Table B.1
New ion-neutral chemical reaction rates and ortho-para branching ratios.
| Chemical reactionsa | α | β | γ | kb | References | NSBRc | |||||
| cm3 s-1 | cm3 s-1 | ||||||||||
|
|
|||||||||||
Formation of H |
|||||||||||
H (o) (o) |
H2(o) | → | H (o) (o) |
H | 1.40(−9) | 0.00 | 0.00 | 1.40(−9) | 1 | 2/3 | |
H (o) (o) |
H2(o) | → | H (p) (p) |
H | 7.00(−10) | 0.00 | 0.00 | 7.00(−10) | 1 | 1/3 | |
H (o) (o) |
H2(p) | → | H (o) (o) |
H | 7.00(−10) | 0.00 | 0.00 | 7.00(−10) | 1 | 1/3 | |
H (o) (o) |
H2(p) | → | H (p) (p) |
H | 1.40(−9) | 0.00 | 0.00 | 1.40(−9) | 1 | 2/3 | |
H (p) (p) |
H2(o) | → | H (o) (o) |
H | 7.00(−10) | 0.00 | 0.00 | 7.00(−10) | 1 | 1/3 | |
H (p) (p) |
H2(o) | → | H (p) (p) |
H | 1.40(−9) | 0.00 | 0.00 | 1.40(−9) | 1 | 2/3 | |
H (p) (p) |
H2(p) | → | H (p) (p) |
H | 2.10(−9) | 0.00 | 0.00 | 2.10(−9) | 1 | 1 | |
|
|
|||||||||||
| Main ortho-to-para conversion reactions of H2 | |||||||||||
H (o) (o) |
H2(o) | → | H (o) (o) |
H2(p) | 9.67(−11) | 0.00 | −0.14 | 9.81(−11) | 2 | ||
H (o) (o) |
H2(o) | → | H (p) (p) |
H2(o) | 4.00(−10) | 0.00 | −0.19 | 4.08(−10) | 2 | ||
H (o) (o) |
H2(o) | → | H (p) (p) |
H2(p) | 1.04(−10) | 0.00 | −0.14 | 1.04(−10) | 2 | ||
H (o) (o) |
H2(p) | → | H (o) (o) |
H2(o) | 8.84(−10) | 0.00 | 170 | 3.66(−17) | 2 | ||
H (o) (o) |
H2(p) | → | H (p) (p) |
H2(o) | 1.50(−9) | 0.00 | 136.2 | 1.82(−15) | 2 | ||
H (p) (p) |
H2(o) | → | H (o) (o) |
H2(o) | 8.03(−10) | 0.00 | 32.6 | 3.08(−11) | 2 | ||
H (p) (p) |
H2(o) | → | H (o) (o) |
H2(p) | 3.46(−10) | 0.00 | −0.69 | 3.71(−10) | 2 | ||
H (p) (p) |
H2(o) | → | H (p) (p) |
H2(p) | 2.98(−10) | 0.00 | −0.69 | 3.19(−10) | 2 | ||
H (p) (p) |
H2(p) | → | H (p) (p) |
H2(p) | 5.88(−10) | 0.00 | 198.2 | 1.45(−18) | 2 | ||
H (p) (p) |
H2(p) | → | H (p) (p) |
H2(p) | 8.16(−10) | 0.00 | 164.9 | 5.63(−17) | 2 | ||
| H+ | H2(o) | → | H+ | H2(p) | 1.82(−10) | 0.13 | −0.02 | 1.17(−10) | 3 | ||
| H+ | H2(p) | → | H+ | H2(o) | 1.64(−9) | 0.13 | 170.5 | 4.15(−17) | 3 | ||
| HCO+ | H2(o) | → | HCO+ | H2(p) | 1.27(−10) | 0.00 | 0.00 | 1.27(−10) | 4 | ||
| HCO+ | H2(p) | → | HCO+ | H2(o) | 1.14(−9) | 0.00 | 170.5 | 4.49(−17) | 4 | ||
|
|
|||||||||||
| Nitrogen hydrides formation | |||||||||||
| N+ | H2(o) | → | NH+ | H | 4.20(−10) | −0.15 | 44.1 | 8.50(−12) | 5 | ||
| N+ | H2(p) | → | NH+ | H | 8.35(−10) | 0.00 | 168.5 | 4.02(−17) | 5 | ||
| NH+ | H2(o) | → | NH (o) (o) |
H | 1.06(−9) | 0.00 | 0.00 | 1.06(−9) | 6 | 5/6 | |
| NH+ | H2(o) | → | NH (p) (p) |
H | 2.13(−10) | 0.00 | 0.00 | 2.13(−10) | 6 | 1/6 | |
| NH+ | H2(p) | → | NH (o) (o) |
H | 6.38(−10) | 0.00 | 0.00 | 6.38(−10) | 6 | 1/2 | |
| NH+ | H2(p) | → | NH (p) (p) |
H | 6.38(−10) | 0.00 | 0.00 | 6.38(−10) | 6 | 1/2 | |
NH (o) (o) |
H2(o) | → | NH (o) (o) |
H | 1.80(−10) | 0.00 | 0.00 | 1.80(−10) | 6 | 2/3 | |
NH (o) (o) |
H2(o) | → | NH (p) (p) |
H | 9.00(−11) | 0.00 | 0.00 | 9.00(−11) | 6 | 1/3 | |
NH (o) (o) |
H2(p) | → | NH (o) (o) |
H | 9.00(−11) | 0.00 | 0.00 | 9.00(−11) | 6 | 1/3 | |
NH (o) (o) |
H2(p) | → | NH (p) (p) |
H | 1.80(−10) | 0.00 | 0.00 | 1.80(−10) | 6 | 2/3 | |
NH (p) (p) |
H2(o) | → | NH (o) (o) |
H | 9.00(−11) | 0.00 | 0.00 | 9.00(−11) | 6 | 1/3 | |
NH (p) (p) |
H2(o) | → | NH (p) (p) |
H | 1.80(−10) | 0.00 | 0.00 | 1.80(−10) | 6 | 2/3 | |
NH (p) (p) |
H2(p) | → | NH (p) (p) |
H | 2.70(−10) | 0.00 | 0.00 | 2.70(−10) | 6 | 1 | |
NH (o) (o) |
H2(o) | → | NH (I = 2) (I = 2) |
H | 1.40(−12) | 0.00 | 0.00 | 1.40(−12) | 6 | 7/12 | |
NH (o) (o) |
H2(o) | → | NH (I = 1) (I = 1) |
H | 8.40(−13) | 0.00 | 0.00 | 8.40(−13) | 6 | 21/60 | |
NH (o) (o) |
H2(o) | → | NH (I = 0) (I = 0) |
H | 1.60(−13) | 0.00 | 0.00 | 1.60(−13) | 6 | 1/15 | |
NH (o) (o) |
H2(p) | → | NH (I = 2) (I = 2) |
H | 1.80(−12) | 0.00 | 0.00 | 1.80(−12) | 6 | 1/4 | |
NH (o) (o) |
H2(p) | → | NH (I = 1) (I = 1) |
H | 6.00(−13) | 0.00 | 0.00 | 6.00(−13) | 6 | 3/4 | |
NH (o) (o) |
H2(p) | → | NH (I = 0) (I = 0) |
H | 0.00 | 0.00 | 0.00 | 0.00 | 6 | 0 | |
NH (p) (p) |
H2(o) | → | NH (I = 2) (I = 2) |
H | 4.00(−13) | 0.00 | 0.00 | 4.00(−13) | 6 | 1/6 | |
NH (p) (p) |
H2(o) | → | NH (I = 1) (I = 1) |
H | 1.68(−12) | 0.00 | 0.00 | 1.68(−12) | 6 | 21/30 | |
NH (p) (p) |
H2(o) | → | NH (I = 0) (I = 0) |
H | 3.20(−13) | 0.00 | 0.00 | 3.20(−13) | 6 | 2/15 | |
NH (p) (p) |
H2(p) | → | NH (I = 2) (I = 2) |
H | 0.00 | 0.00 | 0.00 | 0.00 | 6 | 0 | |
NH (p) (p) |
H2(p) | → | NH (I = 1) (I = 1) |
H | 1.44(−12) | 0.00 | 0.00 | 1.44(−12) | 6 | 3/5 | |
NH (p) (p) |
H2(p) | → | NH (I = 0) (I = 0) |
H | 9.60(−13) | 0.00 | 0.00 | 9.60(−13) | 6 | 2/5 | |
Destruction of nitrogen hydrides by 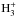 , HCO+, and N2H+ , HCO+, and N2H+ |
|||||||||||
H (p) (p) |
NH | → | NH (p) (p) |
H2(p) | 1.63(−10) | 0.00 | 0.00 | 1.63(−10) | 7 | 1/8 | |
H (p) (p) |
NH | → | NH (o) (o) |
H2(p) | 3.25(−10) | 0.00 | 0.00 | 3.25(−10) | 7 | 1/4 | |
H (p) (p) |
NH | → | NH (p) (p) |
H2(o) | 3.25(−10) | 0.00 | 0.00 | 3.25(−10) | 7 | 1/4 | |
H (p) (p) |
NH | → | NH (o) (o) |
H2(o) | 4.88(−10) | 0.00 | 0.00 | 4.88(−10) | 7 | 3/8 | |
H (p) (p) |
NH2(p) | → | NH (p) (p) |
H2(p) | 7.20(−10) | 0.00 | 0.00 | 7.20(−10) | 7 | 8/5/4 | |
H (p) (p) |
NH2(p) | → | NH (p) (p) |
H2(o) | 7.20(−10) | 0.00 | 0.00 | 7.20(−10) | 7 | 8/5/4 | |
H (p) (p) |
NH2(p) | → | NH (o) (o) |
H2(o) | 3.60(−10) | 0.00 | 0.00 | 3.60(−10) | 7 | 4/5/4 | |
H (p) (p) |
NH2(o) | → | NH (p) (p) |
H2(o) | 8.40(−10) | 0.00 | 0.00 | 8.40(−10) | 7 | 28/5/12 | |
H (p) (p) |
NH2(o) | → | NH (o) (o) |
H2(o) | 4.20(−10) | 0.00 | 0.00 | 4.20(−10) | 7 | 14/5/12 | |
H (p) (p) |
NH2(o) | → | NH (p) (p) |
H2(p) | 2.40(−10) | 0.00 | 0.00 | 2.40(−10) | 7 | 8/5/12 | |
H (p) (p) |
NH2(o) | → | NH (o) (o) |
H2(p) | 3.00(−10) | 0.00 | 0.00 | 3.00(−10) | 7 | 2/12 | |
H (p) (p) |
NH3(p) | → | NH (I = 0) (I = 0) |
H2(p) | 9.10(−10) | 0.00 | 0.00 | 9.10(−10) | 8 | 8/5/16 | |
H (p) (p) |
NH3(p) | → | NH (I = 1) (I = 1) |
H2(p) | 2.28(−9) | 0.00 | 0.00 | 2.28(−9) | 8 | 4/16 | |
H (p) (p) |
NH3(p) | → | NH (I = 0) (I = 0) |
H2(o) | 1.52(−9) | 0.00 | 0.00 | 1.52(−9) | 8 | 8/3/16 | |
H (p) (p) |
NH3(p) | → | NH (I = 1) (I = 1) |
H2(o) | 3.64(−9) | 0.00 | 0.00 | 3.64(−9) | 8 | 32/5/16 | |
H (p) (p) |
NH3(p) | → | NH (I = 2) (I = 2) |
H2(o) | 7.58(−10) | 0.00 | 0.00 | 7.58(−10) | 8 | 4/3/16 | |
H (p) (p) |
NH3(o) | → | NH (I = 0) (I = 0) |
H2(o) | 7.58(−10) | 0.00 | 0.00 | 7.58(−10) | 8 | 4/3/16 | |
H (p) (p) |
NH3(o) | → | NH (I = 1) (I = 1) |
H2(o) | 4.55(−9) | 0.00 | 0.00 | 4.55(−9) | 8 | 8/16 | |
H (p) (p) |
NH3(o) | → | NH (I = 2) (I = 2) |
H2(o) | 1.52(−9) | 0.00 | 0.00 | 1.52(−9) | 8 | 8/3/16 | |
H (p) (p) |
NH3(o) | → | NH (I = 2) (I = 2) |
H2(p) | 1.14(−9) | 0.00 | 0.00 | 1.14(−9) | 8 | 2/16 | |
H (p) (p) |
NH3(o) | → | NH (I = 1) (I = 1) |
H2(p) | 1.14(−9) | 0.00 | 0.00 | 1.14(−9) | 8 | 2/16 | |
H (o) (o) |
NH | → | NH (p) (p) |
H2(o) | 1.63(−10) | 0.00 | 0.00 | 1.63(−10) | 7 | 1/8 | |
H (o) (o) |
NH | → | NH (o) (o) |
H2(o) | 9.75(−10) | 0.00 | 0.00 | 9.75(−10) | 7 | 6/8 | |
H (o) (o) |
NH | → | NH (o) (o) |
H2(p) | 1.63(−10) | 0.00 | 0.00 | 1.63(−10) | 7 | 1/8 | |
H (o) (o) |
NH2(p) | → | NH (o) (o) |
H2(p) | 4.50(−10) | 0.00 | 0.00 | 4.50(−10) | 7 | 1/4 | |
H (o) (o) |
NH2(p) | → | NH (p) (p) |
H2(o) | 9.00(−10) | 0.00 | 0.00 | 9.00(−10) | 7 | 2/4 | |
H (o) (o) |
NH2(p) | → | NH (o) (o) |
H2(o) | 4.50(−10) | 0.00 | 0.00 | 4.50(−10) | 7 | 1/4 | |
H (o) (o) |
NH2(o) | → | NH (p) (p) |
H2(p) | 1.20(−10) | 0.00 | 0.00 | 1.20(−10) | 7 | 4/5/12 | |
H (o) (o) |
NH2(o) | → | NH (p) (p) |
H2(o) | 4.20(−10) | 0.00 | 0.00 | 4.20(−10) | 7 | 14/5/12 | |
H (o) (o) |
NH2(o) | → | NH (o) (o) |
H2(p) | 1.50(−10) | 0.00 | 0.00 | 1.50(−10) | 7 | 1/12 | |
H (o) (o) |
NH2(o) | → | NH (o) (o) |
H2(o) | 1.11(−9) | 0.00 | 0.00 | 1.11(−9) | 7 | 37/5/12 | |
H (o) (o) |
NH3(p) | → | NH (I = 1) (I = 1) |
H2(p) | 1.14(−9) | 0.00 | 0.00 | 1.14(−9) | 8 | 2/16 | |
H (o) (o) |
NH3(p) | → | NH (I = 2) (I = 2) |
H2(p) | 1.14(−9) | 0.00 | 0.00 | 1.14(−9) | 8 | 2/16 | |
H (o) (o) |
NH3(p) | → | NH (I = 0) (I = 0) |
H2(o) | 7.58(−10) | 0.00 | 0.00 | 7.58(−10) | 8 | 4/3/16 | |
H (o) (o) |
NH3(p) | → | NH (I = 1) (I = 1) |
H2(o) | 4.55(−9) | 0.00 | 0.00 | 4.55(−9) | 8 | 8/16 | |
H (o) (o) |
NH3(p) | → | NH (I = 2) (I = 2) |
H2(o) | 1.52(−9) | 0.00 | 0.00 | 1.52(−9) | 8 | 8/3/16 | |
H (o) (o) |
NH3(o) | → | NH (I = 0) (I = 0) |
H2(p) | 2.28(−10) | 0.00 | 0.00 | 2.28(−10) | 8 | 2/5/16 | |
H (o) (o) |
NH3(o) | → | NH (I = 1) (I = 1) |
H2(p) | 5.69(−10) | 0.00 | 0.00 | 5.69(−10) | 8 | 1/16 | |
H (o) (o) |
NH3(o) | → | NH (I = 2) (I = 2) |
H2(p) | 5.69(−10) | 0.00 | 0.00 | 5.69(−10) | 8 | 1/16 | |
H (o) (o) |
NH3(o) | → | NH (I = 0) (I = 0) |
H2(o) | 3.79(−10) | 0.00 | 0.00 | 3.79(−10) | 8 | 2/3/16 | |
H (o) (o) |
NH3(o) | → | NH (I = 1) (I = 1) |
H2(o) | 2.62(−9) | 0.00 | 0.00 | 2.62(−9) | 8 | 23/5/16 | |
H (o) (o) |
NH3(o) | → | NH (I = 2) (I = 2) |
H2(o) | 4.74(−9) | 0.00 | 0.00 | 4.74(−9) | 8 | 25/3/16 | |
| HCO+ | NH | → | NH (p) (p) |
CO | 1.60(−10) | 0.00 | 0.00 | 1.60(−10) | 7 | 1/4 | |
| HCO+ | NH | → | NH (o) (o) |
CO | 4.80(−10) | 0.00 | 0.00 | 4.80(−10) | 7 | 3/4 | |
| HCO+ | NH2(p) | → | NH (p) (p) |
CO | 8.90(−10) | 0.00 | 0.00 | 8.90(−10) | 7 | 1/1 | |
| HCO+ | NH2(o) | → | NH (p) (p) |
CO | 3.00(−10) | 0.00 | 0.00 | 3.00(−10) | 7 | 1/3 | |
| HCO+ | NH2(o) | → | NH (o) (o) |
CO | 5.90(−10) | 0.00 | 0.00 | 5.90(−10) | 7 | 2/3 | |
| HCO+ | NH3(p) | → | NH (I = 0) (I = 0) |
CO | 4.80(−10) | 0.00 | 0.00 | 4.80(−10) | 6 | 1/4 | |
| HCO+ | NH3(p) | → | NH (I = 1) (I = 1) |
CO | 1.40(−9) | 0.00 | 0.00 | 1.40(−9) | 6 | 3/4 | |
| HCO+ | NH3(o) | → | NH (I = 1) (I = 1) |
CO | 7.10(−10) | 0.00 | 0.00 | 7.10(−10) | 6 | 3/8 | |
| HCO+ | NH3(o) | → | NH (I = 2) (I = 2) |
CO | 1.20(−9) | 0.00 | 0.00 | 1.20(−9) | 6 | 5/8 | |
| N2H+ | NH3(p) | → | NH (I = 0) (I = 0) |
N2 | 5.75(−10) | 0.00 | 0.00 | 5.75(−10) | 6 | 1/4 | |
| N2H+ | NH3(p) | → | NH (I = 1) (I = 1) |
N2 | 1.73(−9) | 0.00 | 0.00 | 1.73(−9) | 6 | 3/4 | |
| N2H+ | NH3(o) | → | NH (I = 2) (I = 2) |
N2 | 8.63(−10) | 0.00 | 0.00 | 8.63(−10) | 6 | 3/8 | |
| N2H+ | NH3(o) | → | NH (I = 1) (I = 1) |
N2 | 1.43(−9) | 0.00 | 0.00 | 1.43(−9) | 6 | 5/8 | |
|
|
|||||||||||
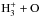
|
|||||||||||
H (o) (o) |
O | → | OH+ | H2(o) | 7.98(−10) | −0.156 | 1.41 | 1.18(−9) | 9 | 1 | |
H (o) (o) |
O | → | H2O+ | H | 3.42(−10) | −0.156 | 1.41 | 5.05(−10) | 9 | ||
H (p) (p) |
O | → | OH+ | H2(o) | 3.99(−10) | −0.156 | 1.41 | 5.89(−10) | 9 | 1/2 | |
H (p) (p) |
O | → | OH+ | H2(p) | 3.99(−10) | −0.156 | 1.41 | 5.89(−10) | 9 | 1/2 | |
H (p) (p) |
O | → | H2O+ | H | 3.42(−10) | −0.156 | 1.41 | 5.05(−10) | 9 | ||
Notes. Numbers in parentheses are power of 10.
o and p stand for ortho and para states respectively. As a spherical top with four identical protons, the ammonium ion exists in three nuclear spin states noted as in Faure et al. (2013): para (I = 0), meta (I = 2), and ortho (I = 1). We note that the meta and ortho species are inverted in Rist et al. (2013).
NSBR stands for nuclear-spin branching ratio. These were combined with the overall rate coefficients taken from the cited references, e.g. Prasad & Huntress (1980). The integer ratios like e.g. 28/5/12 are normalized NSBR and stand for (28/5)/12 i.e. 28/60.
Reference. (1) Langevin rate: 2.10 × 10-9 cm3 s-1; (2) Hugo et al. (2009); (3) Honvault et al. (2011); (4) Langevin rate: 1.52 × 10-9 cm3 s-1; (5) Dislaire et al. (2012); (6) Anicich & Huntress (1986); (7) Prasad & Huntress (1980); (8) Marquette et al. (1989); (9) datasheet by Ian Smith from KIDA (Wakelam et al. 2012).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


