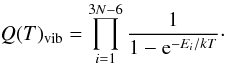| Issue |
A&A
Volume 549, January 2013
|
|
|---|---|---|
| Article Number | A96 | |
| Number of page(s) | 6 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201220632 | |
| Published online | 03 January 2013 | |
Millimeter and submillimeter wave spectra of 13C-glycolaldehydes⋆
Laboratoire de Physique des Lasers, Atomes et Molécules, Bâtiment P5, CNRS,
UMR 8523, Université Lille 1,
59655 Villeneuve d’,
Ascq Cedex,
France
e-mail: Therese.Huet@univ-lille1.fr
Received:
25
October
2012
Accepted:
9
November
2012
Context. Glycolaldehyde (CH2OHCHO) is the simplest sugar and an important intermediate in the path toward forming more complex biologically relevant molecules. Astronomical surveys of interstellar molecules, such as those available with the very sensitive ALMA telescope, require preliminary laboratory investigations of the microwave and submillimeter-wave spectra of molecular species including new isotopologs – to identify these in the interstellar media.
Aims. To achieve the detection of the 13C isotopologs of glycolaldehyde in the interstellar medium, their rotational spectra in the millimeter and submillimeter-wave regions were studied.
Methods. The spectra of 13CH2OHCHO and CH2OH13CHO were recorded in the 150−945 GHz spectral range in the laboratory using a solid-state submillimeter-wave spectrometer in Lille. The observed line frequencies were measured with an accuracy of 30 kHz up to 700 GHz and of 50 kHz above 700 GHz. We analyzed the spectra with a standard Watson Hamiltonian.
Results. About 10 000 new lines were identified for each isotopolog. The spectroscopic parameters were determined for the ground- and the three lowest vibrational states up to 945 and 630 GHz. Previous microwave assignments of 13CH2OHCHO were not confirmed.
Conclusions. The provided line-lists and sets of molecular parameters meet the needs for a first astrophysical search of 13C-glycolaldehydes.
Key words: ISM: molecules / submillimeter: ISM / methods: laboratory / line: identification
Full Tables 3 and 4 are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/549/A96
© ESO, 2013
1. Introduction
Glycolaldehyde (Fig. 1), the smallest sugar, is considered as a probable prebiotic molecule since its first detection in the interstellar medium (ISM) by (Hollis et al. 2000). In a recent paper (Jørgensen et al. 2012) the first detection of 13 transitions of glycolaldehyde around a solar-type young star was presented, which were made with the Atacama Large Millimeter Array (ALMA) observations of the Class 0 protostellar binary IRAS 16293-2422 at 220 GHz (six transitions) and 690 GHz (seven transitions). The order of magnitude increase in line density in these early ALMA data illustrates its huge potential to reveal the full chemical complexity associated with the formation of solar system analogs. Especially the constraints on the chemical formation of glycolaldehyde and other organic species have been discussed. According to Jørgensen et al. (2012), the relative abundances appear to be consistent with UV photochemistry of a CH3OH – CO mixed ice that has undergone mild heating. This conclusion is consistent with previous published results (Bennett & Kaiser 2007) even if possible gas-phase pathways have also been proposed (Jalbout et al. 2007) and discussed (Simakov et al. 2011). In their article, Bennett & Kaiser (2007) paid special attention to the investigation of the glycolaldehyde formation process and its isomers (methyl formate and acetic acid), which obey the same empirical formula C2H4O2. Their relative abundance, for example, was reported by Hollis et al. (2001) to be 52:2:1 (methyl formate:acetic acid:glycolaldehyde) in Sgr B2.
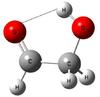 |
Fig. 1 Schematic view of the most stable conformer of glycolaldehyde, stabilized by a weak hydrogen bond OH ... O = C binding the two functional groups. |
In addition to the ability of this hydroxyaldehyde diose to become involved in the production of important biomolecules (glycolaldehyde phosphates, amino acids, etc), the 13C isotopologs of glycolaldehyde are of special importance to astrophysicists. Detecting them in the future will enable us to measure the abundance ratio of 12C over 13C isotopes by measuring the ratio of the abundance of their respective isotopomers. Observating isotopic abundances in the interstellar medium provides an avenue for quantitatively assessing stellar nucleosynthesis and therefore Galactic chemical evolution. The 12C/13C isotope ratio is also considered an important tracer because it reflects the relative degree of primary to secondary processing in stars (Milam et al. 2005).
The present study investigates the rotational structure of the ground states and the three lowest vibrational modes for the two 13C isotopomers of glycolaldehyde. Although they are so important on the astrophysical scale, the two 13C-isotopologs of glycolaldehyde, 13CH2OHCHO and CH2OH13CHO, were studied only once in the past 40 years. Indeed, Marstokk & Møllendal (1973) reported the analysis of spectra recorded in the microwave range (12.4−36.3 GHz) with a spectral accuracy of 0.25 MHz. Ten and eighteen lines were observed in natural abundance and were identified to be 13CH2OHCHO and CH2OH13CHO, respectively. The data were fitted to a standard Watson Hamiltonian, providing a set of molecular parameters including the principal rotational constants and the quartic centrifugal distortion parameters. Marstokk & Møllendal (1973) also re-measured the permanent dipole moment of the parent species: μa = 0.2620 ± 0.002 D, μb = 2.330 ± 0.01 D, and μtot = 2.340 ± 0.01 D. Glycolaldehyde is a fairly light molecule (M = 60) whose typical μb type spectra have their maximum absorption at 820 GHz (at 300 K) and extend far beyond 1 THz. If glycoladehyde and its isotopic species are interesting objects for highly sensitive terahertz radio telescopes such as ALMA or Herschel, the results of Marstokk & Møllendal (1973) are insufficient to provide reliable frequency predictions in the terahertz range. Moreover, as we show below, the previous assignment of one of the 13C species is most probably erroneous and could not be used for astrophysical observations. The deuterated isotopologs of glycolaldehyde have recently been studied in the millimeter-wave region (150−630 GHz) by Bouchez et al. (2012). This spectroscopic study was motivated by a future comparison of the D/H ratios for better understanding the formation of glycolaldehyde in hot cores and hot corinos. According to the relative abundance of the 13C/12C and D/H ratios observed in the ISM, it is probably useful to be able to also identify in the future the two 13C-isotopologs of glycolaldehyde in the future. To this end we studied the spectroscopy of 13CH2OHCHO and CH2OH13CHO in the laboratory in the millimeter-wave and submillimeter-wave ranges. Precise molecular parameters were obtained for the ground and first three excited vibrational states of the two species, providing a line-list for a future detection, with an absolute frequency accuracy better than 30 kHz in the spectral ranges covered by the ALMA and Herschel telescopes.
2. Experiment
The millimeter- and submillimeter-wave spectra of 13C-glycolaldehydes were recorded using the Lille spectrometer based on solid-state sources. The frequency source is a 20 GHz synthesizer (Agilent, model E8257D) that is stabilized on a global positioning system. The output frequency (12.5−18.4 GHz) is multiplied by six and amplified by an active sextupler (Spacek), providing an output power of +15 dBm in the W-band range (75−110 GHz). Passive Schottky multipliers (× 2, × 3, × 5, × 3 × 2, × 3 × 3, Virginia Diodes Inc.) are used in the last stage of the frequency multiplication chain to provide a useful signal in the 150−990 GHz spectral range. The commercial samples of 13CH2OHCHO and CH2OH13CHO (Omicron Biochemicals, Inc., 99% purity, in aqueous solution) were dehydrated and used without additional purification. It was found later that the two dehydrated commercial samples contained a mixture of the two 13C-glycolaldehydes in a ratio equal to 13CHO/13CH2O = 0.59 and 13CH2O/13CHO = 0.67, but without impurity. The absorption cell is a stainless-steel tube (6 cm diameter, 220 cm long). The sample pressure was equal to 20 × 10-6 bars, and slightly increased up to 40 × 10-6 bars in the 700−945 GHz region. To improve the signal sensitivity, the sources were frequency modulated at 10 kHz and lock-in detection with the second harmonic was used. Absorption signals were detected by an InSb liquid He-cooled bolometer (QMC Instruments Ltd.) and processed on a computer. Spectra of the two samples were recorded at room temperature (T = 294 K) in the 150−215, 230−315, 400−530, 500−630, and 700−945 GHz regions with a frequency step of 30, 36, 48, 54, and 76 kHz and with an acquisition time of 35 ms. The line frequencies were obtained by adjusting the second derivative of a Gaussian profile on the observed signals, knowing that the linewidths are limited by Doppler broadening (around 1 MHz at 600 GHz). The absolute accuracy of the line-center frequency is estimated to be better than 30 kHz (50 kHz above 700 GHz) for isolated lines, and can be as high as 100 kHz (150 kHz above 700 GHz) for blended or very weak lines.
3. Analysis
At first, the strongest lines associated with the ground state were considered for both 13CH2OHCHO and CH2OH13CHO. In previous spectroscopic works as well as in the present one, a standard asymmetric-top Hamiltonian with a Watson A-reduction in the Ir-representation was employed. Consequently, the rotational structure is characterized by the JKaKc quantum numbers. The spectral data were fitted by means of the ASFIT1 and ASROT1 suite of programs. Final results are available at the CDS, using the formats of the program suite SPFIT and SPCAT (Pickett 1991), which are commonly used by astrophysicists (Pearson et al. 2010).
At the initial stage of the line assignment, we used predictions based on the principal
rotational constants from Marstokk and Møllendal (Marstokk
& Møllendal 1973) while the value of the quartic and sextic distortion
parameters were fixed to the corresponding values of the CH2OHCHO parent molecule
(Widicus Weaver et al. 2005). We first searched for
the intense R-branch lines of the low Ka
quantum number (Ka = 0,1) with
respect to the selection rule associated with the μb type
transitions (ΔKa = ± 1,
ΔKc = ± 1). Their doublet structure helped
us to recognize them, cf. Fig. 2. Following the
systematic decrease of the splitting within the doublets and the increase of the line
intensity with the increase of the J quantum number, we collected doublets
up to J = 21 for CH2OH13CHO and
J = 20 for 13CH2OHCHO in the 150−215 GHz frequency
range. Meanwhile, the observed minus calculated frequency shift increased quickly, reaching
5.5 MHz at J = 21 for CH2OH13CHO and 43 MHz at
J = 20 for 13CH2OHCHO. Consequently, the new
millimeter-wave lines of each isotopomer were fitted together with the microwave lines
measured by Marstokk and Møllendal (Marstokk &
Møllendal 1973). The initial set of parameters was refined and new and more
accurate predictions were generated. However, we had to exclude four microwave lines from
the fit for CH2OH13CHO because their observed-calculated residuals did
not satisfy the 3σ criteria (where σ is the experimental
measurement uncertainty). We found that the A rotational constant obtained by Marstokk and
Møllendal for 13CH2OHCHO (Marstokk
& Møllendal 1973) could not be associated with the ground state. Therefore
all microwave transitions (Marstokk & Møllendal
1973) were omitted from our analysis. The next step was to proceed with the
assignment by increasing the Ka quantum number.
In this frequency range 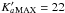 for
for
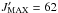 (CH2OH13CHO)
and
(CH2OH13CHO)
and 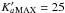 for
for
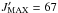 (13CH2OHCHO)
were reached. Afterward, we searched for the Q type transitions and gathered them up to
J′,Ka′ = 64,14
(CH2OH13CHO) and
J′,Ka′ = 66,15
(13CH2OHCHO), cf. Fig. 2.
Pursuing the analysis up to 950 GHz, we assigned R and Q transitions up to
(13CH2OHCHO)
were reached. Afterward, we searched for the Q type transitions and gathered them up to
J′,Ka′ = 64,14
(CH2OH13CHO) and
J′,Ka′ = 66,15
(13CH2OHCHO), cf. Fig. 2.
Pursuing the analysis up to 950 GHz, we assigned R and Q transitions up to
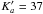 and
J′ = 93 following the same strategy. At the end the weak lines
associated with the μa type of transitions
(ΔKa = 0,
ΔKc = ± 1) were assigned. We finally
determined a set of 20 molecular parameters for each molecule, including centrifugal
distortion parameters up to the octic terms, by fitting a total of 4218 assigned lines for
CH2OH13CHO and 4655 for 13CH2OHCHO, with a
relevant root mean square deviation (rms) of 31 and 29 kHz. The final sets of molecular
parameters are presented in Tables 1 and 2. The number of assigned lines and the maximal values of
the observed quantum numbers are also given.
and
J′ = 93 following the same strategy. At the end the weak lines
associated with the μa type of transitions
(ΔKa = 0,
ΔKc = ± 1) were assigned. We finally
determined a set of 20 molecular parameters for each molecule, including centrifugal
distortion parameters up to the octic terms, by fitting a total of 4218 assigned lines for
CH2OH13CHO and 4655 for 13CH2OHCHO, with a
relevant root mean square deviation (rms) of 31 and 29 kHz. The final sets of molecular
parameters are presented in Tables 1 and 2. The number of assigned lines and the maximal values of
the observed quantum numbers are also given.
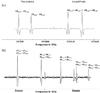 |
Fig. 2 a) Strong R-line doublets associated with Ka = 0,1, observed in the 191 GHz range with a sample containing the two 13C-glycolaldehydes and b) weak Q-lines of 13CH2OHCHO associated with Ka = 22, observed in the 557 GHz range. All signals are assigned to the ground states and are labeled (JKaKc)′ ← − (JKaKc)′′. |
The good quality of the spectra provided by our spectrometer and the high signal-to-noise ratio allowed us to proceed with assigning of the rotational structure of the three lowest excited vibrational modes (Fig. 3). The calculated vibrational energies for the parent molecule are approximately 213 cm-1 (ν1, the C−C torsion mode), 294 cm-1 (ν2, the C−C−O bending mode), and 426 cm-1 (ν3, the O-H torsion mode) (Senent 2004). To start the analysis of ν1 = 1, the rotational constants were roughly predicted using the vibration-rotation constants α of the parent molecule (β = A,B,C) (Widicus Weaver et al. 2005):
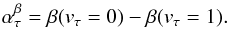 This relation also served
to predict the first set of rotational constants for ν2 = 1 and
ν3 = 1. At the beginning of the analysis, the quartic, sextic,
and octic distortion parameters values were fixed to those of the ground state (see
Tables 1 and 2). This first set of parameters supplied the preliminary predictions. Taking into
account the decrease of the Boltzmann distribution and following the same assignment
strategy as for the ground state, we could assign the rotational structure up to 630 GHz.
The rotational constants and the centrifugal distortion parameters up to the sextic terms
were determined for the three lowest excited modes of the two isotopomers. They are
presented in Tables 1 and 2, for CH2OH13CHO and
13CH2OHCHO. Finally, a full list of assigned lines is given in
Tables 3 and 4 for CH2OH13CHO and 13CH2OHCHO. The
tables contain the rotational assignments, the vibrational assignement, the observed
frequencies, the observed-calculated frequency residuals, and the experimental
uncertainties.
This relation also served
to predict the first set of rotational constants for ν2 = 1 and
ν3 = 1. At the beginning of the analysis, the quartic, sextic,
and octic distortion parameters values were fixed to those of the ground state (see
Tables 1 and 2). This first set of parameters supplied the preliminary predictions. Taking into
account the decrease of the Boltzmann distribution and following the same assignment
strategy as for the ground state, we could assign the rotational structure up to 630 GHz.
The rotational constants and the centrifugal distortion parameters up to the sextic terms
were determined for the three lowest excited modes of the two isotopomers. They are
presented in Tables 1 and 2, for CH2OH13CHO and
13CH2OHCHO. Finally, a full list of assigned lines is given in
Tables 3 and 4 for CH2OH13CHO and 13CH2OHCHO. The
tables contain the rotational assignments, the vibrational assignement, the observed
frequencies, the observed-calculated frequency residuals, and the experimental
uncertainties.
 |
Fig. 3 Example of strong lines of 13CH2OHCHO, i.e. JKaKc = 200,20 − 191,19 (I) and JKaKc = 201,20 − 190,19 (II), observed simultaneously in the ground state and in the first three excited vibrational states in the 200 GHz range at 294 K. |
Determined parameters for the ground state and first lowest excited modes of CH2OH13CHO.
Determined parameters for the ground state and first lowest excited modes of 13CH2OHCHO.
List of the assigned lines for the rotation spectrum of CH2OH13CHO in the ground state and the three first excited vibrational states (ν1 = 1, ν2 = 1, ν3 = 1).
List of the assigned lines for the rotation spectrum of 13CH2OHCHO in the ground state and the three first excited vibrational states (ν1 = 1, ν2 = 1, ν3 = 1).
The tabulated values for the 13C-glycolaldehydes partition function are given in Table 5, with Q(T)tot = Q(T)vibQ(T)rot. Various temperatures are considered. Q(T)rot was calculated as
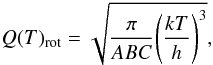 where A,B,
and C represent the rotational contants of the ground state in MHz. As
noted by Widicus Weaver et al. (2005), the
glycolaldehyde partition function increases significantly when taking into account the
vibrational contribution. This is mainly because of the low-frequency vibrational modes.
Unfortunately, no experimental or theoretical data are available for the
13C-glycolaldehydes. Meanwhile, regarding the weak differences in atomic and
molecular masses (one 12C atom is replaced by one 13C atom and
Mtot = 61 instead of Mtot = 60),
we estimated the vibrational partition function using the observed or calculated frequencies
for the main species. The three lowest calculated frequencies
(ν1, ν2,
ν3) were taken from Senent
(2004), the observed band centers (ν5,
ν6, ν8,
ν10–ν18) in the infrared region
were taken from Jetzki et al. (2004) and the others
(ν4, ν7,
ν9) from Carbonniere &
Pouchan (2012). Indeed, these last authors performed a high-level ab initio
calculation limited to the mid-infrared region. Their results agree well with the few
experimental data (Jetzki et al. 2004). Putting the
zero energy at the ground state level, the vibrational partition function was calculated
using the expression
where A,B,
and C represent the rotational contants of the ground state in MHz. As
noted by Widicus Weaver et al. (2005), the
glycolaldehyde partition function increases significantly when taking into account the
vibrational contribution. This is mainly because of the low-frequency vibrational modes.
Unfortunately, no experimental or theoretical data are available for the
13C-glycolaldehydes. Meanwhile, regarding the weak differences in atomic and
molecular masses (one 12C atom is replaced by one 13C atom and
Mtot = 61 instead of Mtot = 60),
we estimated the vibrational partition function using the observed or calculated frequencies
for the main species. The three lowest calculated frequencies
(ν1, ν2,
ν3) were taken from Senent
(2004), the observed band centers (ν5,
ν6, ν8,
ν10–ν18) in the infrared region
were taken from Jetzki et al. (2004) and the others
(ν4, ν7,
ν9) from Carbonniere &
Pouchan (2012). Indeed, these last authors performed a high-level ab initio
calculation limited to the mid-infrared region. Their results agree well with the few
experimental data (Jetzki et al. 2004). Putting the
zero energy at the ground state level, the vibrational partition function was calculated
using the expression
Rotational and vibrational partition functions at various temperatures for the 13C isotopomers of glycolaldehyde (CH2OH13CHO and 13CH2OHCHO) and for their parent molecule. Q(T)tot = Q(T)vibQ(T)rot.
4. Conclusions
The rotational structure of the two 13C-isotopologs of glycolaldehyde was characterized in the laboratory for the ground state and the first three excited vibrational modes up to 945 and 630 GHz. High-quality spectra were continuously recorded with an absolute frequency accuracy better than 30 kHz. The sets of molecular parameters we determined can be used to generate predictions in the range of one to several hundred GHz at the experimental accuracy, without bias. These isotopologs of glycolaldehyde are relevant targets for the ALMA interferometers owing to their high sensitivity and spatial resolution.
Kisiel (2012), programs are available on the http://www.ifpan.edu.pl/~kisiel/asym/asym.htm#asfit
Acknowledgments
The authors gratefully acknowledge Jean-Claude Guillemin (Ecole Nationale Supérieure de Chimie de Rennes, France) for his help in conditioning the samples. This work was supported by the French ANR agency under contract ANR-08-BLAN-0054, by the French CNRS-INSU programme “Action sur Projet Physico-Chimie du Milieu Interstellaire” and by the Centre National d’Etudes Spatiales (CNES).
References
- Bennett, C. J., & Kaiser, R. I. 2007, ApJ, 661, 899 [NASA ADS] [CrossRef] [Google Scholar]
- Bouchez, A., Margulès, L., Motiyenko, R. A., et al. 2012, A&A, 540, A51 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Carbonniere, P., & Pouchan, P. 2012, Theor. Chem. Acc., 131, 1183 [CrossRef] [Google Scholar]
- Hollis, J. M., Lovas, F. J., & Jewell, P. R. 2000, ApJ, 540, 107 [Google Scholar]
- Hollis, J. M., Vogel, S. N., Snyder, L. E., et al. 2001, ApJ, 554, 81 [Google Scholar]
- Jalbout, A. F., Abrell, L., Adamowicz, L., et al. 2007, Astrobiol., 7, 43 [Google Scholar]
- Jetzki, M., Luckhaus, D., & Signorell, R. 2004, Can. J. Chem., 82, 915 [CrossRef] [Google Scholar]
- Jørgensen, J. K., Favre, C., Bisschop, S. E., et al. 2012, ApJ, 757, L4 [NASA ADS] [CrossRef] [Google Scholar]
- Marstokk, K. M., & Møllendal, H. 1973, J. Mol. Struct., 16, 259 [NASA ADS] [CrossRef] [Google Scholar]
- Milam, S. N., Savage, C., Brewster, M. A., Ziurys, L. M., et al. 2005, ApJ, 634, 1126 [NASA ADS] [CrossRef] [Google Scholar]
- Pearson, J. C., Müller, H. S. P., Pickett, H. M., Cohen, E. A., & Drouin, B. J. 2010, J. Quant. Spectr. Rad. Transf., 111, 1614 [NASA ADS] [CrossRef] [Google Scholar]
- Pickett, H. M. 1991, J. Mol. Spectrosc., 148, 371 [Google Scholar]
- Senent, M. L. 2004, J. Phys. Chem. A., 108, 6286 [NASA ADS] [CrossRef] [Google Scholar]
- Simakov, A., Sekiguchi, O., Bunkan, A., C., J., & Uggerud, E. 2011, J. Am. Chem. Soc., 133, 20816 [CrossRef] [Google Scholar]
- Widicus Weaver, S. L., Butler, R. A. H., Drouin, B. J., et al. 2005, ApJ, 158, 188 [Google Scholar]
All Tables
Determined parameters for the ground state and first lowest excited modes of CH2OH13CHO.
Determined parameters for the ground state and first lowest excited modes of 13CH2OHCHO.
List of the assigned lines for the rotation spectrum of CH2OH13CHO in the ground state and the three first excited vibrational states (ν1 = 1, ν2 = 1, ν3 = 1).
List of the assigned lines for the rotation spectrum of 13CH2OHCHO in the ground state and the three first excited vibrational states (ν1 = 1, ν2 = 1, ν3 = 1).
Rotational and vibrational partition functions at various temperatures for the 13C isotopomers of glycolaldehyde (CH2OH13CHO and 13CH2OHCHO) and for their parent molecule. Q(T)tot = Q(T)vibQ(T)rot.
All Figures
 |
Fig. 1 Schematic view of the most stable conformer of glycolaldehyde, stabilized by a weak hydrogen bond OH ... O = C binding the two functional groups. |
| In the text | |
 |
Fig. 2 a) Strong R-line doublets associated with Ka = 0,1, observed in the 191 GHz range with a sample containing the two 13C-glycolaldehydes and b) weak Q-lines of 13CH2OHCHO associated with Ka = 22, observed in the 557 GHz range. All signals are assigned to the ground states and are labeled (JKaKc)′ ← − (JKaKc)′′. |
| In the text | |
 |
Fig. 3 Example of strong lines of 13CH2OHCHO, i.e. JKaKc = 200,20 − 191,19 (I) and JKaKc = 201,20 − 190,19 (II), observed simultaneously in the ground state and in the first three excited vibrational states in the 200 GHz range at 294 K. |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.