| Issue |
A&A
Volume 617, September 2018
|
|
|---|---|---|
| Article Number | A102 | |
| Number of page(s) | 8 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201732293 | |
| Published online | 25 September 2018 | |
Dissociation of methyl formate (HCOOCH3) molecules upon low-energy electron attachment
1
Université de Lyon, Université Claude Bernard Lyon1, CNRS/IN2P3, UMR5822, Institut de Physique Nucléaire de Lyon, 43 Bd du 11 Novembre 1918, 69622 Villeurbanne, France
2
Marie Curie-Sklodowska University, Institute of Physics, Mass Spectrometry Department, Pl. M. C.-Sklodowskiej 1, 20-031, Lublin, Poland
e-mail: Andrzej.Pelc@poczta.umcs.lublin.pl
3
Institut für Ionenphysik und Angewandte Physik, Leopold Franzens Universität Innsbruck, Technikerstr. 25, 6020 Innsbruck, Austria
e-mail: Stephan.Denifl@uibk.ac.at
Received:
14
November
2017
Accepted:
25
April
2018
Context. The methyl formate molecule (HCOOCH3) is considered to be a key molecule in astrochemistry. The abundance of this molecule in space depends on the stability upon irradiation with particles like low-energy electrons.
Aims. We have investigated the decomposition of the molecule upon electron capture in the electron energy range from about 0 eV up to 15 eV. All experimentally obtained fragmentation channels of the molecular anion were investigated by quantum chemical calculations.
Methods. A high resolution electron monochromator coupled with quadrupole mass spectrometer was used for the present laboratory experiment. Quantum chemical calculations of the electron affinities of the generated fragments, the thermodynamic thresholds and the activation barriers for the associated reaction channels were carried out to complement the experimental studies.
Results. Electron attachment is shown to be a purely dissociative process for this molecule and proceeds within two electron energy regions of about 1 eV to 4 eV and from 5 eV to 14 eV. In our experiment five anionic fragments with m/z (and possible stoichiometric structure) 59 (C2H3O2−), 58 (C2H2O2−), 45 (CHO2−) 31 (CH3O−), and 29 (CHO−) were detected. The most abundant anion fragments that are formed through dissociative electron attachment to methyl formate are the complementary anions CH3O− and CHO−, associated with the same single bond cleavage and different survival probability.
Conclusions. The low-energy electron induced dissociation of methyl formate differs from its isomers acetic acid and glycolaldehyde, which leads to possible chemical selectivity in the chemical evolution.
Key words: molecular processes / molecular data / astrochemistry / ISM: molecules / evolution
© ESO 2018
1. Introduction
Many simple organic compounds were identified in outer space. Methyl formate (HCOOCH3) is one of the larger molecules which have been detected in the interstellar medium (ISM) such as molecular cores or interstellar clouds (Blake et al. 1987; Cazaux et al. 2003). These regions are characterized by a molecular density of 106−108 cm−3 and temperatures in the order of 100 K. Abundances of methyl formate in the mentioned ISM regions are expected to be eight orders of magnitude smaller than that of H2 (Garrod & Herbst 2006). In many cases these molecular cores or clouds are associated with protostars which can warm the gas and dust from surrounding regions, reinjecting grain material to the gas phase and causing a variety of chemical reactions between ingredients of the ISM. Moreover, methyl formate was also discovered on comets, Hale-Bopp for example, with a relatively high abundance of 0.01–0.08 of that of water (Despois et al. 2005; Remijan & Hollis 2006).
After discovery of methyl formate in space, studies of its possible formation routes were intensified. It was shown that methyl formate may be formed by a sequence of three ion-molecule reactions. At first, the precursor molecule of methanol is protonated by 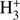 ions. The subsequent reaction of protonated methanol with formaldehyde leads to the formation of the protonated ion of [HC(OH)OCH3]+ which then undergoes dissociative recombination with an electron. In the last step of the sequence methyl formate and atomic hydrogen is formed (Horn et al. 2004; Lawson 2012). Bennet and Kaiser (Bennett & Kaiser 2007) proposed another route for formation of methyl formate from methanol on the surface of grains. In their suggested pathway, the metoxy radical (CH3O) is formed by C−H bond cleavage in methanol. CH3O may then react with the formyl radical (HCO) leading to the formation of methyl formate. The formyl radical may be formed via the reaction of the CO molecule with the H atom. A direct reaction of protonated formic acid with methanol resulting in protonated methyl formate has also been suggested (Laas et al. 2011).
ions. The subsequent reaction of protonated methanol with formaldehyde leads to the formation of the protonated ion of [HC(OH)OCH3]+ which then undergoes dissociative recombination with an electron. In the last step of the sequence methyl formate and atomic hydrogen is formed (Horn et al. 2004; Lawson 2012). Bennet and Kaiser (Bennett & Kaiser 2007) proposed another route for formation of methyl formate from methanol on the surface of grains. In their suggested pathway, the metoxy radical (CH3O) is formed by C−H bond cleavage in methanol. CH3O may then react with the formyl radical (HCO) leading to the formation of methyl formate. The formyl radical may be formed via the reaction of the CO molecule with the H atom. A direct reaction of protonated formic acid with methanol resulting in protonated methyl formate has also been suggested (Laas et al. 2011).
Methyl formate is one of three isomeric forms of C2H4O2, which have been detected in the ISM. The other two isomers are acetic acid (CH3COOH) and glycolaldehyde (HOCH2CHO). In 2001 all three isomers were identified in one space source (Bennett & Kaiser 2007; Hollis et al. 2001). The space angle distributions of these isomers in ISM indicate that they are formed in different way (Remijan et al. 2004). Moreover, measurements of their characteristic spectra have provided information on relative abundances of the isomeric forms of C2H4O2 which were estimated to about 26:0.5:1 (methyl formate:acetic acid:glycolaldehyde; Hollis et al. 2001). This is a surprising result since quantum chemical calculations and experimental data on the standard enthalpy of formation 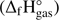 for these three compounds indicated that acetic acid has a lower
for these three compounds indicated that acetic acid has a lower  than methyl formate, with
than methyl formate, with 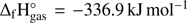 and glycolaldehyde, with
and glycolaldehyde, with 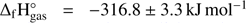 (Linstrom & Mallard 2017; Espinosa-Garcia & Dóbé 2005). This shows that the abundancies of these isomers in the ISM depend not only on thermodynamic properties of the compounds, but also on other chemical and physical reactions leading to their formation and decomposition. Additionally, if we consider conditions in the hot cores of the ISM, processes that occur in the solid phase (dust, surface) as well as in the gas phase must be taken into account.
(Linstrom & Mallard 2017; Espinosa-Garcia & Dóbé 2005). This shows that the abundancies of these isomers in the ISM depend not only on thermodynamic properties of the compounds, but also on other chemical and physical reactions leading to their formation and decomposition. Additionally, if we consider conditions in the hot cores of the ISM, processes that occur in the solid phase (dust, surface) as well as in the gas phase must be taken into account.
One possible reason for such discrepancies in the relative isomeric abundances could be the interaction of these molecules with electrons, atoms, and ions leading to the formation of positive or negative ions as well as to fragmentation of the molecules. These processes play an important role in space as many of the molecules are found in the ISM also in ionic forms (positively or negatively charged). These different charge states of the species cause drastic changes of their chemical and physical properties, for example, their reactivity, and could promote reactions leading to the generation of more complex compounds. Methyl formate contains an ester group and therefore is important in chemical evolution of molecules of organic acids, amines and other compounds. Moreover, as a small ester, HCOOCH3 is also a model compound for large biodiesel esters (Oyeyemi et al. 2014). Ions formed from methyl formate can also be reagents in the formation of larger molecules. For example, the enhanced reactivity of nucleophiles of HOO−, HO−, CH3O− and HCOO− (possible anions formed from HCOOCH3) was demonstrated (Garver et al. 2012). It is also interesting that at extremely low temperatures (like in the ISM) the considerable increase of the reactivity of HCOOCH3 with OH was observed, and resulting reaction products could be a substrate for the subsequent chemical reactions leading to the formation of other compounds (Jimenez et al. 2016). Additionally, the possible radicals, namely HCO and CH3O, generated via fragmentation of methyl formate also have important implications to the chemical evolution (Bacmann & Faure 2016). These two radicals were detected in the ISM and are believed to be precursors of many saturated complex organic molecules (Bacmann & Faure 2016; Gerin et al. 2009; Cernicharo et al. 2012).
The issues mentioned above encourage study of the interaction of atoms, photons, ions, and electrons with methyl formate in order to scrutinize pathways of ion formation via interaction of ionizing radiation with the neutral compound.
It is particularly interesting in this respect to study the interaction of HCOOCH3 with low-energy electrons. Low energy electrons in space may result from the interaction of cosmic rays with gaseous molecules as well as from inelastic interactions of cosmic rays when passing across matter (e.g., ices; Pimblott & LaVerne 2007; Arumainayagam et al. 2010). The low energy electron–molecule interaction will drive chemistry similar to UV and low-energy photon induced processes. However, electrons will induce more reactions than photons due to restrictions of selection rules resulting from spin conservation and dipole interactions.
The formation of anions upon electron attachment can be schematically presented in a hypothetical potential energy diagram of the molecule AB and its anion AB− along the bond distance between A and B, see Fig. 1. In this case, the incoming electron is trapped in the vicinity of the molecule AB forming an (excited) temporary negative ion (TNI) denoted as (AB−)*. Electron attachment is a resonant process, that is, the electron energy must match with an appropriate state of the TNI. In accordance with the Franck-Condon principle, the transition from the continuum state (AB and the electron at infinite distance) to the temporarily bound state (AB−)* is only possible within the A and B atom distances R1 and R2 (or considering the electron energy domain, between E2 and E1), see Fig. 1. Obviously, the energy of the TNI is higher than the ground state of the molecule and electron in the continuum state, so the temporary negative ion is unstable. In the situation shown in Fig. 1, the energy excess in the TNI can be released by two processes, autodetachment or dissociation (dissociative electron attachment (DEA)). The autodetachment process causes the emission of the captured electron. In the DEA process, the TNI dissociates into a thermodynamically stable anion and a neutral fragment(s) (anion B− and neutral A in case of the example shown in Fig. 1). The shape of the B− ion yield corresponds to the Gaussian profile of the vibrational wave function for the neutral ground state reflected by purely repulsive potential (the right part of the Fig. 1; Illenberger & Momigny 1992). The experimentally determined onset is denoted as appearance energy (AE) in the following discussion.
 |
Fig. 1. Schematic potential energy diagram illustrating the formation and dissociation of the TNI. In addition, the resulting ion yield as function of initial electron energy is shown on the right hand side (see text for detailed explanation). In a more advanced description of the DEA process also autodetachment from the TNI, which is possible up to the distance RA between A and B−, is taken into account. This leads to a slight shift of the peak maximum toward lower energies. |
In addition, two important quantities are marked in the Fig. 1, namely the bond dissociation energy (D(A–B)) and electron affinity (EA) of the fragment B. The thermodynamic threshold ΔEr for the potentials depicted in Fig. 1 can be thus expressed as D(A–B) – EA(B). The excess energy imparted into the formed fragments (anion and neutrals) is given by the difference between the threshold of the reaction and the energy of the electron at which the anion formed.
To examine possible reactions in space, the interaction of methyl formate with atoms, ions and photons was studied (Lawson 2012; Garver et al. 2012; Fantuzzi et al. 2011), but almost no studies on the interaction of electrons with HCOOCH3 are available. Photodissociation studies of methyl formate with soft X-rays (532.2 eV and 288.3 eV) have shown formation of several positive ions with the highest intensity of HCO+ (Fantuzzi et al. 2011). In this study, the parent ion as well as 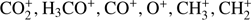 , CH+, and C+ were detected. Ions formed by the separation of atomic or molecular hydrogen from methyl formate were not observed. In mass-spectrometric studies using a magnetic sector mass spectrometer with electron impact ion source, positive ions with m/z ratio equal to 60 (parent ion), 59, 45, 44, 32, 31, 30, 29, 28, 15, and 14 were observed at the electron energy of 50 eV (Raalte & Harrison 1963). Theoretical investigations of elastic electron collisions with HCOOCH3 molecules indicated two resonances in its cross section (de Souza et al. 2016). One resonance was found at the electron energy of about 3 eV and a second broad feature is centered at around 8 eV. These calculations demonstrate that at these two energies the formation of negative ions from methyl formate should occur. In the present experimental study we investigated dissociative electron attachment (DEA) to methyl formate by mass spectrometric detection of formed anions.
, CH+, and C+ were detected. Ions formed by the separation of atomic or molecular hydrogen from methyl formate were not observed. In mass-spectrometric studies using a magnetic sector mass spectrometer with electron impact ion source, positive ions with m/z ratio equal to 60 (parent ion), 59, 45, 44, 32, 31, 30, 29, 28, 15, and 14 were observed at the electron energy of 50 eV (Raalte & Harrison 1963). Theoretical investigations of elastic electron collisions with HCOOCH3 molecules indicated two resonances in its cross section (de Souza et al. 2016). One resonance was found at the electron energy of about 3 eV and a second broad feature is centered at around 8 eV. These calculations demonstrate that at these two energies the formation of negative ions from methyl formate should occur. In the present experimental study we investigated dissociative electron attachment (DEA) to methyl formate by mass spectrometric detection of formed anions.
2. Experimental
The electron attachment spectrometer used in the present studies comprises of a molecular beam source, a high resolution hemispherical electron monochromator (HEM) and a quadrupole mass filter with a pulse counting system for analyzing and detecting the ionic products. The apparatus has been described previously in detail (Denifl et al. 2005). Briefly, as the methyl formate sample is liquid at room temperature and its vapor pressure is relatively high (530 hPa at 289 K and 100 kPa at 305 K; Press 1981), the vapor was directly introduced into the interaction chamber of the HEM by a capillary made of stainless steel and no additional heating was required. The gas flow to the interaction chamber was controlled by the pressure in the main vacuum chamber of the mass spectrometer. In the whole course of the experiment, this pressure was about 10−4 Pa, to ensure collision-free conditions. Prior to the investigations, the sample of methyl formate was purified from residual air by freezing and pumping cycles. The anions generated by the electron attachment process were extracted by a weak electrostatic field into the quadrupole mass spectrometer where they were analyzed and detected by a channeltron secondary electron multiplier. After crossing the collision region, the residual electrons were collected by a Faraday plate; the electron current was monitored during the experiments using a pico-amperemeter.
To determine the energy spread of the HEM and to calibrate the energy scale, the well-known cross section for the formation of Cl−/CCl4 was used. The formation of Cl−/CCl4 is characterized by two main resonances: at 0 eV and 0.8 eV (Matejcik et al. 1995; Klar et al. 2001). The first one can serve for calibration of the electron energy scale and to determine the electron energy spread (the apparent FWHM represents the energy resolution of the electron beam). In the present experiments the FWHM and the electron current were 160 meV and 30 nA, respectively. The used electron energy resolution represents a reasonable compromise between the product ion intensity and the energy spread to resolve resonances in the measured ion yields. The HEM was constantly heated to the temperature of 360 K in order to prevent surface charging. The methyl formate sample of 99% purity was purchased from Sigma Aldrich, Vienna, Austria.
3. Quantum chemical calculations
To complement and support the analysis and interpretation of the experimental results, we used the established high-level extrapolation scheme G4 for the calculation of (adiabatic) electron affinities and thermochemical reaction thresholds (Curtiss et al. 2007). The G4 method achieves mean absolute deviations from experiment of 0.83 kcal mol−1 for a variety of properties (such as atomization energies, or electron and proton affinities) assembled in the G3/05 (G4) test set (Curtiss et al. 2005). The reaction thresholds were obtained as the difference between all ground state free energies of the reaction products (anion and neutral fragments) and reactants (i.e., here, the parent neutral molecule). All calculations were done for room temperature (293.15 K) and the pressure of 0 Pa which resemble the experimental conditions. The computed electron affinities are summarized in Table 1 together with reported experimental values (where available; Linstrom & Mallard 2017). For the cases, where the DEA to methyl formate may have resulted in products involving rearrangement, the potential energy surface was explored to determine the activation energy for the reaction. Transition states (TS) for fragmentation pathways were optimized at the same level of theory and basis set. The frequencies of TS were calculated to confirm that the structures are transition states, that is, local maxima on the potential energy surface. Calculations of the intrinsic reaction coordinates connected the TS to reactants and products. All quantum chemical calculations were carried out using the Gaussian 09 software (Frisch et al. 2009).
Calculated (G4) and experimental electron affinities (EA) for the HCOOCH3 molecule and its neutral fragments.
4. Results and discussion
Our present study shows that electron attachment to methyl formate is a purely dissociative process with the production of five anionic fragments with m/z of 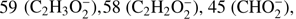 31 (CH3O−), and 29 (CHO−). Moreover, we also measured the trace ion signal for the anion with m/z = 57. Due to the rather low or medium electron affinity of possible fragments (with exception of HCOO; Table 1), the DEA channels are endothermic processes and cannot be accessed at low electron energy, where the capture cross section can be substantially higher. Therefore, the specified ion current intensities measured in this experiment are also rather low. The parent anion
31 (CH3O−), and 29 (CHO−). Moreover, we also measured the trace ion signal for the anion with m/z = 57. Due to the rather low or medium electron affinity of possible fragments (with exception of HCOO; Table 1), the DEA channels are endothermic processes and cannot be accessed at low electron energy, where the capture cross section can be substantially higher. Therefore, the specified ion current intensities measured in this experiment are also rather low. The parent anion 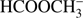 is not observable in the present experiments, where the anions are detected several μs after formation in the ion source. Such absence is in agreement with previous studies of electron attachment to relatively small organic molecules, where the accumulation of excess energy deposited by the extra electron in internal degrees of freedom of the molecule is less probable than the fragmentation of the formed transient negative ion. In addition, the electron affinity of HCOOCH3 was calculated to be negative (−1.24 eV for cis-HCOOCH3 and −1.05 eV for trans-HOOCH3, see Table 1) which additionally restricts the formation of the parent anion to short lifetimes. In previous studies of electron capture by acetic acid (CH3COOH; Sailer et al. 2003) and glycolaldehyde (HOCH2CHO; Ptasińska et al. 2005b), both isomeric forms of C2H4O2, the parent anions were also not detected. In the investigations of DEA to acetic acid, generation of nine different anions with m/z 59, 58, 46, 45, 41, 40, 17, 16, and 14 was reported (Sailer et al. 2003). Even more fragment anions with m/z 59, 58, 45, 43, 41, 31, 29, 25, 17, 16, 13, and 1 were measured in (Ptasińska et al. 2005b) for DEA to glycolaldehyde. In DEA to isomers of C2H4O2 different types of anions are generated as well as significant deviations in resonance energies and ion intensities are observed. For both, acetic acid and glycolaldehyde the OH− anion was detected. In the case of methyl formate we also measured a small signal at m/z = 17 (OH−) which was then identified as a background from the sample investigated before. All anions detected presently were also formed in DEA to glycolaldehyde (Ptasińska et al. 2005b). However, the anion efficiency curves differ significantly in shape. The anion yield curves for all negatively charged fragments observed from HCOOCH3 in the electron energy range of about 0 eV to 15 eV are presented in Fig. 2. The intensities are given in arbitrary but reproducible units, so the ion signals for all anions are comparable. It is clearly visible in Fig. 2 that the anion formation by DEA to methyl formate occurs by resonant electron capture within two broad electron energy ranges, one between about 1 eV to 4 eV, whereas the second one extends from 5 to 14 eV. This result agrees with previous calculations indicating two resonances at around 3 eV and 8 eV (de Souza et al. 2016). In the case of acetic acid, anions were formed at electron energies of about 1 eV to 4 eV, 5 eV to 6 eV (only O−) and 8 eV to 12 eV, whereas in the case of glycolaldehyde the DEA process proceeds at energies of about 0.5 eV to 3 eV and 5 eV to 12 eV (Sailer et al. 2003; Ptasińska et al. 2005b). Table 2 summarizes the present results concerning the resonance peak positions. In the following subsections we discuss the formation of all measured fragment anions in detail.
is not observable in the present experiments, where the anions are detected several μs after formation in the ion source. Such absence is in agreement with previous studies of electron attachment to relatively small organic molecules, where the accumulation of excess energy deposited by the extra electron in internal degrees of freedom of the molecule is less probable than the fragmentation of the formed transient negative ion. In addition, the electron affinity of HCOOCH3 was calculated to be negative (−1.24 eV for cis-HCOOCH3 and −1.05 eV for trans-HOOCH3, see Table 1) which additionally restricts the formation of the parent anion to short lifetimes. In previous studies of electron capture by acetic acid (CH3COOH; Sailer et al. 2003) and glycolaldehyde (HOCH2CHO; Ptasińska et al. 2005b), both isomeric forms of C2H4O2, the parent anions were also not detected. In the investigations of DEA to acetic acid, generation of nine different anions with m/z 59, 58, 46, 45, 41, 40, 17, 16, and 14 was reported (Sailer et al. 2003). Even more fragment anions with m/z 59, 58, 45, 43, 41, 31, 29, 25, 17, 16, 13, and 1 were measured in (Ptasińska et al. 2005b) for DEA to glycolaldehyde. In DEA to isomers of C2H4O2 different types of anions are generated as well as significant deviations in resonance energies and ion intensities are observed. For both, acetic acid and glycolaldehyde the OH− anion was detected. In the case of methyl formate we also measured a small signal at m/z = 17 (OH−) which was then identified as a background from the sample investigated before. All anions detected presently were also formed in DEA to glycolaldehyde (Ptasińska et al. 2005b). However, the anion efficiency curves differ significantly in shape. The anion yield curves for all negatively charged fragments observed from HCOOCH3 in the electron energy range of about 0 eV to 15 eV are presented in Fig. 2. The intensities are given in arbitrary but reproducible units, so the ion signals for all anions are comparable. It is clearly visible in Fig. 2 that the anion formation by DEA to methyl formate occurs by resonant electron capture within two broad electron energy ranges, one between about 1 eV to 4 eV, whereas the second one extends from 5 to 14 eV. This result agrees with previous calculations indicating two resonances at around 3 eV and 8 eV (de Souza et al. 2016). In the case of acetic acid, anions were formed at electron energies of about 1 eV to 4 eV, 5 eV to 6 eV (only O−) and 8 eV to 12 eV, whereas in the case of glycolaldehyde the DEA process proceeds at energies of about 0.5 eV to 3 eV and 5 eV to 12 eV (Sailer et al. 2003; Ptasińska et al. 2005b). Table 2 summarizes the present results concerning the resonance peak positions. In the following subsections we discuss the formation of all measured fragment anions in detail.
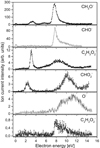 |
Fig. 2. Fragment ions from methyl formate as function of the electron energy. The O− ion yield can be ascribed to DEA to residual H2O present in the chamber. |
Peak positions for the fragment ions obtained by the dissociative electron attachment to HCOOCH3 in the present experiment.
4.1.
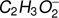 and
and 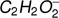
The loss of one or two hydrogen atoms (or a hydrogen molecule) from the methyl formate leads to the formation of two negative ions with the stoichiometric structure of 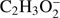 and
and 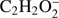 . In the case of methyl formate, these anions are the third (
. In the case of methyl formate, these anions are the third (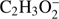 ) and the sixth (
) and the sixth (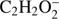 ) in the order of abundance of generated anions. Ions of this type (M−H)− or (M−2H)−, where the H atom or two H (or H2 molecule) are separated from the parent molecule, respectively, are very frequently observed in DEA processes to hydrogen bearing molecules. For example, abundant ion yield of the dehydrogenated parent anion was previously observed in DEA to organic acids and glycolaldehyde (Sailer et al. 2003; Ptasińska et al. 2005b; Pelc et al. 2002, 2004, 2005), nitriles (Tanzer et al. 2015; Pelc et al. 2016), nitrocompounds (Pelc et al. 2007) and biomolecules (Denifl et al. 2008; Vizcaino et al. 2012). As the hydrogen atom could be abstracted from two sites in the methyl formate molecule, the
) in the order of abundance of generated anions. Ions of this type (M−H)− or (M−2H)−, where the H atom or two H (or H2 molecule) are separated from the parent molecule, respectively, are very frequently observed in DEA processes to hydrogen bearing molecules. For example, abundant ion yield of the dehydrogenated parent anion was previously observed in DEA to organic acids and glycolaldehyde (Sailer et al. 2003; Ptasińska et al. 2005b; Pelc et al. 2002, 2004, 2005), nitriles (Tanzer et al. 2015; Pelc et al. 2016), nitrocompounds (Pelc et al. 2007) and biomolecules (Denifl et al. 2008; Vizcaino et al. 2012). As the hydrogen atom could be abstracted from two sites in the methyl formate molecule, the 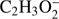 anion may be formed via two DEA channels. These channels, together with the calculated reaction energy thresholds (ΔE
r) are:
anion may be formed via two DEA channels. These channels, together with the calculated reaction energy thresholds (ΔE
r) are: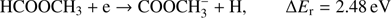 (1a)
(1a)
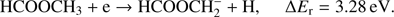 (1b)
(1b)
The present thermochemical calculations for the 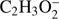 fragment anion indicate that reaction (Eq. (1a)) is accessible at electron energies above 2.48 eV, whereas the channel (Eq. (1b)) is only accessible for electron energies exceeding 3.28 eV. The EA of CHOOCH3 was calculated to be 1.41 eV and EA of HCOOCH2 0.80 eV (Table 1). The measured
fragment anion indicate that reaction (Eq. (1a)) is accessible at electron energies above 2.48 eV, whereas the channel (Eq. (1b)) is only accessible for electron energies exceeding 3.28 eV. The EA of CHOOCH3 was calculated to be 1.41 eV and EA of HCOOCH2 0.80 eV (Table 1). The measured 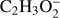 signal shows that this anion is formed within two energy regions: between 2.5 eV and 4 eV, with a resonance at about 3.1 ± 0.1 eV and a second broad energy range from 4.5 eV to 11 eV, with the main maximum of 8.5 ± 0.1 eV. Closer inspection of the ion yield indicates another weakly abundant feature at 5.8 ± 0.1 eV. Additionally, the experimental data show a monotonous increase of the ion yield at energies above 13 eV. This phenomenon may be attributed to the ion pair formation as the ionization energy of H is 13.6 eV. Since in the formation of
signal shows that this anion is formed within two energy regions: between 2.5 eV and 4 eV, with a resonance at about 3.1 ± 0.1 eV and a second broad energy range from 4.5 eV to 11 eV, with the main maximum of 8.5 ± 0.1 eV. Closer inspection of the ion yield indicates another weakly abundant feature at 5.8 ± 0.1 eV. Additionally, the experimental data show a monotonous increase of the ion yield at energies above 13 eV. This phenomenon may be attributed to the ion pair formation as the ionization energy of H is 13.6 eV. Since in the formation of 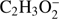 only a single hydrogen bond has to be broken, the anion has the low appearance energy (AE) of 2.7 ± 0.2 eV. The AEs derived from the experimental data for resonances at higher energies are 4.7 ± 0.2 eV and 5.3 ± 0.2 eV, respectively. Taking into account both, the experimental and theoretical data for AE and ΔEr, the first resonance peaking at 3.1 eV likely correspond to reaction (Eq. (1a)). Resonances at higher energies have higher experimental AEs than the ΔEr of both reactions (Eqs. (1a) and (1b)). Therefore both peaks at 5.8 eV and 8.5 eV may be assigned to the simple bond cleavage where the hydrogen atom may be separated also from the methyl group, that is, reaction (Eq. (1b)). The excess energy (difference between the electron energy and ΔEr) in the reaction (Eqs. (1a) and (1b)) may lead to (rovibrational) excitation of the polyatomic anion and kinetic energy of fragments resulting from DEA. Existence of three resonances for two possible reaction paths also suggest, that at higher electron energies the core excited resonance process may be involved. In such a process, the extra electron is bound to an electronically excited state of the neutral molecule. Another possibility for existence of such interfering resonances is electron attachment to different conformers of methyl formate. The HCOOCH3 has two conformation types: cis- and trans- in the C−O−C−O ester dihedral angle (Zeegers-Huyskens & Kryacho 2011). The presently computed difference in the standard enthalpy of formation of methyl formate trans- to cis- conformation transition is −0.21 eV, indicating that the cis- arrangement is more stable in agreement with the experimental value of −4.75 ± 0.19 kcal mol−1 (Blom & Guenthard 1981). For comparison, in the case of acetic acid, the (M−H)− anion exhibits two prominent, energetically overlapping features only at low electron energies. These two resonances have their maxima at 0.75 eV and 1.5 eV (Sailer et al. 2003) which are definitely lower than in the case of methyl formate. These two low energy features were assigned to two different electronic states of the transient anion representing one particle shape resonances with the extra electron occupying the first (LUMO) and second (LUMO+1) virtual orbitals. No resonance at higher energies was detected and therefore only the CH3COO− isomer was formed. The fragmentation of the methyl group of acetic acid was not considered. Formation of (M−H)− anion by DEA to glycoladehyde is possible at two energy regions ranging from about 0.5 eV to 3 eV and from 5 eV to 12 eV via a series of overlapping resonances (Ptasińska et al. 2005b). In the case of glycolaldehyde the resonant energy ranges are similar to the ones observed for electron capture by methyl formate.
only a single hydrogen bond has to be broken, the anion has the low appearance energy (AE) of 2.7 ± 0.2 eV. The AEs derived from the experimental data for resonances at higher energies are 4.7 ± 0.2 eV and 5.3 ± 0.2 eV, respectively. Taking into account both, the experimental and theoretical data for AE and ΔEr, the first resonance peaking at 3.1 eV likely correspond to reaction (Eq. (1a)). Resonances at higher energies have higher experimental AEs than the ΔEr of both reactions (Eqs. (1a) and (1b)). Therefore both peaks at 5.8 eV and 8.5 eV may be assigned to the simple bond cleavage where the hydrogen atom may be separated also from the methyl group, that is, reaction (Eq. (1b)). The excess energy (difference between the electron energy and ΔEr) in the reaction (Eqs. (1a) and (1b)) may lead to (rovibrational) excitation of the polyatomic anion and kinetic energy of fragments resulting from DEA. Existence of three resonances for two possible reaction paths also suggest, that at higher electron energies the core excited resonance process may be involved. In such a process, the extra electron is bound to an electronically excited state of the neutral molecule. Another possibility for existence of such interfering resonances is electron attachment to different conformers of methyl formate. The HCOOCH3 has two conformation types: cis- and trans- in the C−O−C−O ester dihedral angle (Zeegers-Huyskens & Kryacho 2011). The presently computed difference in the standard enthalpy of formation of methyl formate trans- to cis- conformation transition is −0.21 eV, indicating that the cis- arrangement is more stable in agreement with the experimental value of −4.75 ± 0.19 kcal mol−1 (Blom & Guenthard 1981). For comparison, in the case of acetic acid, the (M−H)− anion exhibits two prominent, energetically overlapping features only at low electron energies. These two resonances have their maxima at 0.75 eV and 1.5 eV (Sailer et al. 2003) which are definitely lower than in the case of methyl formate. These two low energy features were assigned to two different electronic states of the transient anion representing one particle shape resonances with the extra electron occupying the first (LUMO) and second (LUMO+1) virtual orbitals. No resonance at higher energies was detected and therefore only the CH3COO− isomer was formed. The fragmentation of the methyl group of acetic acid was not considered. Formation of (M−H)− anion by DEA to glycoladehyde is possible at two energy regions ranging from about 0.5 eV to 3 eV and from 5 eV to 12 eV via a series of overlapping resonances (Ptasińska et al. 2005b). In the case of glycolaldehyde the resonant energy ranges are similar to the ones observed for electron capture by methyl formate.
In the present study we were also able to detect the anion (M−2H)− formed upon DEA to 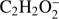 (Fig. 2). This anion is formed with the lowest intensity of about 1:350 of that for the highest one (CH3O−). The present experimental data show a broad resonance ranging from 7.5 eV to 13 eV and peaking at 7.9 ± 0.1 eV. The high energy tail of the peak may also reveal an additional resonance with the maximum at 9.3 ± 0.1 eV. The corresponding appearance energies derived from the measurements for these two resonances are 7.5 ± 0.2 eV and 8.5 ± 0.2 eV, respectively. The formation of
(Fig. 2). This anion is formed with the lowest intensity of about 1:350 of that for the highest one (CH3O−). The present experimental data show a broad resonance ranging from 7.5 eV to 13 eV and peaking at 7.9 ± 0.1 eV. The high energy tail of the peak may also reveal an additional resonance with the maximum at 9.3 ± 0.1 eV. The corresponding appearance energies derived from the measurements for these two resonances are 7.5 ± 0.2 eV and 8.5 ± 0.2 eV, respectively. The formation of 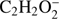 anion may be described by the following reaction, where the molecule dissociates into the anion and two separate H atoms from the methyl group:
anion may be described by the following reaction, where the molecule dissociates into the anion and two separate H atoms from the methyl group: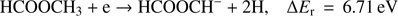 (2a)
(2a)
We also considered the case in which two H bonds are disrupted in different parts of the molecule, i.e. formation of 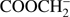 . However,
. However, 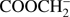 anion is unstable as its EA is negative (Table 1). The calculated threshold of reaction (Eq. (2a)) is below the experimental ones and thus, is thermodynamically accessible. The excess energy may be partitioned into the excitation and/or kinetic energies of fragments. We explored the possibility that instead of a release of two H atoms, a hydrogen molecule H2 could be formed, as it has been identified in DEA to some hydrogen bearing compounds (Ribar et al. 2016; Ptasińska et al. 2005c). For this case, the anion would be formed as:
anion is unstable as its EA is negative (Table 1). The calculated threshold of reaction (Eq. (2a)) is below the experimental ones and thus, is thermodynamically accessible. The excess energy may be partitioned into the excitation and/or kinetic energies of fragments. We explored the possibility that instead of a release of two H atoms, a hydrogen molecule H2 could be formed, as it has been identified in DEA to some hydrogen bearing compounds (Ribar et al. 2016; Ptasińska et al. 2005c). For this case, the anion would be formed as: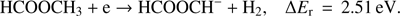 (2b)
(2b)
The formation of this H2 molecule is associated with an activation barrier. Thus, we have investigated the potential energy surface to see if this reaction (Eq. (2b)) would be energetically possible. Figure 3 shows free reaction energy for the H2 elimination in the neutral methyl formate (blue) and in the anionic methyl formate (red). It is obvious that the presence of the extra electron lowers the activation barrier by nearly 1 eV (4.18 eV for neutral and 3.25 eV for anion), which is in agreement with the positive EA of the HCOOCH fragment of 0.95 eV (Table 1). In the case of the methyl formate anion, the elimination of H2 leads to the formation of HCOOCH− anion in cis formation (ΔEr = 2.62 eV) that can lead via rotation to the more stable trans structure (ΔEr = 2.51 eV). In both cases, neutral and anion, the activation barriers are well below the lowest experimental threshold of 7.5 ± 0.2 eV and thus, the formation of H2 molecule is energetically possible, however, further experimental evidence would be needed to prove this proposition.
 |
Fig. 3. G4 schematic free-energy profile (eV) associated with the elimination of H2 molecule from neutral (blue) and anionic (red) methyl formate leading to the formation of HCOOCH fragment and HCOOCH− anion. |
4.2. CH3O− and CHO−
The CH3O− and CHO− anions were found in the ISM and their important role in the formation of complex organic molecules was postulated (Bacmann & Faure 2016). Their formation upon DEA to methyl formate may provide an additional source of their origin, considering they have the highest yields in the case of DEA to methyl formate. The abundance ratio for CH3O− and CHO− is about 3:1, indicating a DEA cross section of the same order of magnitude. We note in this context that the corresponding EAs for these anions are 1.55 eV and 0.33 eV, respectively (Table 1). They are generated by simple C−O bond cleavage leading to one anion and the neutral counterpart, as shown schematically in Fig. 1. In this case CHO and OCH3 compete for the electron. The bond dissociation energy was calculated to be 4.26 eV (G4 energy at 0 K to dissociate neutral methyl formate is 3.76 eV). In order to elucidate the competition between these two channels further, we scanned parts of the potential energy surface of methyl formate. We find that the excess charge asymptotically accumulates at the CH3O moiety upon elongation of the C−O bond length beyond about 2.1 Å, which is in line also with the significantly larger EA of CH3O (1.55 eV) compared to the EA of CHO (0.33 eV). This promotes formation of CH3O− rather than formation of CHO−. As these anions are formed by cleavage of the HCO−OCH3 bond specific for this molecule, it is not surprising that above mentioned anion species were not observed in DEA to the acetic acid, as well as to other organic acids (Sailer et al. 2003; Pelc et al. 2002, 2004). Instead both fragment anions were mentioned as DEA products in case of glycolaldehyde (Ptasińska et al. 2005b), however, the formation of CH3O− from glycolaldehyde requires bond cleavage and H transfer. We note that the formation of both anions CH3O− and CHO− in case of glycolaldehyde (Ptasińska et al. 2005b) showed resonances at different electron energies than in the case of methyl formate, and thus, the three isomers of [C2H4O2] can be distinguished based on the detection of these fragment anions at different electron energies.
The metoxy radical anion CH3O− may be formed via following reaction channels: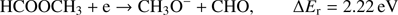 (3a)
(3a)
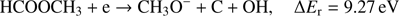 (3b)
(3b)
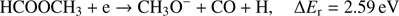 (3c)
(3c)
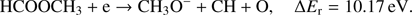 (3d)
(3d)
The calculated values of reaction energy thresholds are in two energy regimes of about 2 eV (2.22 eV and 2.59 eV for channel (Eqs. (3a) and (3c)) and additionally above 9 eV (9.27 eV and 10.17 eV for reaction (Eqs. (3b) and (3d)), respectively). This also shows that reaction route (Eq. (3a)), where only one bond is broken is energetically favored. The present ion yield reveals three main resonance features in the energy ranges of about 2 eV–4 eV, 4.5 eV–6.5 eV and 7 eV–11 eV. The corresponding peak maxima are 3.4 ± 0.1 eV, 5.6 ± 0.1 eV and 7.6 ± 0.1 eV. Moreover, the anion yield shows a weak peak at energies close to 0 eV. Considering the thermochemistry of the reaction channels (Eqs. (3a)–(3d)), this peak can be assigned as DEA to some impurity leading to the formation of an anion with the same nominal mass of CH3O− at this electron energy. The asymmetric shape and the long tail of the resonance peak ranged from about 7 eV to 11 eV suggest the existence of another weak resonance peaking at energy of 8.3 ± 0.1 eV. The estimated appearance energies of these four resonances (excluding the one close to 0 eV) are 2.5 ± 0.2 eV, 4.3 ± 0.2 eV, 7.0 ± 0.2 eV and 7.4 ± 0.2 eV, respectively. By comparison of the experimental AEs with the theoretical ΔEr, only reaction channels (Eqs. (3a) and (3c)) are accessible. The other reaction routes (Eqs. (3b) and (3d)) have their calculated thresholds about 2 eV above the highest AE measured for the CH3O− anion. The first two resonances at 3.3 eV and 5.6 eV may be thus attributed to reaction (Eqs. (3a) and (3c)), respectively. The excess energy deposited in the transient negative ion for the next two resonances may lead to: (i) electronic excitation where the energy will be used for excitation and kinetic energy of fragments, (ii) change to different conformer forms and/or (iii) structural reorganization of the CH3O− anion into another structure. Regarding the third possibility, the anion structure of 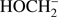 may be considered, but this fragment has negative EA (see Table 1) making its formation unfavorable.
may be considered, but this fragment has negative EA (see Table 1) making its formation unfavorable.
The complementary anion to CH3O− with respect to the breaking of C−O bond is the CHO− anion. The following reaction channels may be considered for its formation with the respective calculated reaction thresholds: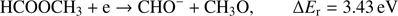 (4a)
(4a)
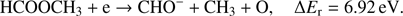 (4b)
(4b)
Similarly to the formation of CH3O− via channel (Eq. (3a)), also the complementary reaction channel (Eq. (4a)) involving single bond breaking needs the lowest amount of energy (3.43 eV) for activation. The reaction (Eq. (4b)) where two bonds need to be broken requires about the double amount of the energy compared to (Eq. (4a)). The measured CHO− ion signal shows that this ion is formed in the broad energy region from about 7 eV to 12 eV. Like in the case of the CH3O− anion, a sample impurity leads to the weak peak observed at about 0 eV. In the energy region of 7 eV–12 eV we observe one major resonance at 7.8 ± 0.1 eV. Additionally, the closer inspection of the ion yield indicates another resonance at 8.8 ± 0.1 eV, which is more pronounced than for the CH3O− channel. The appearance energy of main resonance was estimated to 7.2 ± 0.2 eV. Within the calculated thermochemical thresholds this resonance may be assigned to both reaction channels (Eqs. (4a) and (4b)) as their ΔEr = 3.43 eV and 6.92 eV, respectively. The AE of the resonance at 8.8 eV is estimated to 7.8 ± 0.2 eV, hence again also in the formation of CHO− at this electron energy both reaction (Eqs. (4a) and (4b)) are energetically open. However, further studies are required to clearly distinguish which channel is responsible for specified DEA resonance.
4.3.
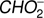 and O−
and O−
The 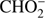 anion is formed with rather low efficiency in DEA to methyl formate. The ratio of the ion current for this anion and the most intense anion CH3O− is about 1/50. The
anion is formed with rather low efficiency in DEA to methyl formate. The ratio of the ion current for this anion and the most intense anion CH3O− is about 1/50. The 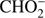 anion was also detected in previous studies of low energy electron interaction with acetic acid and glycolaldehyde as well as with other organic acids (Sailer et al. 2003; Ptasińska et al. 2005b; Pelc et al. 2002, 2004, 2005). With respect ot the three isomers (methyl formate, acetic acid, glycolaldehyde), the
anion was also detected in previous studies of low energy electron interaction with acetic acid and glycolaldehyde as well as with other organic acids (Sailer et al. 2003; Ptasińska et al. 2005b; Pelc et al. 2002, 2004, 2005). With respect ot the three isomers (methyl formate, acetic acid, glycolaldehyde), the 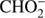 anion can be formed by a simple bond cleavage only in the case of methyl formate. Moreover, the anion
anion can be formed by a simple bond cleavage only in the case of methyl formate. Moreover, the anion 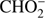 with structure of HCOO− was always one of the most effectively generated anions in DEA to simplest organic acids. We note a high positive EA of HCOO to be 3.6 eV (Table 1). In the case of organic acids, the HCOO− anion was observed at low electron energies between 1 eV and 4 eV corresponding to single bond breaking in the organic acid molecule. In the case of methyl formate the HCOO− anion may be formed most likely via following reaction channels:
with structure of HCOO− was always one of the most effectively generated anions in DEA to simplest organic acids. We note a high positive EA of HCOO to be 3.6 eV (Table 1). In the case of organic acids, the HCOO− anion was observed at low electron energies between 1 eV and 4 eV corresponding to single bond breaking in the organic acid molecule. In the case of methyl formate the HCOO− anion may be formed most likely via following reaction channels: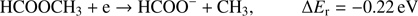 (5a)
(5a)
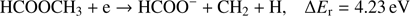 (5b)
(5b)
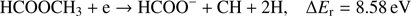 (5c)
(5c)
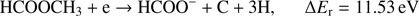 (5d)
(5d)
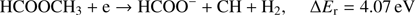 (5e)
(5e)
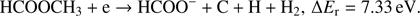 (5f)
(5f)
Reaction (Eq. (5a)) is the only exothermic DEA channel (ΔEr = −0.22 eV) in the case of methyl formate and thus possible at low electron energies. In fact, the HCOO− ion yield reveals a low energy resonance with maximum at 2.3 ± 0.1 eV (Fig. 2). The AE of this resonance is 1.6 ± 0.2 eV. Hence, we assign this resonance to the DEA process (Eq. (5a)). The HCOO− is also formed at higher electron energies from about 7 eV to 12 eV where another prominent ion yield is observed. The more detailed analysis of this ion yield reveals three different resonances with maxima at 8.2 ± 0.1 eV, 10.1 ± 0.1 eV and 10.9 ± 0.1 eV. The AEs obtained for these high energy resonances are 7.4 ± 0.2 eV, 8.2 ± 0.2 eV and 8.8 ± 0.2 eV, respectively. The anion formation at the 8.2 eV and 10.1 eV resonances may therefore correspond to the DEA processes in methyl formate, where the methyl group undergoes additional fragmentation (Eqs. (5b) and (5c)). The ion yield of HCOO− increases further at energies above about 12 eV which may be assigned to reaction (Eq. (5d)). Nevertheless, at such high energies also the ion pair formation is energetically accessible as the ionization energy for CH3 is 9.84 eV (Linstrom & Mallard 2017). Since a step-wise dissociation can be expected, one can consider the elimination of H2 molecule in the first step as shown in Fig. 3 leading to the anion HCOOCH− followed by a cleavage of a single bond to form HCOO−. The calculated activation barrier of 3.25 eV is well below the ΔEr for both reactions 5e and 5f, considering the formation of H2 molecule. However, the detection of the neutrals would be required to prove this possibility. We note that the 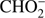 anion may be also formed in another structure, namely COOH−. This anion can arise from the reactions similar to the HCOO− case with dissociation to the same neutral fragments like in the reactions (Eqs. (5a)–(5e)). The energetically favorable channel with generation of COOH− and methyl radical is associated with calculated energetic threshold of 1.28 eV, therefore it is accessible for all measured resonances. However, the generation of the COOH− anion requires strong rearrangement of bonds in the parent molecule, and hence seems much less probable than the HCOO− formation associated with a single bond cleavage. Moreover, the values of calculated EAs for HCOO and COOH fragments (Table 1) firmly promote the formation of
anion may be also formed in another structure, namely COOH−. This anion can arise from the reactions similar to the HCOO− case with dissociation to the same neutral fragments like in the reactions (Eqs. (5a)–(5e)). The energetically favorable channel with generation of COOH− and methyl radical is associated with calculated energetic threshold of 1.28 eV, therefore it is accessible for all measured resonances. However, the generation of the COOH− anion requires strong rearrangement of bonds in the parent molecule, and hence seems much less probable than the HCOO− formation associated with a single bond cleavage. Moreover, the values of calculated EAs for HCOO and COOH fragments (Table 1) firmly promote the formation of 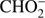 anion in the structure of HCOO− instead of COOH−.
anion in the structure of HCOO− instead of COOH−.
The atomic oxygen anion has already been detected in studies of low energy electron interactions with organic acids including acetic acid and glycolaldehyde (Sailer et al. 2003; Ptasińska et al. 2005b; Pelc et al. 2002, 2004). In the present experiment, we were able to detect signals at m/z = 16, which would correspond to formation of O− formation. The ion yield exhibits three resonances with maxima at 7.0 ± 0.1 eV, 8.9 ± 0.1 eV and 11.3 ± 0.1 eV. Peak position as well as shape of the resonance yield (three resonances with raising intensity) resembles that of O− formed upon DEA to water (Fedor et al. 2006) and hence we assign the present measured O− yield to an impurity. We note that O− was the fifth-strongest anion among twelve fragment anions reported in DEA to the glycolaldehyde monomer (Ptasińska et al. 2005b). The O− anion generation was also reported in DEA to acetic acid (Sailer et al. 2003) where O− was observed at two resonances energies of about 5.5 eV and 9.5 eV, though its abundance was second-weakest among all fragment anions reported. Calculations for the acetic acid anion indicated that O− is formed by cleavage of the C−O bond after H abstraction, while the direct cleavage of the C=O bond does not lead to formation of the O− anion (Meneses et al. 2017). These predictions on the dissociation dynamics are in line with the present results for methyl formate, where the formation of O− seems to be completely blocked by the presence of the methyl group. A similar effect in electron-induced chemistry was previously shown for single hydrogen loss in DEA to nucleobases (Ptasińska et al. 2005a) as well as in few fragmentation channels of nitroimidazoles upon DEA (Tanzer et al. 2014). However, in the latter study this effect was restricted to electron energies below 2 eV.
5. Conclusions
We studied dissociative electron attachment to methyl formate in the gas phase using electron attachment spectroscopy. Methyl formate is an important astrochemical molecule with a possible role in the formation of more complex compounds like for example amino acids. The efficiency of anion formation upon electron attachment as well as the study of decomposition processes of methyl formate anion are therefore crucial points that must be addressed regarding chemical evolution processes. Indeed, pronounced differences with respect to the decomposition of the two isomers, acetic acid and glycolaldehyde can be observed here. We detected five anionic species in the electron energy range of about 0 eV to 15 eV. The most abundant anion fragments formed through DEA to methyl formate are the complementary anions CH3O− and CHO− associated with single bond cleavage and a competition of the two fragments for the extra electron. This bond dissociation energy was calculated to be 4.26 eV. We note that the formation of CH3O− and CHO differs for the three isomers [C2H4O2] making it possible to distinguish them based on the detection of these fragment anions at different electron energies.
Additionally, we measured the 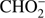 anion. These three anion species were found in the ISM and their important role in the formation of complex organic molecules is postulated. Interestingly, reaction channels which lead to formation of O− and OH− seem to be completely blocked in the case of methyl formate, demonstrating that the dissociation pathways are strongly dependent on the isomeric structure of C2H4O2.
anion. These three anion species were found in the ISM and their important role in the formation of complex organic molecules is postulated. Interestingly, reaction channels which lead to formation of O− and OH− seem to be completely blocked in the case of methyl formate, demonstrating that the dissociation pathways are strongly dependent on the isomeric structure of C2H4O2.
Acknowledgments
This work was partially supported by the FWF (P22665), the COST Action CM1401 and NCN project 2013/11/B/ST10/00250. L.F. is thankful for the support from the Labex-LIO (lyon Institute of Origins).
References
- Arumainayagam, C., Lee, H.-L., Nelson, R., Haines, D., & Gunawardane, R. 2010, Surf. Sci. Rep., 65, 1 [NASA ADS] [CrossRef] [Google Scholar]
- Bacmann, A., & Faure, A. 2016, A&A, 587, A130 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bennett, C., & Kaiser, R. 2007, ApJ, 661, 899 [NASA ADS] [CrossRef] [Google Scholar]
- Blake, G., Sutton, E., Masson, C., & Phillips, T. 1987, ApJ, 315, 621 [NASA ADS] [CrossRef] [Google Scholar]
- Blom, C., & Guenthard, H. 1981, Chem. Phys. Lett., 84, 267 [NASA ADS] [CrossRef] [Google Scholar]
- Cazaux, S., Tielens, A. G. G. M., Ceccarelli, C., et al. 2003, ApJ, 593, L51 [NASA ADS] [CrossRef] [Google Scholar]
- Cernicharo, J., Marcelino, N., Roueff, E., et al. 2012, ApJ, 759, L43 [NASA ADS] [CrossRef] [Google Scholar]
- Curtiss, L., Redfern, P., & Raghavachari, K. 2005, J. Chem. Phys., 123, 124107 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Curtiss, L., Redfern, P., & Raghavachari, K. 2007, J. Chem. Phys., 126, 084108 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Denifl, S., Ptasinska, S., Sonnweber, B., et al. 2005, J. Chem. Phys., 10, 104308 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Denifl, S., Zappa, F., Mauracher, A., et al. 2008, Chem. Phys. Chem., 9, 1387 [CrossRef] [PubMed] [Google Scholar]
- de Souza, G., da Silva, L., de Sousa, W., et al. 2016, Phys. Rev. A, 93, 032711 [NASA ADS] [CrossRef] [Google Scholar]
- Despois, D., Biver, N., Bockelée-Morvan, D., & Crovisier, J. 2005, IAU Symp., 231, 469 [NASA ADS] [Google Scholar]
- Espinosa-Garcia, J., & Dóbé, S. 2005, J. Mol. Struct. THEOCHEM, 713, 119 [CrossRef] [Google Scholar]
- Fantuzzi, F., Pilling, S., Santos, A., et al. 2011, MNRAS, 417, 2631 [NASA ADS] [CrossRef] [Google Scholar]
- Fedor, J., Cicman, P., Coupier, B., et al. 2006, Phys. B: At. Mol. Opt. Phys., 39, 3935 [NASA ADS] [CrossRef] [Google Scholar]
- Frisch, M., Trucks, G., Schlegel, H., et al. 2009, Gaussian 09 Revision C.01 (Wallingford CT: Gaussian Inc.) [Google Scholar]
- Garrod, R., & Herbst, E. 2006, A&A, 457, 927 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Garver, J., Yang, Z., Wehres, N., et al. 2012, Int. J. Mass Spectrom., 330, 182 [CrossRef] [Google Scholar]
- Gerin, M., Goicoechea, J., Pety, J., & Hily-Blant, P. 2009, A&A, 494, 977 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Hollis, J., Vogel, S., Snyder, L., Jewell, P., & Lovas, F. 2001, ApJ, 554, L81 [Google Scholar]
- Horn, A., Møllendal, H., Sekiguchi, O., et al. 2004, ApJ, 611, 605 [NASA ADS] [CrossRef] [Google Scholar]
- Illenberger, E., & Momigny, J. 1992, Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization (Springer Verlag) [Google Scholar]
- Jimenez, E., Antinolo, M., Ballesteros, B., Canosa, A., & Albaladejo, J. 2016, Phys. Chem. Chem. Phys., 18, 2183 [CrossRef] [Google Scholar]
- Klar, D., Ruf, M., & Hotop, H. 2001, Int. J. Mass Spectrom. Ion Process., 205, 93 [CrossRef] [Google Scholar]
- Laas, J., Garrod, R., Herbst, E., & Weaver, S. W. 2011, ApJ, 728, 71 [NASA ADS] [CrossRef] [Google Scholar]
- Lawson, P., Osborne, D., Jr., & Adams, N. 2012, J. Phys. Chem. A., 116, 2880 [CrossRef] [Google Scholar]
- Linstrom, P., & Mallard, W. 2017, NIST Chemistry WebBook, NISTStandard Reference Database Number 69, http://webbook.nist.gov/chemistry [Google Scholar]
- Matejcik, S., Kiendler, A., Stamatovic, A., & Märk, T. 1995, Int. J. Mass Spectrom. Ion Process., 149, 311 [NASA ADS] [CrossRef] [Google Scholar]
- Meneses, G., Widmann, C., Cunha, T., et al. 2017, Phys. Chem. Chem. Phys., 19, 1083 [CrossRef] [Google Scholar]
- Oyeyemi, V., Keith, J., & Carter, E. 2014, J. Phys. Chem. A, 118, 7392 [CrossRef] [Google Scholar]
- Pelc, A., Sailer, W., Scheier, P., Mason, N. J., & Märk, T. D. 2002, Eur. Phys. J. D, 20, 441 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Pelc, A., Sailer, W., Scheier, P., Illenberger, E., & Märk, T. D. 2004, Chem. Phys. Lett., 392, 465 [NASA ADS] [CrossRef] [Google Scholar]
- Pelc, A., Sailer, W., Scheier, P., & Märk, T. 2005, Vacuum, 78, 631 [NASA ADS] [CrossRef] [Google Scholar]
- Pelc, A., Sailer, W., Scheier, P., & Märk, T. D. 2007, Vacuum, 81, 1180 [NASA ADS] [CrossRef] [Google Scholar]
- Pelc, A., Huber, S. E., Matias, C., Czupyt, Z., & Denifl, S. 2016, J. Phys. Chem. A, 120, 903 [CrossRef] [Google Scholar]
- Pimblott, S., & LaVerne, J. 2007, Radiat. Phys. Chem., 76, 1244 [NASA ADS] [CrossRef] [Google Scholar]
- Press, C. 1981, CRC Handbook of Chemistry and Physics (Tallahassee, FL: Inc Florida) [Google Scholar]
- Ptasińska, S., Denifl, S., Scheier, P., Illenberger, E., & Märk, T. D. 2005a, Angew. Chem. Int. Ed., 44, 6941 [CrossRef] [Google Scholar]
- Ptasińska, S., Limao-Vieira, P., Denifl, S., Scheier, P., & Märk, T. 2005b, Chem. Phys. Lett., 401, 227 [NASA ADS] [CrossRef] [Google Scholar]
- Ptasińska, S., Denifl, S., Mróz, B., et al. 2005c, J. Chem. Phys., 123, 124302 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Raalte, D. V., & Harrison, A. 1963, Can. J. Chem., 41, 2054 [CrossRef] [Google Scholar]
- Remijan, A. J., & Hollis, J. 2006, ApJ, 640, 842 [NASA ADS] [CrossRef] [Google Scholar]
- Remijan, A., Shiao, Y., Friedel, D., Meier, D., & Snyder, L. 2004, ApJ, 617, 384 [NASA ADS] [CrossRef] [Google Scholar]
- Ribar, A., Fink, K., Li, Z., et al. 2016, Phys. Chem. Chem. Phys., 19, 6406 [CrossRef] [Google Scholar]
- Sailer, W., Pelc, A., Probst, M., et al. 2003, Chem. Phys. Lett., 378, 250 [NASA ADS] [CrossRef] [Google Scholar]
- Tanzer, K., Feketeova, L., Puschnigg, B., et al. 2014, Angew. Chem. Int. Ed., 53, 2 [CrossRef] [Google Scholar]
- Tanzer, K., Pelc, A., Huber, S., Czupyt, Z., & Denifl, S. 2015, J. Chem. Phys., 142, 034301 [NASA ADS] [CrossRef] [Google Scholar]
- Vizcaino, V., Puschnigg, B., Huber, S. E., et al. 2012, New. J. Phys., 14, 043017 [NASA ADS] [CrossRef] [Google Scholar]
- Zeegers-Huyskens, T., & Kryacho, E. 2011, J. Phys. Chem. A, 115, 12586 [CrossRef] [Google Scholar]
All Tables
Calculated (G4) and experimental electron affinities (EA) for the HCOOCH3 molecule and its neutral fragments.
Peak positions for the fragment ions obtained by the dissociative electron attachment to HCOOCH3 in the present experiment.
All Figures
 |
Fig. 1. Schematic potential energy diagram illustrating the formation and dissociation of the TNI. In addition, the resulting ion yield as function of initial electron energy is shown on the right hand side (see text for detailed explanation). In a more advanced description of the DEA process also autodetachment from the TNI, which is possible up to the distance RA between A and B−, is taken into account. This leads to a slight shift of the peak maximum toward lower energies. |
| In the text | |
 |
Fig. 2. Fragment ions from methyl formate as function of the electron energy. The O− ion yield can be ascribed to DEA to residual H2O present in the chamber. |
| In the text | |
 |
Fig. 3. G4 schematic free-energy profile (eV) associated with the elimination of H2 molecule from neutral (blue) and anionic (red) methyl formate leading to the formation of HCOOCH fragment and HCOOCH− anion. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


