Table 5
Detected lines of 13C-HCOOCH3vt = 1 towards Orion KL.
| Spec. | J | K a | K c | p | J ′ |
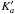
|
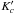
|
p ′ | Calc. freq. | E u | S ij μ 2 | Obs. freq. | v LSR |
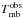
|
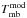
|
Blend |
| (MHz) | (K) | (D2) | (MHz) | (km s-1) | (K) | (K) | ||||||||||
|
|
||||||||||||||||
| A-13C1 | 12 | 1 | 12 | + | 11 | 1 | 11 | + | 130 348.229 | 229.4 | 32.1 | 130 349.3 | 4.6±1.2 | 0.06 | 0.03 | |
| E-13C1 | 12 | –1 | 12 | 11 | –1 | 11 | 130 424.730 | 229.5 | 32.2 | 130 424.3 | 8.1±1.2 | 0.04 | 0.03 | |||
| A-13C2 | 11 | 3 | 9 | + | 10 | 3 | 8 | + | 130 674.060 | 231.2 | 26.8 | 130 673.6 | 8.0±1.1 | 0.07 | 0.03 | CH3CH2C15N |
| E-13C2 | 11 | 5 | 6 | 10 | 5 | 5 | 131 497.371 | 340.9 | 23.2 | 131 497.9 | 5.7±1.1 | 0.03 | 0.02 | |||
| E-13C2 | 11 | –3 | 9 | 10 | –3 | 8 | 131 600.450 | 329.8 | 26.6 | 131 600.6 | 6.6±1.1 | 0.03 | 0.02 | |||
| A-13C2 | 11 | 4 | 8 | – | 10 | 4 | 7 | – | 131 626.157 | 235.9 | 25.2 | 131 626.6 | 6.0±1.1 | 0.07 | 0.02 | (CH3)2CO |
| A-13C2 | 11 | 4 | 7 | + | 10 | 4 | 6 | + | 132 401.079 | 236.0 | 25.2 | 132 400.5 | 8.3±1.1 | 0.04 | 0.02 | CH3CHDCN |
| A-13C1 | 11 | 3 | 9 | + | 10 | 3 | 8 | + | 132 807.823 | 231.8 | 27.4 | 132 806.7 | 9.6±1.1 | 0.07 | 0.03 | |
| A-13C1 | 11 | 9 | 2 | – | 10 | 9 | 1 | – | 132 945.107 | 279.3 | 9.86 | 132 946.6 | 3.7±1.1 | 0.07 | 0.02 | CCCS |
| A-13C1 | 11 | 9 | 3 | + | 10 | 9 | 2 | + | 132 945.107 | 279.3 | 9.86 | † | 3.7±1.1 | |||
| E-13C2 | 11 | 3 | 8 | 10 | 3 | 7 | 136 042.486 | 330.6 | 26.8 | 136 042.9 | 6.2±1.1 | 0.03 | 0.03 | |||
| E-13C1 | 11 | 3 | 8 | 10 | 3 | 7 | 138 640.538 | 232.8 | 27.6 | 138 640.6 | 6.9±1.1 | 0.04 | 0.03 | |||
| A-13C2 | 13 | 1 | 13 | + | 12 | 1 | 12 | + | 138 830.565 | 235.5 | 34.0 | 138 829.7 | 8.9±1.1 | 0.06 | 0.04 | |
| E-13C1 | 12 | –2 | 11 | 11 | –2 | 10 | 139 241.862 | 234.5 | 31.5 | 139 239.2 | 12.6±1.1 | 0.06 | 0.03 | CH2DCH2CN | ||
| E-13C1 | 13 | –1 | 13 | 12 | –1 | 12 | 140 917.742 | 236.2 | 35.0 | 140 918.6 | 5.3±1.1 | 0.04 | 0.04 | |||
| A-13C1 | 13 | 0 | 13 | + | 12 | 0 | 12 | + | 140 937.135 | 236.2 | 34.8 | 140 937.6 | 6.1±1.1 | 0.13 | 0.04 | CH2DOH |
| E-13C1 | 13 | 0 | 13 | 12 | 0 | 12 | 141 008.471 | 236.2 | 35.0 | 141 007.8 | 8.3±1.1 | 0.09 | 0.04 | |||
| E-13C1 | 12 | 1 | 11 | 11 | 1 | 10 | 141 542.530 | 234.2 | 31.6 | 141 543.0 | 6.1±1.1 | 0.05 | 0.04 | |||
| A-13C2 | 12 | 3 | 10 | + | 11 | 3 | 9 | + | 142 240.894 | 238.0 | 29.5 | 142 240.3 | 8.2±1.1 | 0.04 | 0.03 | |
| E-13C2 | 12 | 4 | 8 | 11 | 4 | 7 | 144 894.428 | 341.8 | 28.2 | 144 895.6 | 4.6±1.0 | 0.06 | 0.03 | |||
| E-13C2 | 12 | –4 | 9 | 11 | –4 | 8 | 144 984.322 | 341.4 | 28.2 | 144 983.7 | 8.4±1.0 | 0.05 | 0.03 | |||
| E-13C1 | 12 | 7 | 5 | 11 | 7 | 4 | 145 262.992 | 266.0 | 21.5 | 145 262.9 | 7.1±1.0 | 0.07 | 0.02 | U | ||
| A-13C1 | 12 | 7 | 6 | + | 11 | 7 | 5 | + | 145 282.393 | 265.1 | 21.5 | 145 282.9 | 5.9±1.0 | 0.07 | 0.04 | |
| A-13C1 | 12 | 7 | 5 | – | 11 | 7 | 4 | – | 145 282.519 | 265.1 | 21.5 | † | 6.2±1.0 | |||
| E-13C1 | 12 | 5 | 7 | 11 | 5 | 6 | 146 170.669 | 250.0 | 27.0 | 146 170.3 | 7.7±1.0 | 0.15 | 0.03 | H13COOCH3 | ||
| E-13C1 | 12 | 4 | 8 | 11 | 4 | 7 | 147 500.782 | 244.1 | 28.9 | 147 501.6 | 5.4±1.0 | 0.15 | 0.07 | |||
| E-13C1 | 12 | –4 | 9 | 11 | –4 | 8 | 147 501.246 | 243.7 | 28.7 | † | 6.3±1.0 | |||||
| A-13C1 | 13 | 2 | 12 | – | 12 | 2 | 11 | – | 149 734.053 | 241.5 | 34.0 | 149 734.5 | 6.0±1.0 | 0.05 | 0.04 | |
| A-13C1 | 12 | 2 | 10 | + | 11 | 2 | 9 | + | 150 951.028 | 237.3 | 31.4 | 150 950.6 | 7.9±1.0 | 0.04 | 0.04 | |
| E-13C1 | 14 | 0 | 14 | 13 | –1 | 13 | 151 328.687 | 243.5 | 6.07 | 151 331.6 | 1.3±1.0 | 0.11 | 0.05 | U | ||
| A-13C1 | 14 | 1 | 14 | + | 13 | 1 | 13 | + | 151 329.124 | 243.4 | 37.5 | † | 2.2±1.0 | |||
| E-13C1 | 14 | 0 | 14 | 13 | 0 | 13 | 151 452.158 | 243.5 | 37.7 | 151 451.6 | 8.1±1.0 | 0.04 | 0.05 | |||
| E-13C2 | 13 | –3 | 11 | 12 | –3 | 10 | 154 267.593 | 344.1 | 32.4 | 154 267.7 | 6.8±1.0 | 0.08 | 0.04 | CH3CHO | ||
| A-13C2 | 13 | 7 | 7 | + | 12 | 7 | 6 | + | 154 902.510 | 272.0 | 24.4 | 154 902.4 | 7.2±1.0 | 0.24 | 0.05 | SO2ν2 = 1 |
| A-13C2 | 13 | 7 | 6 | – | 12 | 7 | 5 | – | 154 902.822 | 272.0 | 24.4 | † | 7.8±1.0 | |||
| A-13C1 | 13 | 3 | 11 | + | 12 | 3 | 10 | + | 156 106.621 | 246.2 | 33.2 | 156 107.9 | 4.6±1.0 | 0.15 | 0.04 | HCOOCH3νt = 1 |
| E-13C1 | 13 | –3 | 11 | 12 | –3 | 10 | 156 705.635 | 246.5 | 33.3 | 156 705.5 | 7.3±1.0 | 0.11 | 0.04 | CH3CH2C15N | ||
| E-13C1 | 13 | 10 | 3 | 12 | 10 | 2 | 156 879.965 | 307.6 | 14.4 | 156 880.5 | 6.0±1.0 | 0.05 | 0.01 | |||
| E-13C1 | 13 | 9 | 4 | 12 | 9 | 3 | 157 026.174 | 294.9 | 18.4 | 157 026.5 | 6.4±1.0 | 0.09 | 0.02 | HCOOCH3 | ||
| A-13C1 | 13 | 10 | 4 | – | 12 | 10 | 3 | – | 157 221.146 | 306.4 | 14.4 | 157 224.1 | 1.4±1.0 | 0.15 | 0.05 | U |
| A-13C1 | 13 | 10 | 3 | + | 12 | 10 | 2 | + | 157 221.146 | 306.4 | 14.4 | † | 1.4±1.0 | |||
| E-13C1 | 13 | 8 | 5 | 12 | 8 | 4 | 157 227.514 | 283.6 | 22.0 | † | 13.6±1.0 | 0.02 | ||||
| E-13C2 | 13 | –4 | 10 | 12 | –4 | 9 | 157 232.535 | 348.9 | 30.8 | 157 231.6 | 8.9±1.0 | 0.11 | 0.04 | CH3OH | ||
| A-13C2 | 13 | 4 | 9 | + | 12 | 4 | 8 | + | 158 076.398 | 250.5 | 31.1 | 158 076.5 | 6.9±0.9 | 0.10 | 0.04 | |
| A-13C1 | 13 | 5 | 9 | + | 12 | 5 | 8 | + | 158 310.862 | 257.0 | 30.0 | 158 311.6 | 5.7±0.9 | 0.14 | 0.04 | 13CH3OH |
| E-13C2 | 14 | –2 | 13 | 13 | –2 | 12 | 158 376.417 | 346.9 | 36.1 | 158 376.5 | 6.8±0.9 | 0.07 | 0.05 | |||
| E-13C1 | 13 | –6 | 8 | 12 | –6 | 7 | 158 492.862 | 264.3 | 27.8 | 158 492.8 | 7.1±0.9 | 0.13 | 0.03 | U | ||
| E-13C1 | 13 | –5 | 9 | 12 | –5 | 8 | 159 175.181 | 257.1 | 30.1 | 159 175.2 | 6.9±0.9 | 0.10 | 0.04 | CH3CH2OCOH | ||
| E-13C2 | 14 | 1 | 13 | 13 | 1 | 12 | 159 621.190 | 346.8 | 36.1 | 159 620.4 | 8.4±0.9 | 0.06 | 0.05 | CH3CH2OCOH | ||
| E-13C1 | 13 | –4 | 10 | 12 | –4 | 9 | 159 907.354 | 251.3 | 31.4 | 159 906.5 | 8.6±0.9 | 0.10 | 0.04 | U | ||
| E-13C1 | 13 | 3 | 10 | 12 | 3 | 9 | 165 795.305 | 248.1 | 33.6 | 165 796.4 | 5.1±0.9 | 0.15 | 0.05 | CH3C13CH | ||
| A-13C2 | 14 | 10 | 4 | + | 13 | 10 | 3 | + | 166 582.134 | 313.8 | 18.1 | 166 581.5 | 8.2±0.9 | 0.08 | 0.04 | |
| A-13C2 | 14 | 10 | 5 | – | 13 | 10 | 4 | – | 166 582.134 | 313.8 | 18.1 | † | 8.2±0.9 | |||
| E-13C2 | 14 | 8 | 6 | 13 | 8 | 5 | 166 627.702 | 389.3 | 25.0 | 166 627.5 | 7.4±0.9 | 0.08 | 0.03 | |||
| A-13C2 | 14 | 6 | 9 | – | 13 | 6 | 8 | – | 167 331.309 | 271.4 | 30.3 | 167 330.3 | 8.9±0.9 | 0.12 | 0.04 | U |
| A-13C2 | 14 | 6 | 8 | + | 13 | 6 | 7 | + | 167 355.684 | 271.4 | 30.3 | 167 356.5 | 5.6±0.9 | 0.10 | 0.04 | |
| E-13C1 | 14 | –3 | 12 | 13 | –3 | 11 | 168 050.461 | 254.5 | 36.1 | 168 050.1 | 7.7±0.9 | 0.07 | 0.05 | |||
| A-13C2 | 14 | 5 | 9 | – | 13 | 5 | 8 | – | 168 269.790 | 264.3 | 32.1 | 168 270.3 | 6.1±0.9 | 0.16 | 0.04 | HCOO13CH3 |
| A-13C1 | 14 | 7 | 8 | + | 13 | 7 | 7 | + | 169 803.580 | 280.8 | 28.4 | 169 802.4 | 9.1±0.9 | 0.14 | 0.06 | U |
| A-13C1 | 14 | 7 | 7 | – | 13 | 7 | 6 | – | 169 804.698 | 280.8 | 28.4 | † | 11.1±0.9 | |||
| A-13C2 | 16 | 0 | 16 | + | 15 | 0 | 15 | + | 169 839.735 | 258.4 | 41.7 | 169 839.4 | 7.6±0.9 | 0.22 | 0.06 | CH CH2CN CH2CN |
| E-13C2 | 16 | –1 | 16 | 15 | –1 | 15 | 169 881.101 | 356.9 | 42.0 | 169 880.5 | 8.1±0.9 | 0.10 | 0.06 | |||
| E-13C2 | 14 | 2 | 12 | 13 | 2 | 11 | 171 156.971 | 351.0 | 35.9 | 171 157.6 | 5.9±0.9 | 0.05 | 0.06 | |||
| E-13C2 | 18 | –2 | 17 | 17 | –2 | 16 | 200 012.238 | 382.3 | 46.5 | 200 011.0 | 8.9±0.7 | 0.11 | 0.08 | |||
| E-13C2 | 16 | 3 | 13 | 15 | 3 | 12 | 200 350.331 | 372.6 | 40.9 | 200 351.0 | 5.9±0.7 | 0.08 | 0.07 | |||
| E-13C1 | 17 | –3 | 15 | 16 | –3 | 14 | 201 246.113 | 281.9 | 44.4 | 201 244.8 | 8.9±0.7 | 0.21 | 0.08 | CH3CH2OH | ||
| A-13C1 | 16 | 4 | 12 | + | 15 | 4 | 11 | + | 202 078.548 | 278.5 | 40.7 | 202 078.1 | 7.7±0.7 | 0.33 | 0.07 | U |
| A-13C2 | 17 | 11 | 7 | + | 16 | 11 | 6 | + | 202 460.334 | 355.2 | 26.1 | 202 460.6 | 6.7±0.7 | 0.15 | 0.06 | |
| A-13C2 | 17 | 11 | 6 | – | 16 | 11 | 5 | – | 202 460.334 | 355.2 | 26.1 | † | 6.7±0.7 | |||
| E-13C1 | 16 | 4 | 12 | 15 | 4 | 11 | 202 695.684 | 279.0 | 44.4 | 202 695.6 | 7.1±0.7 | 0.25 | 0.08 | U | ||
| A-13C2 | 17 | 9 | 8 | – | 16 | 9 | 7 | – | 202 701.664 | 328.6 | 32.4 | 202 701.7 | 6.9±0.7 | 0.16 | 0.09 | |
| A-13C2 | 17 | 9 | 9 | + | 16 | 9 | 8 | + | 202 701.650 | 328.6 | 32.4 | † | 6.9±0.7 | |||
| A-13C1 | 18 | 1 | 17 | – | 17 | 1 | 16 | – | 202 854.699 | 285.1 | 47.6 | 202 854.4 | 7.5±0.7 | 0.14 | 0.08 | |
| E-13C1 | 18 | 1 | 17 | 17 | 1 | 16 | 203 103.686 | 285.3 | 47.8 | 203 099.3 | 13.5±0.7 | 0.04 | 0.09 | DCOOCH3 | ||
| A-13C2 | 17 | 4 | 14 | – | 16 | 4 | 13 | – | 203 145.664 | 285.9 | 42.2 | 203 146.8 | 5.3±0.7 | 0.25 | 0.07 | SO17O |
| HCCC15N | ||||||||||||||||
| A-13C1 | 19 | 1 | 19 | + | 18 | 1 | 18 | + | 203 678.875 | 287.3 | 51.0 | 203 679.3 | 6.4±0.7 | 0.16 | 0.09 | U |
| A-13C1 | 19 | 0 | 19 | + | 18 | 0 | 18 | + | 203 682.196 | 287.3 | 51.0 | 203 683.2 | 5.6±0.7 | 0.10 | 0.09 | |
| E-13C1 | 19 | –1 | 19 | 18 | –1 | 18 | 203 734.744 | 287.4 | 51.3 | 203 733.1 | 9.5±0.7 | 0.14 | 0.09 | U | ||
| E-13C1 | 19 | 0 | 19 | 18 | 0 | 18 | 203 737.849 | 287.4 | 51.3 | † | 14.0±0.7 | 0.09 | ||||
| A-13C1 | 17 | 2 | 15 | + | 16 | 2 | 14 | + | 203 884.346 | 281.3 | 44.4 | 203 884.2 | 7.2±0.7 | 0.23 | 0.08 | HCOOCH3νt = 1 |
|
|
||||||||||||||||
| H13COOCH3 | ||||||||||||||||
| A-13C2 | 17 | 6 | 12 | – | 16 | 6 | 11 | – | 204 024.731 | 299.0 | 39.3 | 204 024.3 | 7.6±0.7 | 0.09 | 0.06 | CH3COOCH3 |
| A-13C1 | 17 | 10 | 8 | – | 16 | 10 | 7 | – | 205 972.338 | 342.4 | 30.3 | 205 972.2 | 7.2±0.7 | 0.39 | 0.12 | H2CS |
| A-13C1 | 17 | 10 | 7 | + | 16 | 10 | 6 | + | 205 972.338 | 342.4 | 30.3 | † | 7.2±0.7 | |||
| A-13C1 | 17 | 7 | 11 | + | 16 | 7 | 10 | + | 206 865.964 | 308.8 | 38.3 | 206 867.3 | 5.1±0.7 | 0.04 | 0.06 | CH3COOCH3 |
| A-13C1 | 17 | 6 | 12 | – | 16 | 6 | 11 | – | 207 553.192 | 300.4 | 40.4 | 207 553.6 | 6.5±0.7 | 0.14 | 0.07 | U |
| A-13C2 | 19 | 2 | 18 | – | 18 | 2 | 17 | – | 210 097.941 | 293.7 | 48.9 | 210 097.2 | 8.0±0.7 | 0.18 | 0.09 | |
| E-13C2 | 19 | –2 | 18 | 18 | –2 | 17 | 210 345.827 | 392.4 | 49.2 | 210 346.0 | 6.8±0.7 | 0.18 | 0.09 | |||
| A-13C2 | 20 | 0 | 20 | + | 19 | 1 | 19 | + | 211 082.205 | 296.0 | 8.83 | 211 087.2 | 0.0±0.7 | 0.19 | 0.10 | |
| A-13C2 | 20 | 1 | 20 | + | 19 | 1 | 19 | + | 211 085.095 | 296.0 | 52.3 | † | 4.1±0.7 | |||
| A-13C2 | 20 | 0 | 20 | + | 19 | 0 | 19 | + | 211 087.344 | 296.0 | 52.3 | † | 7.3±0.7 | |||
| A-13C2 | 20 | 1 | 20 | + | 19 | 0 | 19 | + | 211 090.234 | 296.0 | 8.83 | † | 11.4±0.7 | |||
| E-13C2 | 20 | 0 | 20 | 19 | –1 | 19 | 211 134.083 | 394.5 | 8.60 | 211 137.3 | 2.4±0.7 | 0.24 | 0.09 | NH2CHO | ||
| E-13C2 | 20 | –1 | 20 | 19 | –1 | 19 | 211 136.716 | 394.5 | 52.6 | † | 6.1±0.7 | |||||
| A-13C2 | 17 | 3 | 14 | – | 16 | 3 | 13 | – | 211 372.277 | 284.0 | 43.3 | 211 376.0 | 1.8±0.7 | 0.16 | 0.08 | CH2CH13CN/U |
| E-13C2 | 18 | 2 | 16 | 17 | 2 | 15 | 211 377.266 | 388.8 | 46.0 | † | 8.8±0.7 | 0.08 | ||||
| A-13C1 | 19 | 1 | 18 | – | 18 | 1 | 17 | – | 213 261.995 | 295.3 | 50.2 | 213 262.2 | 6.7±0.7 | 0.13 | 0.09 | |
| A-13C1 | 18 | 2 | 16 | + | 17 | 2 | 15 | + | 213 882.009 | 291.5 | 47.0 | 213 884.7 | 3.2±0.7 | 0.23 | 0.09 | 13CH3CH2CN |
| CH2CHCN ν11 = 2 | ||||||||||||||||
| E-13C1 | 20 | 0 | 20 | 19 | –1 | 19 | 214 193.599 | 297.6 | 9.04 | 214 197.2 | 1.9±0.7 | 0.34 | 0.13 | |||
| E-13C1 | 20 | –1 | 20 | 19 | –1 | 19 | 214 195.705 | 297.6 | 54.0 | † | 4.9±0.7 | |||||
| E-13C1 | 20 | 0 | 20 | 19 | 0 | 19 | 214 197.429 | 297.6 | 54.0 | † | 7.3±0.7 | |||||
| E-13C1 | 20 | –1 | 20 | 19 | 0 | 19 | 214 199.535 | 297.6 | 9.04 | † | 10.2±0.7 | |||||
| A-13C2 | 18 | 10 | 9 | – | 17 | 10 | 8 | – | 214 572.059 | 351.5 | 32.9 | 214 572.2 | 6.8±0.7 | 0.27 | 0.09 | 13C17O |
| A-13C2 | 18 | 10 | 8 | + | 17 | 10 | 7 | + | 214 572.060 | 351.5 | 32.9 | † | 6.8±0.7 | |||
| E-13C1 | 17 | 3 | 14 | 16 | 3 | 13 | 215 322.039 | 286.0 | 44.6 | 215 324.7 | 3.3±0.7 | 0.11 | 0.09 | U | ||
| E-13C2 | 18 | –4 | 15 | 17 | –4 | 14 | 215 326.475 | 395.1 | 45.2 | † | 9.5±0.7 | 0.08 | ||||
| E-13C2 | 18 | –6 | 13 | 17 | –6 | 12 | 217 440.211 | 408.2 | 42.5 | 217 441.0 | 6.0±0.7 | 0.16 | 0.07 | |||
| A-13C1 | 18 | 4 | 15 | – | 17 | 4 | 14 | – | 218 060.270 | 297.8 | 46.2 | 218 057.1 | 11.4±0.7 | 0.20 | 0.08 | c-C3H2 |
| E-13C1 | 18 | 8 | 10 | 17 | 8 | 9 | 218 648.438 | 330.1 | 39.3 | 218 647.0 | 8.9±0.7 | 0.10 | 0.06 | U | ||
| E-13C1 | 18 | –9 | 10 | 17 | –9 | 9 | 219 017.617 | 340.8 | 36.8 | 219 017.2 | 7.6±0.7 | 0.10 | 0.06 | |||
| A-13C2 | 19 | 3 | 17 | + | 18 | 3 | 16 | + | 219 092.328 | 300.8 | 48.4 | 219 092.2 | 7.2±0.7 | 0.13 | 0.09 | |
| E-13C2 | 18 | 5 | 13 | 17 | 5 | 12 | 219 883.920 | 401.8 | 43.3 | 219 885.9 | 4.3±0.7 | 0.13 | 0.08 | CH2CHCN ν11 = 2 | ||
| U | ||||||||||||||||
| E-13C1 | 19 | –3 | 17 | 18 | –3 | 16 | 222 753.709 | 302.8 | 49.8 | 222 753.4 | 7.5±0.7 | 0.09 | 0.09 | U | ||
| E-13C1 | 18 | 5 | 13 | 17 | 5 | 12 | 224 161.641 | 305.0 | 44.4 | 224 160.9 | 7.9±0.7 | 0.14 | 0.08 | CH3COOCH3 | ||
| E-13C2 | 18 | 4 | 14 | 17 | 4 | 13 | 225 917.084 | 397.1 | 45.7 | 225 917.1 | 7.0±0.7 | 0.14 | 0.09 | |||
| A-13C2 | 19 | 10 | 10 | – | 18 | 10 | 9 | – | 226 614.150 | 362.4 | 36.4 | 226 614.6 | 6.4±0.7 | 0.42 | 0.10 | CH2CHCN ν15 = 1 |
| A-13C2 | 19 | 10 | 9 | + | 18 | 10 | 8 | + | 226 614.152 | 362.4 | 36.4 | † | 6.4±0.7 | |||
| E-13C2 | 19 | –9 | 11 | 18 | –9 | 10 | 227 459.769 | 448.7 | 39.1 | 227 459.6 | 7.3±0.7 | 0.18 | 0.06 | |||
| A-13C1 | 18 | 4 | 14 | + | 17 | 4 | 13 | + | 229 246.345 | 299.9 | 46.5 | 229 247.1 | 6.0±0.7 | 0.24 | 0.09 | CH3CH2CN |
| E-13C2 | 19 | 6 | 13 | 18 | 6 | 12 | 229 294.250 | 419.7 | 45.2 | 229 294.6 | 6.5±0.7 | 0.30 | 0.08 | U | ||
| E-13C2 | 19 | –6 | 14 | 18 | –6 | 13 | 229 951.441 | 419.2 | 45.2 | 229 952.1 | 6.2±0.7 | 0.11 | 0.08 | |||
| E-13C1 | 18 | 4 | 14 | 17 | 4 | 13 | 230 063.581 | 300.4 | 46.8 | 230 064.6 | 5.7±0.7 | 0.21 | 0.10 | U | ||
| A-13C1 | 19 | 14 | 6 | – | 18 | 14 | 5 | – | 230 100.629 | 427.4 | 23.6 | 230 100.8 | 6.8±0.7 | 0.15 | 0.14 | |
| A-13C1 | 19 | 14 | 5 | + | 18 | 14 | 4 | + | 230 100.629 | 427.4 | 23.6 | † | 6.8±0.7 | |||
| E-13C2 | 19 | –5 | 15 | 18 | –5 | 14 | 230 101.552 | 412.2 | 46.0 | † | 8.0±0.7 | |||||
| A-13C1 | 19 | 9 | 11 | + | 18 | 9 | 10 | + | 230 720.161 | 351.4 | 40.1 | 230 719.6 | 7.7±0.7 | 0.15 | 0.12 | |
| A-13C1 | 19 | 9 | 10 | – | 18 | 9 | 9 | – | 230 720.299 | 351.4 | 40.1 | † | 7.9±0.7 | |||
| A-13C2 | 22 | 0 | 22 | + | 21 | 1 | 21 | + | 231 708.149 | 317.7 | 9.76 | 231 709.6 | 5.2±0.6 | 0.22 | 0.21 | |
| A-13C2 | 22 | 1 | 22 | + | 21 | 1 | 21 | + | 231 709.053 | 317.7 | 57.7 | † | 6.3±0.6 | |||
| A-13C2 | 22 | 0 | 22 | + | 21 | 0 | 21 | + | 231 709.768 | 317.7 | 57.7 | † | 7.3±0.6 | |||
| A-13C2 | 22 | 1 | 22 | + | 21 | 0 | 21 | + | 231 710.673 | 317.7 | 9.76 | † | 8.4±0.6 | |||
| A-13C1 | 20 | 2 | 18 | + | 19 | 2 | 17 | + | 234 053.346 | 313.5 | 52.3 | 234 054.0 | 6.2±0.6 | 0.29 | 0.10 | |
| E-13C1 | 22 | 0 | 22 | 21 | –1 | 21 | 235 113.385 | 319.7 | 10.0 | 235 114.0 | 6.2±0.6 | 0.35 | 0.24 | |||
| E-13C1 | 22 | –1 | 22 | 21 | –1 | 21 | 235 114.014 | 319.7 | 59.4 | † | 7.0±0.6 | |||||
| E-13C1 | 22 | 0 | 22 | 21 | 0 | 21 | 235 114.538 | 319.7 | 59.4 | † | 7.7±0.6 | |||||
| E-13C1 | 22 | –1 | 22 | 21 | 0 | 21 | 235 115.167 | 319.7 | 10.0 | † | 8.5±0.6 | |||||
| E-13C2 | 20 | –8 | 13 | 19 | –8 | 12 | 240 214.055 | 449.0 | 44.6 | 240 213.9 | 7.2±0.6 | 0.24 | 0.07 | |||
| A-13C2 | 22 | 1 | 21 | – | 21 | 1 | 20 | – | 241 080.593 | 327.0 | 56.9 | 241 082.0 | 5.2±0.6 | 0.24 | 0.10 | |
| E-13C1 | 20 | –4 | 17 | 19 | –4 | 16 | 241 374.048 | 320.9 | 51.9 | 241 373.5 | 7.7±0.6 | 0.12 | 0.10 | CH3CH2OCOH | ||
| E-13C1 | 22 | –2 | 21 | 21 | –2 | 20 | 244 776.097 | 329.3 | 58.6 | 244 774.9 | 8.5±0.6 | 0.21 | 0.11 | |||
| E-13C1 | 22 | 1 | 21 | 21 | 1 | 20 | 244 800.362 | 329.3 | 58.6 | 244 799.9 | 7.5±0.6 | 0.68 | 0.21 | CH3CH CN CN
|
||
| H13COOCH3 | ||||||||||||||||
| A-13C2 | 21 | 9 | 13 | + | 20 | 9 | 12 | + | 251 115.944 | 373.3 | 45.4 | 251 115.9 | 7.1±0.6 | 0.13 | 0.13 | |
| A-13C2 | 21 | 9 | 12 | – | 20 | 9 | 11 | – | 251 116.550 | 373.3 | 45.4 | † | 7.8±0.6 | |||
| A-13C2 | 24 | 0 | 24 | + | 23 | 1 | 23 | + | 252 327.721 | 341.5 | 10.7 | 252 327.2 | 7.6±0.6 | 0.44 | 0.26 | |
| A-13C2 | 24 | 1 | 24 | + | 23 | 0 | 23 | + | 252 328.505 | 341.5 | 10.7 | † | 8.5±0.6 | |||
| A-13C2 | 24 | 1 | 24 | + | 23 | 1 | 23 | + | 252 328.001 | 341.5 | 63.0 | † | 7.9±0.6 | |||
| A-13C2 | 24 | 0 | 24 | + | 23 | 0 | 23 | + | 252 328.225 | 341.5 | 63.0 | † | 8.2±0.6 | |||
| A-13C2 | 21 | 7 | 14 | – | 20 | 7 | 13 | – | 252 689.210 | 352.5 | 49.4 | 252 689.8 | 6.3±0.6 | 0.18 | 0.08 | |
| E-13C2 | 21 | 7 | 14 | 20 | 7 | 13 | 252 703.017 | 451.8 | 49.4 | 252 704.8 | 4.8±0.6 | 0.14 | 0.08 | CH3COOCH3 | ||
| A-13C2 | 21 | 6 | 16 | – | 20 | 6 | 15 | – | 253 342.936 | 344.1 | 51.0 | 253 343.5 | 6.4±0.6 | 0.15 | 0.09 | |
| A-13C1 | 21 | 14 | 8 | – | 20 | 14 | 7 | – | 254 444.432 | 451.2 | 25.5 | 254 444.7 | 6.7±0.6 | 0.15 | 0.07 | |
| A-13C1 | 21 | 14 | 7 | + | 20 | 14 | 6 | + | 254 444.432 | 451.2 | 25.5 | † | 6.7±0.6 | |||
| A-13C1 | 21 | 14 | 7 | + | 20 | 14 | 7 | – | 254 444.432 | 451.2 | 6.22 | † | 6.7±0.6 | |||
| A-13C1 | 21 | 14 | 8 | – | 20 | 14 | 6 | + | 254 444.432 | 451.2 | 6.22 | † | 6.7±0.6 | |||
| E-13C1 | 21 | 10 | 11 | 20 | 10 | 10 | 254 626.092 | 388.9 | 44.1 | 254 625.8 | 7.4±0.6 | 0.22 | 0.06 | |||
| E-13C1 | 22 | 2 | 20 | 21 | 2 | 19 | 254 899.597 | 337.9 | 58.0 | 254 899.8 | 6.8±0.6 | 0.15 | 0.11 | |||
| A-13C1 | 21 | 9 | 13 | + | 20 | 9 | 12 | + | 255 417.206 | 375.3 | 46.5 | 255 417.1 | 7.1±0.6 | 0.07 | 0.14 | |
| A-13C1 | 11 | 5 | 7 | + | 10 | 4 | 6 | + | 255 417.848 | 242.4 | 2.44 | † | 7.9±0.6 | |||
| A-13C1 | 21 | 9 | 12 | – | 20 | 9 | 11 | – | 255 418.028 | 375.3 | 46.5 | † | 8.1±0.6 | |||
| A-13C2 | 21 | 5 | 16 | – | 20 | 5 | 15 | – | 261 016.105 | 338.3 | 52.3 | 261 016.8 | 6.2±0.6 | 0.43 | 0.10 | |
| A-13C2 | 22 | 12 | 10 | + | 21 | 12 | 9 | + | 262 404.906 | 427.7 | 40.9 | 262 404.6 | 7.3±0.6 | 0.18 | 0.10 | |
| A-13C2 | 22 | 12 | 11 | – | 21 | 12 | 10 | – | 262 404.906 | 427.7 | 40.9 | † | 7.3±0.6 | |||
| A-13C2 | 22 | 3 | 19 | – | 21 | 3 | 18 | – | 263 026.207 | 342.3 | 56.1 | 263 025.8 | 7.5±0.6 | 0.14 | 0.10 | |
| E-13C2 | 22 | 3 | 19 | 21 | 3 | 18 | 263 484.906 | 441.3 | 56.1 | 263 484.6 | 7.4±0.6 | 0.10 | 0.11 | |||
| E-13C1 | 23 | –3 | 21 | 22 | –3 | 20 | 264 936.868 | 350.6 | 60.7 | 264 936.9 | 7.0±0.6 | 0.13 | 0.12 | |||
| E-13C1 | 7 | –7 | 1 | 6 | –6 | 1 | 264 937.868 | 236.2 | 3.22 | † | 8.1±0.6 | |||||
| A-13C1 | 24 | 2 | 23 | – | 23 | 2 | 22 | – | 265 443.450 | 354.0 | 63.8 | 265 442.1 | 8.5±0.6 | 0.11 | 0.11 | DCOOCH3 |
| A-13C2 | 22 | 6 | 17 | – | 21 | 6 | 16 | – | 265 640.606 | 356.9 | 53.7 | 265 640.8 | 6.8±0.6 | 0.14 | 0.08 | CH3COOCH3 |
| E-13C1 | 25 | –1 | 25 | 24 | 0 | 24 | 266 482.702 | 356.6 | 11.5 | 266 483.3 | 6.3±0.6 | 0.35 | 0.29 | |||
| E-13C1 | 25 | 0 | 25 | 24 | 0 | 24 | 266 482.602 | 356.6 | 67.6 | † | 6.2±0.6 | |||||
| E-13C1 | 25 | 0 | 25 | 24 | –1 | 24 | 266 482.416 | 356.6 | 11.5 | † | 6.0±0.6 | |||||
| E-13C1 | 25 | –1 | 25 | 24 | –1 | 24 | 266 482.517 | 356.6 | 67.6 | † | 6.1±0.6 | |||||
| A-13C1 | 22 | 10 | 13 | – | 21 | 10 | 12 | – | 267 355.733 | 400.6 | 47.3 | 267 355.8 | 6.9±0.6 | 0.19 | 0.13 | |
| A-13C1 | 22 | 10 | 12 | + | 21 | 10 | 11 | + | 267 355.808 | 400.6 | 47.3 | † | 7.0±0.6 | |||
| E-13C2 | 23 | –4 | 20 | 22 | –4 | 19 | 270 150.708 | 454.7 | 58.5 | 270 152.0 | 5.6±0.6 | 0.17 | 0.11 | |||
| A-13C2 | 23 | 3 | 20 | – | 22 | 3 | 19 | – | 272 723.937 | 355.4 | 58.5 | 272 723.7 | 7.3±0.6 | 0.15 | 0.10 | |
| A-13C2 | 26 | 0 | 26 | + | 25 | 0 | 25 | + | 272 941.805 | 367.2 | 68.3 | 272 941.8 | 7.0±0.5 | 0.17 | 0.27 | |
| A-13C2 | 26 | 1 | 26 | + | 25 | 0 | 25 | + | 272 941.891 | 367.2 | 11.7 | † | 7.1±0.5 | |||
| A-13C2 | 26 | 0 | 26 | + | 25 | 1 | 25 | + | 272 941.649 | 367.2 | 11.7 | † | 6.9±0.5 | |||
| A-13C2 | 26 | 1 | 26 | + | 25 | 1 | 25 | + | 272 941.735 | 367.2 | 68.3 | † | 7.0±0.5 | |||
| A-13C2 | 23 | 16 | 8 | – | 22 | 16 | 7 | – | 274 076.953 | 515.3 | 31.4 | 274 077.0 | 7.0±0.5 | 0.11 | 0.05 | |
| A-13C2 | 23 | 16 | 7 | + | 22 | 16 | 6 | + | 274 076.953 | 515.3 | 31.4 | † | 7.0±0.5 | |||
| A-13C2 | 23 | 15 | 8 | – | 22 | 15 | 7 | – | 274 124.220 | 494.7 | 35.1 | 274 125.7 | 5.4±0.5 | 0.21 | 0.06 | U |
| A-13C2 | 23 | 15 | 9 | + | 22 | 15 | 8 | + | 274 124.220 | 494.7 | 35.1 | † | 5.4±0.5 | |||
| E-13C1 | 23 | –4 | 20 | 22 | –4 | 19 | 274 172.892 | 358.8 | 60.0 | 274 173.3 | 6.6±0.5 | 0.23 | 0.11 | |||
| A-13C2 | 23 | 11 | 12 | – | 22 | 11 | 11 | – | 274 666.966 | 425.6 | 47.0 | 274 666.7 | 7.3±0.5 | 0.24 | 0.12 | |
| A-13C2 | 23 | 11 | 13 | + | 22 | 11 | 12 | + | 274 666.961 | 425.6 | 47.0 | † | 7.3±0.5 | |||
| A-13C2 | 23 | 10 | 14 | – | 22 | 10 | 13 | – | 274 998.925 | 411.7 | 49.4 | 274 998.3 | 7.7±0.5 | 0.35 | 0.13 | CH3OCH3 |
| A-13C2 | 23 | 10 | 13 | + | 22 | 10 | 12 | + | 274 999.054 | 411.7 | 49.4 | † | 7.9±0.5 | |||
| E-13C1 | 23 | 3 | 20 | 22 | 3 | 19 | 276 849.213 | 358.5 | 60.1 | 276 849.6 | 6.5±0.5 | 0.24 | 0.11 | |||
| E-13C1 | 26 | –1 | 26 | 25 | –1 | 25 | 276 936.143 | 369.9 | 70.3 | 276 934.7 | 8.5±0.5 | 0.31 | 0.29 | CH3CH2OH | ||
| E-13C1 | 26 | 0 | 26 | 25 | 0 | 25 | 276 936.189 | 369.9 | 70.3 | † | 8.6±0.5 | |||||
| E-13C1 | 26 | 0 | 26 | 25 | –1 | 25 | 276 936.089 | 369.9 | 12.0 | † | 8.5±0.5 | |||||
| E-13C1 | 26 | –1 | 26 | 25 | 0 | 25 | 276 936.244 | 369.9 | 12.0 | † | 8.7±0.5 | |||||
| E-13C2 | 24 | –4 | 21 | 23 | –4 | 20 | 280 723.536 | 468.2 | 61.1 | 280 722.7 | 7.9±0.5 | 0.22 | 0.11 | |||
Notes. Emission lines of 13C-HCOOCH3vt = 1 present in the spectral scan of Orion-KL from the IRAM 30-m radio-telescope. Column (1) indicates the species, being A-13C1: A-H13COOCH3vt = 1, E-13C1: E-H13COOCH3vt = 1, A-13C2: A-HCOO13CH3vt = 1, and E-13C2: E-HCOO13CH3vt = 1, Cols. (2)−(9) indicate the line transition, Col. (10) gives the predicted frequency obtained with the Hamiltonian parameters of this work for 13C2-MF and from Carvajal et al. (2010) for 13C1-MF, Col. (11) upper level energy, Col. (12) line strength, Col. (13) observed frequency assuming a vLSR of 7 km s-1, Col. (14) the radial velocity, Col. (15) observed main-beam temperature, Col. (16) modelled main-beam temperature, and Col. (17) blends. † Blended with previous line.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


