Fig. 1
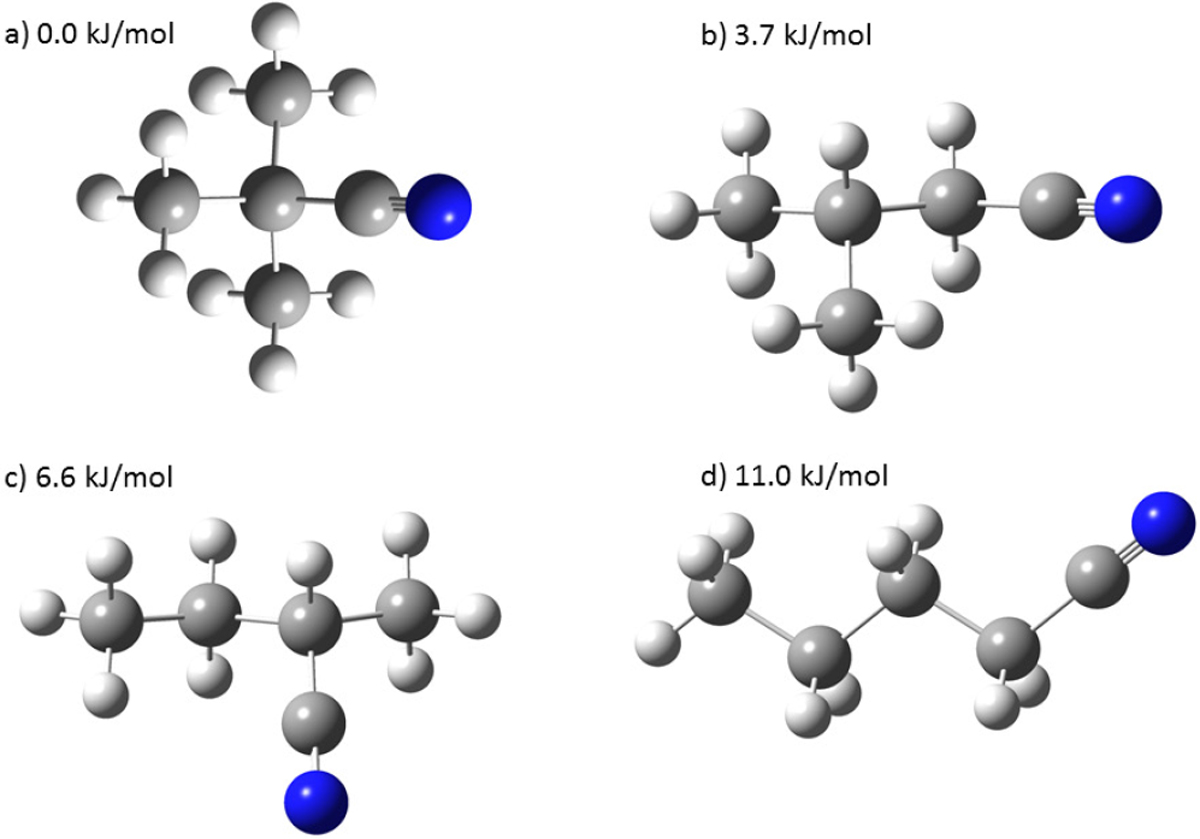
Structures and relative energies of the four different isomers of butyl cyanide. Here, the lowest energy conformers are shown. Panel a: the lowest energy isomer is tertiary butyl cyanide (0.0 kJ mol−1). Panel b: 3-methylbutyronitrile (here the anti-conformer is shown) has an energy difference of 3.7 kJ mol−1. Both conformers, the anti- and gauche-conformer, are essentially isoenergetic. Panel c: 2-cyano-anti-butane is about 6.6 kJ mol−1 higher in energy. Panel d: the highest energy isomer is the straight-chain butyl cyanide (here the anti–anti-conformer is shown) with an energy of 11.0 kJ mol−1 higher than the lowest energy isomer tertiary butyl cyanide. Blue and grey spheres indicate N and C atoms, respectively, and white spheres indicate H atoms.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


