Fig. A.1.
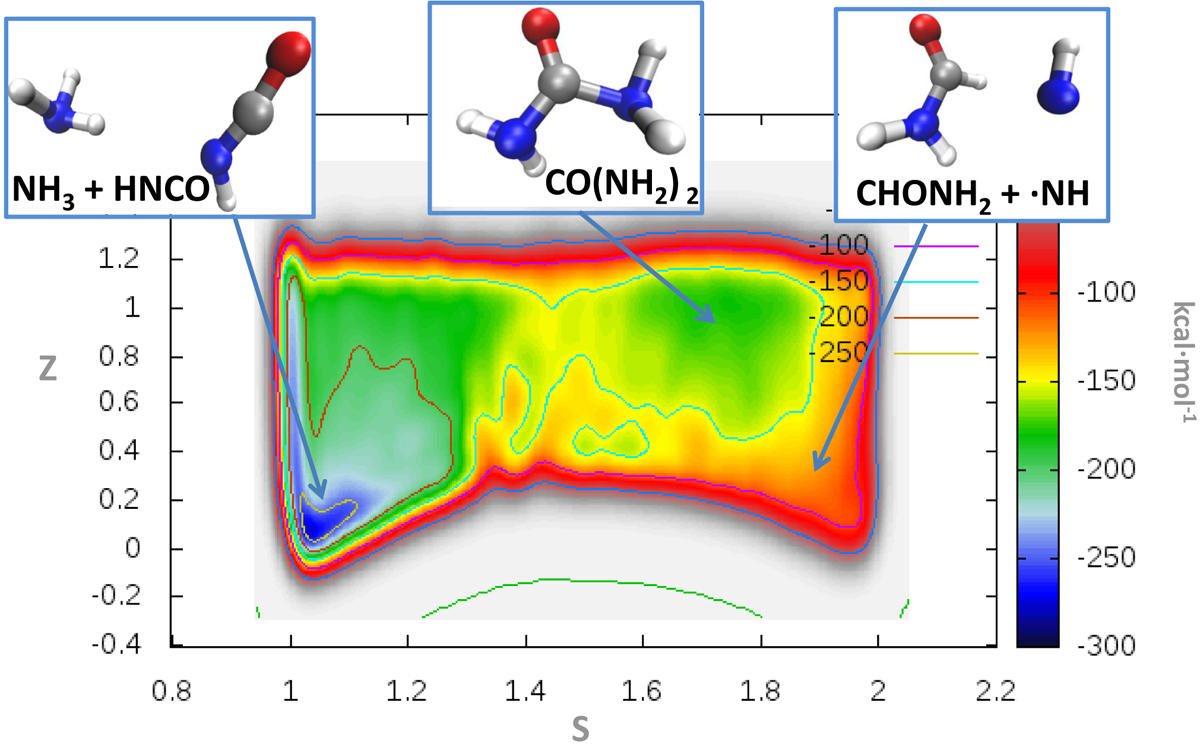
Free-energy landscape of the synthesis of formamide and •NH from isocyanic acid and ammonia at T = 63 K (i.e., reaction (2) of the main text). During the chemical conversion of the reactants into the products the system spontaneously explores a (meta)stability basin ascribed to the urea molecule (CO(NH2)2) at (S, Z) ~ (1.8, 1.0). Incidentally, this basin is more stable than that related to the expected products (i.e., formamide and •NH).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


