Fig. 6.
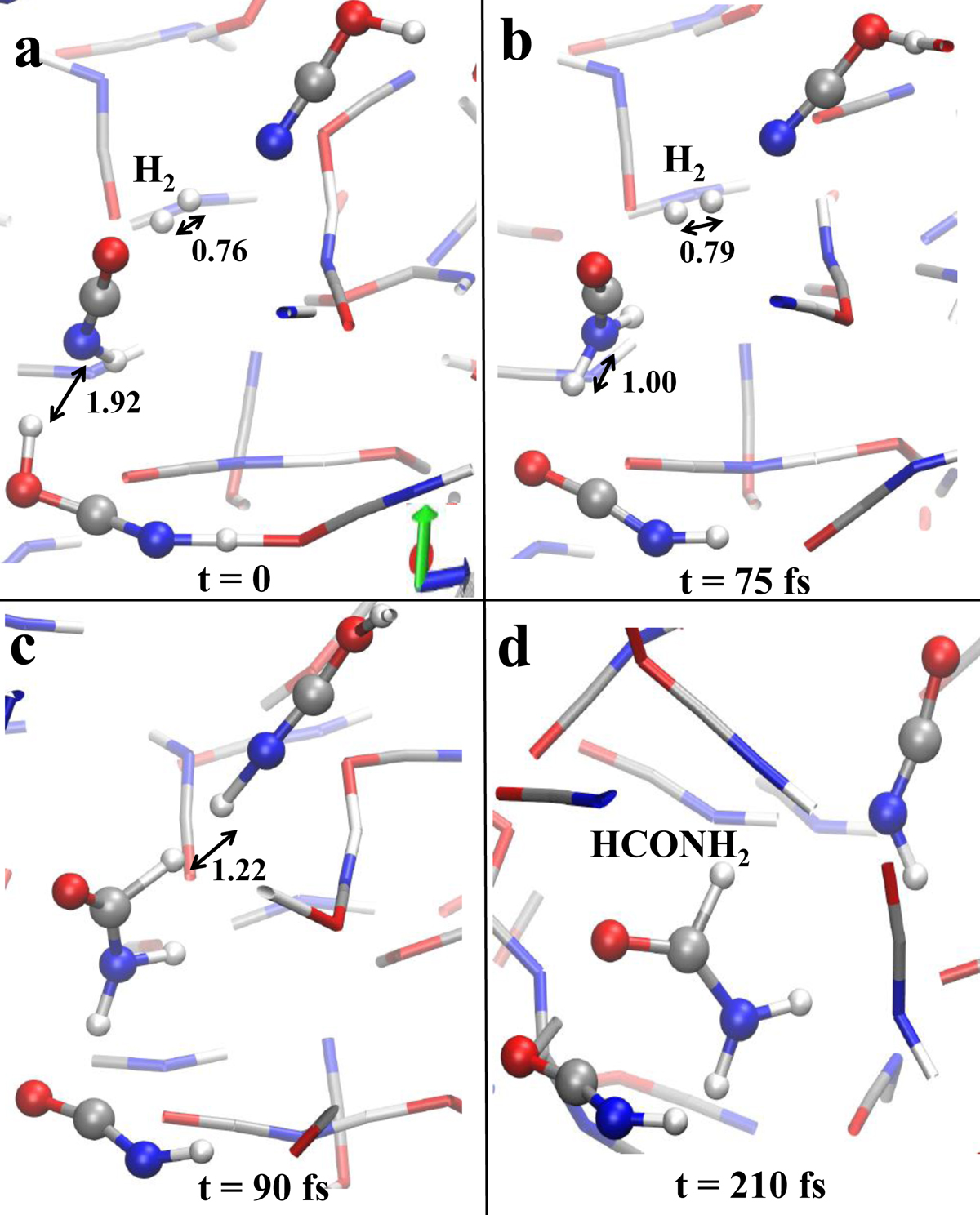
Formamide synthesis in a mixture of HNCO and H2 under the effect of an electric field strength of 0.40 V Å−1. The field direction coincides with the blue arrow at the bottom of panel a. Red, silver, blue, and white colorings refer to oxygen, carbon, nitrogen, and hydrogen atoms, respectively. Some distances (in Å) have been shown in order to better follow the overall mechanism. In panel a, a proton transfer is going to be triggered between a hydrogen cyanate cation and HNCO. This process leads to the transient formation of a H2NCO+ (panel b). Nearby to this latter species, a di-hydrogen molecule dissociates in the fs time-scale by donating one proton and two electrons to H2NCO+ and one proton to a neighbor hydrogen cyanate (panel c). This way, in a hundred of fs, a formamide molecule is formed (panel c) whereas the newly created hydrogen cyanate cation releases its excess proton in favor of the solvent (panel d). Hence, in 210 fs the system locally composed of one hydrogen cyanate cation, one isocyanic acid molecule, one di-hydrogen, and one hydrogen cyanate molecule (panel a) evolves into two isocyanic acid molecules and one molecule of formamide.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


