The reaction of Cl+ with H2 readily forms HCl+, which has been detected in absorption in Galactic diffuse clouds (De Luca et al. 2012) harboring a significant amount (3–5%) of the gas-phase chlorine. In turn, HCl+ can further react exothermically with H2 to form chloronium (H2Cl+; first observed by Lis et al. 2010). Chloronium itself can react with free electrons to either form hydrogen chloride (HCl; historically the first chlorine-bearing molecule detected in the ISM; Blake et al. 1985) or release neutral chlorine. There are several pathways to destroy HCl through photodissociation, photoionization, or reactions with He+, 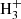 , and C+. Methyl chloride, CH3Cl, was also recently identified in the young stellar object IRAS 16293 − 2422 and the gaseous coma of comet 67P/Churyumov–Gerasimenko (Fayolle et al. 2017), suggesting that chlorine chemistry in space also extends to more complex species (see, e.g., Acharyya & Herbst 2017 for gas-grain chemical models of chlorine chemistry).
, and C+. Methyl chloride, CH3Cl, was also recently identified in the young stellar object IRAS 16293 − 2422 and the gaseous coma of comet 67P/Churyumov–Gerasimenko (Fayolle et al. 2017), suggesting that chlorine chemistry in space also extends to more complex species (see, e.g., Acharyya & Herbst 2017 for gas-grain chemical models of chlorine chemistry).
From the nucleosynthetic point of view, elemental chlorine is produced on both short timescales in core-collapse supernovae, and long timescales in asymptotic giant branch (AGB) stars and Type Ia supernovae (p.164, Clayton 2003). Chlorine is produced during oxygen burning, from fast reactions with the more abundant alpha elements, in stars massive enough to ignite it. In core-collapse supernovae excess neutrons cause a great increase in chlorine abundance, and hence they produce the majority of chlorine in the universe through explosive nucleosynthesis. Type Ia supernovae produce smaller amounts of chlorine through explosive oxygen burning.
Chlorine can be found in two stable isotopes, 35Cl and 37Cl, with different sources producing different relative abundances. Low metallicity core-collapse supernovae, like the ones that likely enriched the pre-Solar nebula, produce a 35Cl/37Cl ratio of ∼3 as measured in the Solar system (Asplund et al. 2009). Higher metallicity core-collapse supernovae can produce lower ratios (Kobayashi et al. 2006, 2011). The isotopic ratios produced in AGB stars also depend heavily on metallicity, with low metallicities producing lower 35Cl/37Cl values (Cristallo et al. 2011).
Molecular absorbers at intermediate redshift (z ∼ 1) are powerful probes of the physico-chemical properties of distant galaxies. They offer a cosmological perspective on the chemical evolution of the Universe, for example with measurements of isotopic ratios at look-back times on the order of half or more of the present age of the Universe, but unfortunately, only a handful of such absorbers have been identified so far (e.g., Combes 2008; Wiklind et al. 2018). In this paper, we collect observations of chlorine-bearing species in the two best studied and most molecule-rich redshifted radio molecular absorbers, with the goal of investigating their chemical properties and measuring the 35Cl/37Cl isotopic ratio in different absorbing sightlines.
The molecular absorber located in front of the quasar PKS 1830−211 is a face-on spiral galaxy at a redshift z = 0.88582 (hereafter labeled MA0.89). The intervening galaxy lenses the quasar into two bright and compact images, leading to two independent sightlines in the southwest and northeast where absorption is detected (hereafter MA0.89 SW and MA0.89 NE), with impact parameters of ∼2 kpc and 4 kpc, respectively, in the absorber. These two compact, lensed images are embedded in a faint structure, reminiscent of an Einstein ring, seen at low radio frequencies (Jauncey et al. 1991). A wealth of molecules and their rare isotopologs have been detected in this absorber, especially in MA0.89 SW, which is characterized by a large H2 column density (∼2 × 1022 cm−2) and moderate gas density ∼103 cm−3 (e.g. Wiklind & Combes 1996; Muller et al. 2011, 2013, 2014a). MA0.89 NE is characterized by a lower H2 column density (∼1 × 1021 cm−2) and more diffuse gas composition as shown by the enhancement of the relative abundances of hydrides such as OH+, H2O+, and H2Cl+ (e.g., Muller et al. 2016a).
The second molecular absorber (hereafter labeled MA0.68), located in front of the quasar B 0218 + 357, shares similar properties with MA0.89. It is a nearly face-on spiral galaxy at an intermediate redshift z = 0.68466, also lensing the background quasar into two main compact images and an Einstein ring, seen at low radio frequencies and centered on the faintest image. Molecular absorption has only been detected in one line of sight (toward the brightest image), at an impact parameter of ∼2 kpc from the absorber’s center (Wiklind & Combes 1995; Muller et al. 2007). A number of molecules and their isotopologs have also been observed in MA0.68 (e.g. Wallström et al. 2016), which has an H2 column density (∼8 × 1021 cm−2) that is intermediate between MA0.89 SW and MA0.89 NE.
With these two absorbers, we are thus exploring three independent lines of sight, with different redshifts, galactocentric distances, H2 column densities, visual extinctions, metallicities, local radiation fields, cosmic-ray ionization rates, etc. Our observations and results are presented in Sects. 2 and 3. We discuss our findings in terms of chemical properties derived from chlorine-bearing species and 35Cl/37Cl isotopic ratios in Sect. 4.
2. Observations
Observations of the two absorbers were obtained with the Atacama Large Millimeter/submillimeter Array (ALMA) between 2014 and 2017. A summary of these observations is given in Table 1. Details of the observational setups and data analysis are described below for each absorber separately.
Summary of ALMA observations.
MA0.68. Observations of H2Cl+ were obtained on 2016 October 22 and 2017 May 02. During the first run, the precipitable water vapor content was ∼2.4 mm. The array was in a configuration where the longest baseline was 1.7 km. The bandpass calibrator was J 0237+2848 (∼2.2 Jy at 100 GHz) and the gain calibrator was J 0220+3241 (∼0.2 Jy at 100 GHz). During the second run, the precipitable water vapor content was < 1 mm. The array’s longest baseline was 1.1 km. The bandpass calibrator was J0510+1800 (∼2.6 Jy at 100 GHz) and the gain calibrator J 0205+3212 (∼0.9 Jy at 100 GHz). The data calibration was done with the CASA package following standard procedures. We further improved the data quality with a step of self-calibration of the phase, using a model of two point-sources for MA0.68. When combined, the two datasets result in a synthesized beam of ∼1.0″ × 0.6″ (P.A. = −176°) at 100 GHz, slightly larger than the separation of 0.3″ between the two lensed images of the quasar. However, we extracted the spectra along each line of sight by directly fitting each visibility set separately using the CASA-Python task UVMULTIFIT (Martí-Vidal et al. 2014), with a model of two point-sources. This is possible in the Fourier plane, thanks to the high signal-to-noise ratio of the data and the known and simple geometry of the source. The final spectrum was the weighted average of the individual spectra.
MA0.89. We complemented previous observations of the ortho and para forms of H2Cl+ toward MA0.89 presented by Muller et al. (2014a) and Le Gal et al. (2017) with new observations of HCl obtained with ALMA during three observing runs: in 2014 May 03, 2014 May 06, and 2014 Jun 30. Both HCl isotopologs were observed in the same spectral window, and within the same receiver tuning as the para-H2O+ data presented by Muller et al. (2017). The data reduction followed the same method as for MA0.68 data described above (self-calibration on the quasar, extraction of the spectra from visibility fitting, and weighted-averaged final spectrum).
3. Results
The spectra observed along the three lines of sight are shown in Fig. 1. H2Cl+ is detected in all sightlines, unsurprisingly since it is ubiquitous in the Galactic diffuse and translucent gas (Lis et al. 2010; Neufeld et al. 2012). On the other hand, HCl is detected in only one of the two sightlines toward PKS 1830−211 (MA0.89 SW), with a stringent upper limit toward the other. It was not observed toward MA0.68.
 |
Fig. 1. Absorption spectra of chlorine-bearing species observed toward MA0.89 SW (top three panels), MA0.89 NE (middle three panels), and MA0.68 (bottom panel). Hyperfine structure is indicated in green for 35Cl-isotopologs of each line toward MA0.89 SW. Hyperfine structure was deconvolved by fitting multiple Gaussian velocity components; these fits are shown in red on top of spectra, with residuals shown in blue. On the right, deconvolved opacity profiles (assuming optically thin lines with fc = 1, as explained in Sect. 3), normalized to their peak opacity, are shown, together with opacity profiles of some other species for comparison. The profile of p-H2O+ is of the strongest hyperfine component of the 110–101 transition, at rest frequency of 607 GHz. The profile of o-H2S is the 110–101 transition, at rest frequency of 168.8 GHz, which was observed simultaneously with o-H2Cl+ in the same tuning. |
The H2Cl+ absorptions reach at most a few percent of the continuum level for all sightlines, so we must determine whether the absorption is optically thin and whether the source covering factor, fc, is less than unity. In MA0.89 SW, the ground state transition of species like CH+ and H2O is strongly saturated and reaches a depth of nearly 100% of the continuum level, implying that fc ≈ 1 for these species (Muller et al. 2014a, 2017). In MA0.89 NE, the situation is not so clear, but the deepest absorption reaches a depth of several tens of percent of the continuum level (Muller et al. 2017). For MA0.68, the covering factor of the 557 GHz water line is known to be large and close to unity (Combes & Wiklind 1997), as confirmed by recent ALMA data (Muller, priv. comm.). The HCl absorption reaches a depth of ∼10% of the continuum level toward MA0.89 SW, so without further evidence we consider fc = 1 for all species and all sightlines – hence an optically thin line regime – and our derived column densities (Table 2) can be viewed, strictly speaking, as lower limits.
Summary of results and line-of-sight properties.
When detected, both H2Cl+ and HCl show both their 35Cl- and 37Cl-isotopologs, observed simultaneously in the same spectral window (see a summary of the spectroscopic parameters of both species in Table A.1). Considering optically thin lines and given the very small difference in their relative weight, the abundance ratio of the 35Cl- and 37Cl-isotopologs should reflect the 35Cl/37Cl isotopic ratio (see Sect. 4.2).
In MA0.89, the ground-state transitions of both the ortho and para forms of H2Cl+ were observed. It should be noted that, from the same data, Le Gal et al. (2017) determined ortho-to-para ratios (OPR) in agreement with the spin statistical weight 3:1 for both sightlines, within the uncertainties. In MA0.68, only ortho-H2Cl+ was observed. We adopted an OPR = 3 for the calculations of H2Cl+ total column densities in all sightlines.
Finally, both H2Cl+ and HCl harbor a hyperfine structure that needs to be deconvolved before comparing intrinsic absorption profiles. This was done by fitting an intrinsic normalized profile, composed by the sum of individual Gaussian velocity components and convolved with the hyperfine structure (see Table A.1), using χ2 minimization and taking the errors to be 1σ. Both the 35Cl- and 37Cl-isotopologs were treated in a common fit, with the 35Cl/37Cl ratio as a free parameter. In order to assess whether the hyperfine structure deconvolution was robust, we fitted the ortho and para lines of H2Cl+ separately for MA0.89 SW. We obtained a good solution, with residuals at the noise level, with four Gaussian velocity components for this line of sight. For MA0.89 NE and MA0.68 the detections have lower signal-to-noise ratios, so we limited the number of Gaussian components to one for sake of simplicity. These fits still yield residuals consistent with the noise level. All the velocity profiles in Fig. 1 were normalized to the peak opacity to enable a straightforward comparison.
There is a striking difference in the opacity profiles of H2Cl+ and HCl toward MA0.89 SW: the former has prominent line wings, at velocities |v| = 10 − 50 km s−1, while the latter is mostly a narrow component centered at v = 0 km s−1 with FWHM ∼ 20 km s−1. In addition to the chlorine-bearing species of interest here, we also show in Fig. 1 the opacity profiles for some other species observed at the same epoch (to prevent effects from time variations of the absorption profile, see Muller & Guélin 2008; Muller et al. 2014a). In MA0.89 SW, we find that the profile of H2Cl+ best matches that of H2O+, while the profile of HCl best matches that of H2S. Similarly, in MA0.68, we observe that the FWHM of the H2Cl+ opacity is noticeably larger than that of H2S, suggesting again that the two species trace different gas components.
Using the column density ratios between the two lines of sight of MA0.89, γSW/NE, Muller et al. (2016a); Muller et al. (2017) could classify the different species in two simple categories: those with low γSW/NE (≲5), for example ArH+, OH+, H2Cl+, and H2O+, which are known to trace gas with low molecular hydrogen fraction, fH21, of 1–10% (see, e.g., Neufeld & Wolfire 2016), and those with γSW/NE above 20, for example CH, HF, HCO+, HCN, and H2S, tracing gas with much higher molecular hydrogen fraction, > 30% and up to 100%. Since MA0.89 NE is dominated by gas with low fH2 the non-detection of HCl in this line of sight, with a large γSW/NE > 90, suggests that HCl is a tracer of high-fH2 gas, in agreement with chemical predictions (see Sect. 4). The line of sight towards MA0.68 is not yet fully characterized, but the estimated column density of H2 and relative abundance of H2Cl+ suggest that it is intermediate between MA0.89 NE and SW in term of molecular hydrogen fraction.
4. Discussion
4.1. Chemistry
Given the lack of observational constraints other than molecular abundances in the lines of sight through MA0.68 and MA0.89, and the fact that they sample different clouds and potentially different gas components, we have limited ourselves to a simple analytical model to explore the chlorine chemistry and how it varies with a few key parameters. For diffuse cloud conditions, HCl and H2Cl+ are linked in a simple way, as HCl results from one specific channel of the dissociative recombination of H2Cl+ and is principally destroyed by photodissociation:
with reaction rates k1 = 7.5 × 10−8(T/300)−0.5 cm3 s−1 (see Appendix B) and k2 = 1.8 × 10−9 G0exp(−2.9AV) s−1, where G0 is the scaling factor of the interstellar UV radiation field (ISRF), expressed in Draine units (Heays et al. 2017). Under these conditions HCl is almost completely destroyed.
From these two reactions, we can write, at steady-state:
and, introducing the fractional ionization xe = n(e−)/nH and neglecting the AV dependence of the photodissociation rate as in a diffuse line of sight AV ≪ 1, we get
A large [H2Cl+] / [HCl] ratio is obtained for large values of G0/nH as suggested by Neufeld & Wolfire (2009).
We can perform a similar analysis for the [OH+] / [H2O+] ratio by considering the main formation process of H2O+ via the OH+ + H2 reaction and its destruction by dissociative recombination and reaction with molecular hydrogen:
with reaction rates k5 = 1.7 × 10−9 cm3 s−1, k6 = 4.3 × 10−7(T/300)−0.5 cm3 s−1, and k7 = 6.4 × 10−10 cm3 s−1. Substituting the fractional ionization xe and the molecular fraction fH2 we get
Using the derived molecular fractions from Muller et al. (2016a), we can estimate the fractional ionization from the [OH+] / [H2O+] ratio in the two MA0.89 lines of sight, assuming T = 100 K. The [H2Cl+] / [HCl] ratio then allows for the derivation of G0, again following Muller et al. (2016a) in the assumption of nH = 35 cm−3.
The calculated values are reported in Table 3. As the derivation does not consider any dependence on the visual extinction, the derived values of the radiation field correspond to lower limits. We note that the SW line of sight has a significantly higher molecular fraction, and hence a larger total visual extinction (AV; see Sect. 4.1.1), than the NE line of sight, so its ISRF is likely to be underestimated in our reasoning. The results depend strongly on the value of AV, but as an example: for a cloud of AV = 1 in MA0.89 SW, we calculate G0 = 9. This suggests that both MA0.89 SW and MA0.89 NE have more intense radiation fields than in the Solar neighborhood.
Abundance ratios and derived parameters for diffuse gas in MA0.89 SW and MA0.89 NE.
Our simple model is broadly consistent with the results of Neufeld & Wolfire (2009), though we note that the chlorine chemical network still has some uncertainties, as discussed in Appendix B, which can significantly impact the model results. Neufeld & Wolfire (2009) find a column density ratio [H2Cl+] / [HCl] ∼1, as we measure in MA0.89, for a two-sided slab model with G0 = 10 and nH = 315 cm−3 at AV ∼ 1. However, the predicted column densities of these molecules are significantly smaller than the observed ∼1013 cm−2 in our lines of sight.
4.1.1. Properties of each sightline
Here we discuss more specifically our results towards MA0.68 and MA0.89 and compare the physical and chemical properties in the different sightlines.
MA0.89 SW. This sightline is rich in molecular species, with almost 50 different species detected so far (e.g., Muller et al. 2011, 2014a), and the H2 column density is relatively high, at ∼1022 cm−2. We can estimate the total visual extinction AV, tot in MA0.89 SW using Bohlin’s law (Bohlin et al. 1978) and the H and H2 column densities (Chengalur et al. 1999; Muller et al. 2014a). We find AV, tot ≈ 20 mag, which is shared among a number of individual clouds or kinematical structures. Obviously, there is at least one, most likely a few, cloud(s) with AV high enough (∼1) in the line of sight for HCl to be detectable. Indeed, the line profile of HCl indicates two peaks close to v ∼ 0 km s−1. Those two velocity components were previously noticed as two distinct clouds with different chemical setups, as they show opposite peaks in their CF+ vs. CH3OH absorption (Muller et al. 2016b), suggesting chemical segregation.
The multi-phase composition of the absorbing gas in this sightline has been clearly revealed by the recent observations of hydrides with ALMA (Muller et al. 2014a, 2016b, 2017). Accordingly, the absorption from H2Cl+ and HCl can be simply understood as tracing gas with different molecular fraction. In Fig. 2, we see a clear difference between the core and wings of the line profiles: in the line center the gas is denser or better shielded and HCl dominates, with a [H2Cl+] / [HCl] ratio of ∼0.5, while in the wings the ratio increases to ≳1 and H2Cl+ dominates. This is consistent with different gas properties (i.e., fH2) between the core and wings.
 |
Fig. 2. Abundance ratio of H2Cl+ (derived from ortho and para forms, shown in top and bottom panels, respectively) over HCl across absorption profile toward MA0.89 SW. We use measured ortho-to-para ratio of three to obtain total H2Cl+ column density. Abundance ratio is calculated only for channels where signal-to-noise ratio of normalized profile of both H2Cl+ and HCl is higher than five. |
MA0.89 NE. Only H2Cl+ is detected in this line of sight, which is characterized by gas at low fH2. The [H2Cl+] / [HCl] abundance ratio has a lower limit of ∼17, more than one order of magnitude higher than in the SW line of sight. Following the method outlined above, we estimate a total AV ≈ 2 mag. Based on a statistical analysis, Muller & Guélin (2008) argued that the large time variations of the opacity profiles2 imply a relatively small number of individual clouds in the line of sight, ≲5, and that the bulk of the absorption should arise from clouds not much smaller than the background continuum source, ∼1 pc. ALMA observations of the CH+ absorption (Muller et al. 2017) reveal a complex velocity profile which can be fitted with ∼10 different Gaussian velocity components, some with widths as small as a few km s−1, spanning a total velocity interval between −300 km s−1 and −100 km s−1. Taking a simplified model of ∼10 individual clouds along the line of sight results in each having an average AV of ∼0.2. From the chemical predictions, we indeed expect some production of H2Cl+ at this level of AV but very little HCl, consistent with the non-detection of HCl.
MA0.68. Here, we estimate a total AV ≈ 8 magnitudes. The overall absorption profile is narrow compared to that of MA0.89 and we estimate the number of clouds to be ∼3 based on the number of distinct velocity features in the profile (e.g. Wallström et al. 2016), suggesting characteristics intermediate between MA0.89 SW and MA0.89 NE. The abundance of v relative to H2 is also intermediate between the MA0.89 sightlines.
4.1.2. Elemental chlorine and abundances of chlorine-bearing molecules
Models of chlorine chemistry (e.g., Neufeld & Wolfire 2009) find that in the presence of H2 most chlorine resides in the form of the neutral atom Cl. Observationally, a strong correlation is found between the column densities of Cl and H2, in both the local ISM (Jura 1974; Sonnentrucker 2006; Moomey et al. 2012) and in high-redshift damped Lyman-α absorption systems (Balashev et al. 2015) over three orders of magnitude in H2 column densities, with a relation3
This trend is found to be independent of the overall gas metallicity, suggesting that neutral chlorine could be an excellent tracer of H2.
Assuming that the correlation in Eq. (9) also holds for our molecular absorbers at intermediate redshifts, we can infer the column density of neutral chlorine corresponding to the column density of H2 in the different sightlines. With the observed column densities of H2Cl+ and HCl, we can then estimate the fraction of chlorine in these molecules (assuming neutral chlorine is vastly dominant and summing the 35Cl and 37Cl-isotopologs). Accordingly, we estimate that about 0.7%, 1.8%, and 0.9% of chlorine is in the form of H2Cl+ for MA0.89 SW, MA0.89 NE, and MA0.68, respectively. For MA0.89 SW, we also estimate that about 0.9% of Cl is in HCl. These values are uncertain by about an order of magnitude (due to dispersion of the NCl − NH2 correlation and uncertainty in the H2 column density), and averaged over the whole sightline.
4.1.3. HCl+ and CH3Cl
Besides H2Cl+ and HCl, we here discuss briefly some other chlorine-bearing species, already detected or potentially present in the ISM in our intermediate-redshift absorbers. As previously mentioned, HCl+ is the first chlorine-bearing species to be formed in the chlorine-chemistry network from the reaction of Cl+ and H2, in gas with a low enough fH2 to maintain some Cl+, and as such, HCl+ is an excellent tracer of the diffuse gas component. HCl+ has a complex spectrum, with multiple ground-state hyperfine transitions around 1.44 THz (De Luca et al. 2012). Unfortunately, those transitions fall out of ALMA bands for a redshift of 0.89 and HCl+ cannot be observed toward PKS 1830−211. It could in principle be observed toward B 0218+357 in ALMA Band 10, although with the observational challenge of a weak background continuum source at low elevation.
Another chlorine-bearing molecule, recently detected in the ISM, is methyl chloride (CH3Cl). It has several hyperfine transitions which were covered in a deep spectral scan in the 7 mm band with the Australia Telescope Compact Array toward PKS 1830−211 (Muller et al. 2011). No features related to CH3Cl (rest frequency near 79.8 GHz) are detected down to a few per mil of the continuum level. Simulated absorption spectra for the conditions appropriate for the MA0.89 absorber yield a peak optical depth of τ = 1.95 × 10−3 for the hyperfine-structure blend, with an upper limit on the column density of N(CH3Cl) = 5 × 1012 cm−2. CH3Cl was recently identified in the young stellar object IRAS 16293 − 2422 by Fayolle et al. (2017), who infer a CH3Cl column density from these high-resolution ALMA data under the assumption of a fixed excitation temperature of 102 K of 4.6 × 1014 cm−2 over a 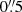 beam. A previous observation of HCl toward the same source by Peng et al. (2010) yielded well-constrained optical depths in the J = 1 − 0 transition but referred to a much larger beam size of
beam. A previous observation of HCl toward the same source by Peng et al. (2010) yielded well-constrained optical depths in the J = 1 − 0 transition but referred to a much larger beam size of 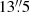 . If the HCl result is re-interpreted for a fixed excitation temperature of 102 K, the resulting column density of HCl would be 8.9 × 1014 cm−2, and if the ratio of column densities is simply scaled by the ratio of beam areas, then the abundance ratio would be [CH3Cl] / [HCl] = 7 × 10−4. This is likely to be an underestimate, because the HCl-emitting source probably does not fill the
. If the HCl result is re-interpreted for a fixed excitation temperature of 102 K, the resulting column density of HCl would be 8.9 × 1014 cm−2, and if the ratio of column densities is simply scaled by the ratio of beam areas, then the abundance ratio would be [CH3Cl] / [HCl] = 7 × 10−4. This is likely to be an underestimate, because the HCl-emitting source probably does not fill the 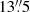 beam. Even so, the [CH3Cl] / [HCl] ratio in IRAS 16293 − 2422 is not inconsistent with the value (4 ± 2)×10−3 determined from the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis (ROSINA) measurements of the gaseous coma of comet 67P/Churyumov–Gerasimenko (Fayolle et al. 2017). This [CH3Cl] / [HCl] ratio is consistent with our upper limit on CH3Cl in MA0.89 SW.
beam. Even so, the [CH3Cl] / [HCl] ratio in IRAS 16293 − 2422 is not inconsistent with the value (4 ± 2)×10−3 determined from the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis (ROSINA) measurements of the gaseous coma of comet 67P/Churyumov–Gerasimenko (Fayolle et al. 2017). This [CH3Cl] / [HCl] ratio is consistent with our upper limit on CH3Cl in MA0.89 SW.
4.2. 35Cl/37Cl isotopic ratio
The 35Cl/37Cl isotopic ratio has been studied in a range of Galactic sources ranging from circumstellar envelopes to molecular clouds and star-forming regions. The measured ratios vary between ∼1 and 5, though most fall around 2.5 (Maas & Pilachowski 2018) with uncertainties large enough to be consistent with the solar system ratio of 3.13 (Lodders et al. 2009). This value is measured from meteorites, and hence is indicative of the conditions at the formation of the solar system. Galactic chemical evolution models by Kobayashi et al. (2011) predict that the chlorine isotopic ratio in the solar system neighborhood has decreased, with increasing metallicity, since its formation to about 1.8 at the present day and Solar metallicity.
From H2Cl+ absorption observed in 2012 in MA0.89 SW, Muller et al. (2014b) found a ratio 35Cl/37Cl = 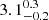 , consistent with the Solar system value. With the better quality of these new 2014 data, we revise this ratio (2.99 ± 0.05) and we are also able to measure it in MA0.89 NE (3.3 ± 0.3). These two measurements, at galactocentric radii of 2 and 4 kpc, respectively, are consistent each other, suggesting there are only small effects from metallicity or stellar population differences in the disk of MA0.89. In addition, we obtain another measurement from the HCl isotopologs in MA0.89 SW, 3.28 ± 0.08, relatively consistent with previous values and suggesting a good mixing between the different gas components with low and high fH2.
, consistent with the Solar system value. With the better quality of these new 2014 data, we revise this ratio (2.99 ± 0.05) and we are also able to measure it in MA0.89 NE (3.3 ± 0.3). These two measurements, at galactocentric radii of 2 and 4 kpc, respectively, are consistent each other, suggesting there are only small effects from metallicity or stellar population differences in the disk of MA0.89. In addition, we obtain another measurement from the HCl isotopologs in MA0.89 SW, 3.28 ± 0.08, relatively consistent with previous values and suggesting a good mixing between the different gas components with low and high fH2.
In MA0.68, we find a 35Cl/37Cl ratio of 2.2 ± 0.3, from H2Cl+ isotopologs. Wallström et al. (2016) found overall very similar C, N, O, and S isotopic ratios between MA0.68 and MA0.89 SW, which show clear evolution effects compared to the ratios found in the Solar neighborhood. The 35Cl/37Cl ratio is the first to deviate between MA0.89 SW and MA0.68, and it would be interesting to confirm this difference with further observations.
A 35Cl/37Cl ratio around three reflects nucleosynthesis products mainly from massive stars and core-collapse supernovae (Kobayashi et al. 2011). On the other hand, a 35Cl/37Cl ratio of ∼2 requires nucleosynthesis by either low-metallicity AGB stars or higher-metallicity Type II supernovae. As AGB stars affect their environment on long timescales, the first possibility requires MA0.68 to be a relatively old galaxy. For the second possibility, higher-metallicity Type II supernovae require short timescales and an intrinsically more metal-rich galaxy. The simplest explanation of the difference in chlorine isotopic ratios between MA0.89 and MA0.68 might be that MA0.68 has an intrinsically higher metallicity.
5. Summary and conclusions
We investigate the absorption of chlorine-bearing molecules in two molecular absorbers (three independent lines of sight with different properties) at intermediate redshift, MA0.89 located toward PKS 1830−211 (zabs = 0.89) and MA0.68 towards B 0218+357 (zabs = 0.68). H2Cl+ was observed, and detected, toward all sightlines. HCl was observed only toward PKS 1830−211, but detected only in one of the two sightlines.
Our results and conclusions are summarized as follows:
-
The comparison of the absorption spectra of chlorine-bearing species between the different sightlines and with that of other species, namely H2O+ and H2S, provides us with a simple classification based on molecular fraction (fH2). H2Cl+ and HCl trace the gas component with low and high fH2 gas, respectively. This picture is consistent with predictions from chemical modeling, where HCl requires higher visual extinction (AV ∼ 1) than H2Cl+ to form.
-
These two chlorine-bearing species can hence be used together to characterize the column of absorbing gas. In particular, toward MA0.89 SW, we find that the [H2Cl+] / [HCl] abundance ratio varies from ∼0.5 at the line center, to ∼2 in the line wings, reflecting the multi-phase composition of the gas along the line of sight. The same ratio has a lower limit more than one order of magnitude higher toward MA0.89 NE.
-
The detection of the 35Cl- and 37Cl-isotopologs allows us to measure the 35Cl/37Cl isotopic ratio at look-back times of about half the present age of the Universe. We find basically the same values at galactocentric radii of 2 kpc (35Cl/37Cl = 2.99 ± 0.05 from H2Cl+ and 3.28 ± 0.08 from HCl) and 4 kpc (35Cl/37Cl = 3.3 ± 0.3) in the disk of MA0.89 and in the Solar neighborhood (35Cl/37Cl ∼ 3), while we find a lower ratio (35Cl/37Cl = 2.2 ± 0.3) in MA0.68. This could be interpreted as MA0.68 having an intrinsically higher metallicity. It would be interesting to obtain more measurements of the 35Cl/37Cl isotopic ratio, including at higher redshifts, to investigate whether this isotopic ratio is a useful tracer of evolution.
-
The comparison of the observed abundances for H2Cl+ and HCl with that predicted from chemical modeling suggests the need for a stronger interstellar radiation field in the disk of these absorbers than in the Solar neighborhood. Evidence for a slightly increased cosmic-ray ionization rate of atomic hydrogen was also found in MA0.89 (Muller et al. 2016a), potentially related to the higher star formation activity at these intermediate redshifts.
In short, the two chlorine-bearing species H2Cl+ and HCl are sensible probes of the ISM in redshifted absorbers. They offer diagnostics of the molecular fraction, gas composition, and nucleosynthesis enrichment via the 35Cl/37Cl ratio. Their ground-state transitions are readily observable, for instance with ALMA, in a wide range of redshifts (z ∼ 0 − 6), making them interesting tools to probe physico-chemical properties and evolution effects in the young Universe.
From an independent least-squares fit of the data from Moomey et al. (2012) and Balashev et al. (2015).
Acknowledgments
This paper uses the following ALMA data: ADS/JAO.ALMA#2012.1.00056.S ADS/JAO.ALMA#2013.1.00020.S ADS/JAO.ALMA#2016.1.00031.S. ALMA is a partnership of ESO (representing its member states), NSF (USA) and NINS (Japan), together with NRC (Canada) and NSC and ASIAA (Taiwan) and KASI (Republic of Korea), in cooperation with the Republic of Chile. The Joint ALMA Observatory is operated by ESO, AUI/NRAO and NAOJ. S.H.J.W. acknowledges support by the Ministry of Science and Technology of Taiwan under grants MOST104-2628-M-001-004-MY3 and MOST107-2119-M-001-031-MY3, and from Academia Sinica under AS-IA-106-M03.
References
- Acharyya, K., & Herbst, E. 2017, ApJ, 850, 105 [NASA ADS] [CrossRef] [Google Scholar]
- Araki, M., Furuya, T., & Saito, S. 2001, J. Mol. Spectrosc., 210, 132 [NASA ADS] [CrossRef] [Google Scholar]
- Asplund, M., Grevesse, N., Sauval, A. J., & Scott, P. 2009, ARA&A, 47, 481 [NASA ADS] [CrossRef] [Google Scholar]
- Balashev, S. A., Noterdaeme, P., Klimenko, V., et al. 2015, A&A, 575, L8 [Google Scholar]
- Blake, G. A., Keene, J., & Phillips, T. G. 1985, ApJ, 295, 501 [NASA ADS] [CrossRef] [Google Scholar]
- Bohlin, R. C., Savage, B. D., & Drake, J. F. 1978, ApJ, 224, 132 [NASA ADS] [CrossRef] [Google Scholar]
- Cazzoli, G., & Puzzarini, C. 2004, J. Mol. Spectrosc., 226, 161 [NASA ADS] [CrossRef] [Google Scholar]
- Chengalur, J. N., de Bruyn, A. G., & Narasimha, D. 1999, A&A, 343, L79 [NASA ADS] [Google Scholar]
- Clayton, D. 2003, Handbook of Isotopes in the Cosmos – Hydrogen to Gallium No. 1 (Cambridge: Cambridge University Press), 1 [Google Scholar]
- Combes, F. 2008, Ap&SS, 313, 321 [NASA ADS] [CrossRef] [Google Scholar]
- Combes, F., & Wiklind, T. 1997, ApJ, 486, L79 [NASA ADS] [CrossRef] [Google Scholar]
- Cristallo, S., Piersanti, L., Straniero, O., et al. 2011, ApJS, 197, 1 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- De Luca, M., Gupta, H., Neufeld, D., et al. 2012, ApJ, 751, L37 [NASA ADS] [CrossRef] [Google Scholar]
- Fayolle, E. C., Öberg, K. I., Jørgensen, J. K., et al. 2017, Nat. Astron., 1, 703 [NASA ADS] [CrossRef] [Google Scholar]
- Heays, A. N., Bosman, A. D., & van Dishoeck, E. F. 2017, A&A, 602, A105 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Jauncey, D. L., Reynolds, J. E., Tzioumis, A. K., et al. 1991, Nature, 352, 132 [NASA ADS] [CrossRef] [Google Scholar]
- Jungen, C., & Pratt, S. T. 2009, Phys. Rev. Lett., 102, 023201 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Jura, M. 1974, ApJ, 190, L33 [NASA ADS] [CrossRef] [Google Scholar]
- Kawaguchi, K., Muller, S., Black, J. H., et al. 2016, ApJ, 822, 9 [NASA ADS] [CrossRef] [Google Scholar]
- Kobayashi, C., Umeda, H., Nomoto, K., Tominaga, N., & Ohkubo, T. 2006, ApJ, 653, 1145 [NASA ADS] [CrossRef] [Google Scholar]
- Kobayashi, C., Karakas, A. I., & Umeda, H. 2011, MNRAS, 414, 3231 [NASA ADS] [CrossRef] [Google Scholar]
- Le Gal, R., Xie, C., Herbst, E., et al. 2017, A&A, 608, A96 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Lis, D., Pearson, J., Neufeld, D., et al. 2010, A&A, 521, A4 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Lodders, K., Palme, H., & Gail, H. P. 2009, Landolt Börnstein, VI/4B, 560 [Google Scholar]
- Maas, Z. G., & Pilachowski, C. A. 2018, AJ, 156, 2 [NASA ADS] [CrossRef] [Google Scholar]
- Martí-Vidal, I., Vlemmings, W. H. T., Muller, S., & Casey, S. 2014, A&A, 563, A136 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Moomey, D., Federman, S. R., & Sheffer, Y. 2012, ApJ, 744, 174 [NASA ADS] [CrossRef] [Google Scholar]
- Muller, S., & Guélin, M. 2008, A&A, 491, 739 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Müller, H. S. P., Thorwirth, S., Roth, D. A., & Winnewisser, G. 2001, A&A, 370, L49 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Guélin, M., Combes, F., & Wiklind, T. 2007, A&A, 468, 53 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Beelen, A., Guélin, M., et al. 2011, A&A, 535, A103 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Beelen, A., Black, J. H., et al. 2013, A&A, 551, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Black, J. H., Guélin, M., et al. 2014a, A&A, 566, L6 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Combes, F., Guélin, M., et al. 2014b, A&A, 566, A112 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Müller, H. S. P., Black, J. H., et al. 2016a, A&A, 595, A128 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Muller, S., Kawaguchi, K., Black, J. H., & Amano, T. 2016b, A&A, 589, A5 [EDP Sciences] [Google Scholar]
- Muller, S., Müller, H. S. P., Black, J. H., et al. 2017, A&A, 606, A109 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Neufeld, D. A., & Wolfire, M. G. 2009, ApJ, 706, 1594 [NASA ADS] [CrossRef] [Google Scholar]
- Neufeld, D. A. D., & Wolfire, M. G. M. 2016, ApJ, 826, 183 [NASA ADS] [CrossRef] [Google Scholar]
- Neufeld, D. A., Goicoechea, J. R., Sonnentrucker, P., et al. 2010, A&A, 521, L10 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Neufeld, D. A., Roueff, E., Snell, R. L., et al. 2012, ApJ, 748, 37 [NASA ADS] [CrossRef] [Google Scholar]
- Novotný, O., Buhr, H., Geppert, W., et al. 2018, ApJ, 862, 166 [NASA ADS] [CrossRef] [Google Scholar]
- Peng, R., Yoshida, H., Chamberlin, R. A., et al. 2010, ApJ, 723, 218 [NASA ADS] [CrossRef] [Google Scholar]
- Sonnentrucker, P. 2006, ApJ, 4, 115 [NASA ADS] [CrossRef] [Google Scholar]
- Sonnentrucker, P., Neufeld, D. A., Phillips, T. G., et al. 2010, A&A, 521, L12 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wallström, S., Muller, S., & Guélin, M. 2016, A&A, 595, A96 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Wiens, J. P., Miller, T. M., Shuman, N. S., & Viggiano, A. A. 2016, J. Chem. Phys., 145, 244312 [NASA ADS] [CrossRef] [Google Scholar]
- Wiklind, T., & Combes, F. 1995, A&A, 299, 382 [NASA ADS] [Google Scholar]
- Wiklind, T., & Combes, F. 1996, Nature, 379, 139 [Google Scholar]
- Wiklind, T., Combes, F., & Kanekar, N. 2018, ApJ, 864, 73 [NASA ADS] [CrossRef] [Google Scholar]
Appendix A: Spectroscopic data
Spectroscopic data for H2Cl+ and HCl.
Appendix B: Considerations on the chemical network of chlorine-bearing species
Neufeld & Wolfire (2009) have investigated the chlorine chemistry in different photodissociation region models where the thermal balance and chemical equilibrium are solved simultaneously for different densities, illumination conditions and visual magnitudes, and for two different values of the cosmic ionization rate: 1.8 × 10−17 s−1 and 1.8 × 10−16 s−1. That study allows for the derivation of different chlorine-chemistry regimes which affect the [H2Cl+] / [HCl] ratio, where a large value is obtained for high radiation field and rather low density conditions.
However, the dissociative recombination (DR) of H2Cl+ induces a major uncertainty in the chlorine chemical network, as discussed by Kawaguchi et al. (2016), which severely impacts the abundances of H2Cl+ and HCl, and their ratio, as shown below. Three exothermic channels are available in the reaction:
The values of the different reaction rate coefficients used in the Neufeld & Wolfire (2009) studies were unpublished results where the branching ratio towards HCl is taken as 10% and the total DR rate coefficient is taken as 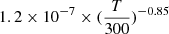 cm3 s−1. That value was challenged by Kawaguchi et al. (2016) who report a total DR rate coefficient of (0.46 ± 0.05)×10−7 cm3 s−1 at 209 K from infrared time-resolved spectroscopy experiments. Last, but not least, Novotný et al. (2018) studied the dissociative recombination of the deuterated counterpart D2Cl+ in an ion storage ring experiment and obtained a total DR rate coefficient of
cm3 s−1. That value was challenged by Kawaguchi et al. (2016) who report a total DR rate coefficient of (0.46 ± 0.05)×10−7 cm3 s−1 at 209 K from infrared time-resolved spectroscopy experiments. Last, but not least, Novotný et al. (2018) studied the dissociative recombination of the deuterated counterpart D2Cl+ in an ion storage ring experiment and obtained a total DR rate coefficient of 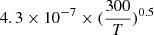 cm3 s−1. The derived coefficient is claimed to be most reliable at low temperatures relevant for astrochemical applications, in contrast to other experiments performed at room temperature. In addition, the branching ratio towards DCl was found to be 8.7%. The dissociative recombination rate of H2Cl+ is expected to be larger according to previous experiments on other hydrogenated or deuterated molecular ions, which is also anticipated from theoretical considerations (Jungen & Pratt 2009). Indeed, Wiens et al. (2016) obtain a DR rate coefficient of (1.1 ± 0.3) × 10−7 cm3 s−1 for D2Cl+ and (2.6 ± 0.8) × 10−7 cm3 s−1 for H2Cl+ in an afterglow measurement at 300 K. Novotný et al. (2018) emphasize that their measurement of D2Cl+ DR at 300 K is within the error bar of the storage ring experimental result but question the corresponding temperature extrapolation at low temperature. Summing up all these facts, we assume that the DR rate coefficient of H2Cl+ can be evaluated from the D2Cl+ measurement of Novotný et al. (2018), assuming the same [D2Cl+] / [H2Cl+] ratio than that obtained by Wiens et al. (2016) at 300 K. The corresponding values are reported in Table B.1.
cm3 s−1. The derived coefficient is claimed to be most reliable at low temperatures relevant for astrochemical applications, in contrast to other experiments performed at room temperature. In addition, the branching ratio towards DCl was found to be 8.7%. The dissociative recombination rate of H2Cl+ is expected to be larger according to previous experiments on other hydrogenated or deuterated molecular ions, which is also anticipated from theoretical considerations (Jungen & Pratt 2009). Indeed, Wiens et al. (2016) obtain a DR rate coefficient of (1.1 ± 0.3) × 10−7 cm3 s−1 for D2Cl+ and (2.6 ± 0.8) × 10−7 cm3 s−1 for H2Cl+ in an afterglow measurement at 300 K. Novotný et al. (2018) emphasize that their measurement of D2Cl+ DR at 300 K is within the error bar of the storage ring experimental result but question the corresponding temperature extrapolation at low temperature. Summing up all these facts, we assume that the DR rate coefficient of H2Cl+ can be evaluated from the D2Cl+ measurement of Novotný et al. (2018), assuming the same [D2Cl+] / [H2Cl+] ratio than that obtained by Wiens et al. (2016) at 300 K. The corresponding values are reported in Table B.1.
α factor corresponding to dissociative recombination rate of H2Cl+ expressed as 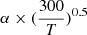 cm3 s−1.
cm3 s−1.
These values correspond to a total destruction channel of H2Cl+ by electrons which is about seven times more efficient than that taken in Neufeld & Wolfire (2009) at 300 K whereas the branching ratio to HCl is similar. The ratio between the two estimates reduces to a factor of four at 50 K and a factor of two at 10 K, due to the different slopes assumed for the energy dependence of the DR cross sections. Given these uncertainties, a detailed chemical modeling appears superfluous.
All Tables
Abundance ratios and derived parameters for diffuse gas in MA0.89 SW and MA0.89 NE.
α factor corresponding to dissociative recombination rate of H2Cl+ expressed as 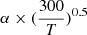 cm3 s−1.
cm3 s−1.
All Figures
 |
Fig. 1. Absorption spectra of chlorine-bearing species observed toward MA0.89 SW (top three panels), MA0.89 NE (middle three panels), and MA0.68 (bottom panel). Hyperfine structure is indicated in green for 35Cl-isotopologs of each line toward MA0.89 SW. Hyperfine structure was deconvolved by fitting multiple Gaussian velocity components; these fits are shown in red on top of spectra, with residuals shown in blue. On the right, deconvolved opacity profiles (assuming optically thin lines with fc = 1, as explained in Sect. 3), normalized to their peak opacity, are shown, together with opacity profiles of some other species for comparison. The profile of p-H2O+ is of the strongest hyperfine component of the 110–101 transition, at rest frequency of 607 GHz. The profile of o-H2S is the 110–101 transition, at rest frequency of 168.8 GHz, which was observed simultaneously with o-H2Cl+ in the same tuning. |
| In the text | |
 |
Fig. 2. Abundance ratio of H2Cl+ (derived from ortho and para forms, shown in top and bottom panels, respectively) over HCl across absorption profile toward MA0.89 SW. We use measured ortho-to-para ratio of three to obtain total H2Cl+ column density. Abundance ratio is calculated only for channels where signal-to-noise ratio of normalized profile of both H2Cl+ and HCl is higher than five. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.



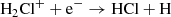
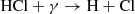
![$$ \begin{aligned} [\mathrm{H}_2\mathrm{Cl}^+]/ [\mathrm{{HCl}}] = \frac{k_2}{k_1 n_{e^-}} \end{aligned} $$](/articles/aa/full_html/2019/09/aa35860-19/aa35860-19-eq10.gif)
![$$ \begin{aligned} \frac{[\mathrm{H}_2\mathrm{Cl}^+]}{[\mathrm{{HCl}}]} \sim 1.4 \times 10^{-2} \frac{G_0}{n_{\rm {H}} x_e} \left(\frac{T}{100}\right)^{0.5}\cdot \end{aligned} $$](/articles/aa/full_html/2019/09/aa35860-19/aa35860-19-eq11.gif)
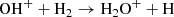
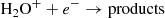
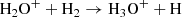
![$$ \begin{aligned} \frac{[\mathrm{OH}^+]}{[\mathrm{H}_2\mathrm{O}^{+}]}&= \frac{k_6 [e^-] + k_7 [\mathrm{H}_2]}{k_5 [\mathrm{H}_2]} = \frac{2 k_6 x_e + k_7 {f}_{\mathrm{H}_2}}{k_5 {f}_{\mathrm{H}_2}}, \nonumber \\&\quad {\sim }8.8 \times 10^2 \frac{x_e}{{f}_{\mathrm{H}_2}} \left(\frac{100}{T}\right)^{0.5} + 0.38 . \end{aligned} $$](/articles/aa/full_html/2019/09/aa35860-19/aa35860-19-eq15.gif)
![$$ \begin{aligned} \mathrm log\,[N_{Cl}] = (0.79 \pm 0.06) \times \mathrm log\,[N_{H_2}] - (2.13 \pm 1.15) . \end{aligned} $$](/articles/aa/full_html/2019/09/aa35860-19/aa35860-19-eq16.gif)