Fig. 6
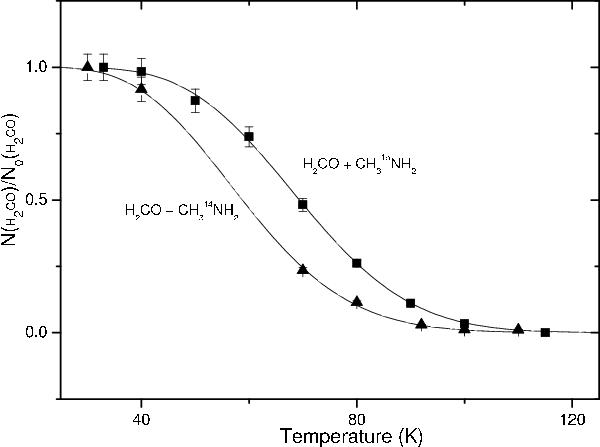
Formaldehyde thermal evolution as a function of temperature in water ice containing CH3NH214N (triangle), and 15N (square) (H2O/CH3NH2/CH2O = 1/2/0.1). The rate coefficients are given in the form k = ν0exp( − E/RT), where T is the ice temperature (K), ν0 the pre-exponential factor (s-1), and E the activation energy (J mol-1). The corresponding rate coefficients are k14N = 1.5 ± 0.1 × 10-2exp( − 1100 ± 50/RT) and k15N = 2.6 ± 0.3 × 10-2exp( − 1800 ± 80/RT).
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


