| Issue |
A&A
Volume 543, July 2012
|
|
|---|---|---|
| Article Number | A135 | |
| Number of page(s) | 5 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/201219713 | |
| Published online | 11 July 2012 | |
Analysis of the terahertz rotational spectrum of the three mono-13C ethyl cyanides (13C–CH3CH2CN)⋆
1 Laboratoire de Physique des Lasers, Atomes et Molécules, UMR CNRS 8523, Université Lille 1, 59655 Villeneuve d’Ascq Cedex, France
e-mail: cyril.richard@phlam.univ-lille1.fr, laurent.margules@univ-lille1.fr
2 Institut des Sciences Chimiques de Rennes, UMR 6226 CNRS – ENSCR, Avenue du Général Leclerc, CS 50837, 35708 Rennes Cedex 7, France
Received: 30 May 2012
Accepted: 12 June 2012
Context. Millimeter- and submillimeter-wave spectra of regions such as the Orion molecular cloud show many rotational-torsional lines that are caused by the emission of complex organic molecules (COM). Previous laboratory investigations have been conducted for three isotopologues of ethyl cyanide up to 360 GHz, and subsequently, several hundred lines of the three isotopologues have been detected in Orion IRc2 using the IRAM 30 m radiotelescope.
Aims. In this survey we present the analysis based on a Watson Hamiltonian for an asymmetric one-top rotor of the 13C-substituted ethyl cyanide 13CH3CH2CN, CH313CH2CN and CH3CH213CN in the frequency range 480–650 GHz and 780–990 GHz.
Methods. The rotational spectra of the three species were measured with a submillimeter spectrometer (50–990 GHz) using solid-state sources.
Results. A new set of spectroscopic parameters was determined from a least-squares fit procedure for each isotopologue. These parameters permit a new accurate prediction of rotational lines suitable for an astrophysical detection in the submillimeter wave range.
Key words: line: identification / methods: laboratory / molecular data / techniques: spectroscopic / submillimeter: ISM / ISM: molecules
Full Tables B.1–B.3 are only available at the CDS via anonymous ftp to cdsarc.u-strasbg.fr (130.79.128.5) or via http://cdsarc.u-strasbg.fr/viz-bin/qcat?J/A+A/543/A135
© ESO, 2012
1. Introduction
Ethyl cyanide, also known as propanenitrile or propionitrile, has been observed in abundance in hot molecular clouds in the range 40–950 GHz (Johnson et al. 1977; Blake et al. 1987, 1996; Schilke et al. 2001; White et al. 2003; Comito et al. 2005). The three 13C isotopologues of CH3CH2CN have been observed in Orion (Demyk et al. 2007). Furthermore, 193, 161 and 208 lines, respectively, from the 13CH3CH2CN, CH313CH2CN and the CH3CH213CN in the region of 8–40 GHz and 160–360 GHz, have also been assigned and fitted.
This paper proposes a re-investigation of the ground-state rotational spectrum of 13C-substituted ethyl cyanide (CH3CH2CN) and we present the analysis of spectra of up to 990 GHz for both μa and μb transitions.
2. Experimental details
2.1. Synthesis of the three species
Into a three-necked flask equipped with a stirring bar, a reflux condenser, and a nitrogen inlet, we introduced triethylene glycol (20 mL), potassium cyanide (0.7 g, 10.7 mmol), and ethyl iodide-2-13C (1 g, 6.4 mmol). The mixture was heated to 110 °C and stirred at this temperature for one hour. After cooling at room temperature, the flask was fitted on a vacuum line equipped with two U-tubes. The high boiling compounds were condensed in the first trap cooled at −30 °C and propanenitrile-3-13C (13CH3CH2CN) was selectively condensed in the second trap cooled at −90 °C.
Spectroscopic constants of the ground-vibrational state of 13C–CH3CH2CN, A-reduction.
The same approach was used with propanenitrile-2-13C (CH313CH2CN) (1 g, 6.4 mmol).
Propanenitrile-1-13C (CH3CH213CN) was prepared with the same approach starting from K13CN, (1 g, 15 mmol) and an excess of ethyl iodide (2.7 g, 17.3 mmol). The nuclear magnetic resonance spectroscopy (NMR) of the three isotopologues is given in Appendix A.
2.2. Lille – submillimeter wave spectrometer
The submillimeter-wave measurements were performed using the Lille spectrometer (150–990 GHz) (Motiyenko et al. 2010). The sources are only solid-state devices. The frequency of the Agilent synthesizer (12.5–17.5 GHz) was first multiplied by six and amplified by a Spacek active sextupler providing the output power of + 15 dBm in the W-band range (75–110 GHz). This power is sufficiently high to use passive Schottky multipliers (×2, × 3, × 5, × 2 × 3, × 3 × 3) from Virginia Diodes Inc. in the next stage of frequency multiplication chain. As a detector we used an InSb liquid He-cooled bolometer from QMC Instruments Ltd. to improve the sensitivity of the spectrometer, the sources were frequency modulated at 10 kHz. The absorption cell was a stainless-steel tube (6 cm diameter, 220 cm long). The sample pressure during measurements was about 1.5 Pa (15 μbar) and the linewidth was limited by Doppler broadening. These measurements were performed at room temperature. The measurement accuracy for isolated lines is estimated to be better than 30 kHz up to 630 GHz and 50 kHz above, owing to the Doppler effect. However, if the lines were blended or had a poor signal-to-noise ratio, they were given a lighter weight.
Spectroscopic constants of the ground-vibrational state of 13C–CH3CH2CN, S-reduction.
3. Analysis
Ethyl cyanide is a near-prolate assymetric top (κ = −0.96 MHz), it has a large a-dipole moment component (μa = 3.85 D) and a smaller b-dipole moment component (μb = 1.23 D) (Heise et al. 1973). In consequence, the spectrum contains both a- and b-type transitions.
For each isotopologue of 13C–CH3CH2CN, preliminary predictions and fits of rotational spectra were carried out with programs ASROT and ASFIT (Kisiel 2001), using the standard rotational Hamiltonian in Watson’s A- and S-reduction in Ir representation (Watson 1977). This analysis was undertaken as a subsequent work of Demyk et al. (2007) in the region of 8−40 GHz and 160–360 GHz. Previous parameters and assigned transitions were used for the initial prediction in A-reduction. Then, the prediction was improved step by step while adding new identified lines. Once all new lines were assigned, the spectroscopic parameters were also determined in S-reduction with the same set of data. Even if in the original set of spectroscopic parameters the use of five quartics and seven sextic distortion constants was sufficient to fit the data, the addition of five or eight octic constants was necessary in our survey. As discussed in the previous work, these three species show a very dense spectrum and some lines are either blended or distorted. In consequence, some distorted lines are not included in the fit but blended transitions, with their relative intensities, are assigned as such. In addition, we assumed that the value of the dipole moment of the 13C-isotopologues can be approximated to the value of the 12C species: the spectrum contains mainly aR-branch and bR- and bQ- branch.
The spectroscopic parameters and their uncertainty are presented in Tables 1 and 2 in A- and S-reduction.
For 13CH3CH2CN, 1845 lines (1653 new lines) were fitted, including 1081 (946 new lines) μa and 764 (707 new lines) μb transitions: a short example is listed in Table B.1 and all experimental frequencies of the three isotopologues are available in electronic form at the CDS. One line from the previous study was removed from the final fit because of higher residuals (more than 4σ). The lines were fitted with a J value up to 99 and a Ka value up to 28. The standard deviation of the fit in A-reduction is 39 kHz and all parameters were well determined except for LJJK. Fixing its value to the value given by Brauer et al. (2009) for the 12C species, LJJK = −0.0814 μHz, made the fit poorer. In consequence, its value was fixed to zero. We used the same set of data for the S-reduction and derived a fit with a standard deviation of 35 kHz and well determined S-parameters.
1835 μa and μb transitions (1684 new lines) were assigned and included in the fit for CH313CH2CN, 1007 and 678 new μa and μb lines, respectively. Nevertheless, ten lines from the previous work were removed. Table B.2 gives a short example of the measured transitions. The total of the transitions was fitted with 0 ≤ J ≤ 100 and a Ka value up to 30. The fit in A- and S-reduction gives a standard deviation of 38 kHz and 36 kHz, respectively. All parameters are well determined in both cases.
For CH3CH213CN, the fit includes 1976 distinct lines (1771 new lines), 1164 μa (1083 new lines) and 812 μb (688 new lines): a short example is listed in Table B.3. Three lines were removed from the original fit and the J value up to 100 and the Ka value up to 33. The result of the fit in A-reduction has a standard deviation of 38 kHz and all the parameters are well determined. For the S-reduction the standard deviation is the same and the S-parameters are also well determined.
4. Discussion
The ground-state rotational spectrum of 13C-substituted ethyl cyanide (CH3CH2CN) was measured and analysed in a new frequency range, up to 1 THz. Data from this current investigation were combined with published data from Demyk et al. (2007) into a global fit for each species. As a rule, some lines were excluded from the fit if their residual was higher than 4σ. All experimental frequencies are given in Tables B.1 − B.3 and are only available in electronic form at the CDS. As we could expect, regarding the previous work on the 12C species (Brauer et al. 2009), both A- and S-reduction of this work gave similar results. One of these fits (fit of 13CH3CH2CN) is slightly better in S-reduction (35 kHz vs. 39 kHz), but there are three additional parameters and the two condition numbers are very similar. Therefore, no valid conclusion can be inferred from this result. For the other isotopologues, condition numbers are about twice as good in S-reduction for fits giving similar (36 kHz vs. 38 kHz) or equal (38 kHz) standard deviations, but again, fewer parameters are used in A-reduction (21 vs. 23).
Comparison between observed frequency and preliminary calculation of CH313CH2CN for several J and Ka values.
Using the previous set of data from Demyk et al. (2007) to build preliminary predictions in the ALMA or Herschel frequency range (respectively 84–720 GHz and 500–2000 GHz), significant discrepancies between calculated and observed lines were noticed. Table 3 presents the frequency difference between the initial predictions based upon Demyk et al. (2007) and measurements of this study for several transitions. The significance of the difference does not permit an astrophysical detection at 300 GHz and above even for the lines that are the most suitable for detection, i.e., the lines with a low Ka, and it is noticeable that the error increases with J and Ka. For example, the transition 713,68 ← 703,67 is shifted by around 18 MHz compared to our measurements. It was therefore very important to extend the measures up to 1 THz to improve the set of spectroscopic parameters, in turn obtain a more accurate set of data lines. The data are now also predictable up to higher frequency. Still, it is important to keep in mind that at high J and Ka transitions (J > 100, Ka > 35), the propagation of the uncertainties becomes non-negligible and the decic distortion constants need to be determined. Therefore, extrapolated data should be used with caution.
 |
Fig. 1 Stick spectrum of 13CH3CH2CN at 150 K (above) and 300 K (below) from 0 to 2 THz. The a-type spectrum is represented in grey while the b-type spectrum is shown in black. This simulation is based upon the spectroscopic constants determined in this work and was provided by the ASROT program (Kisiel 2001). |
An additional reason to extend the measurement up to 1 THz is given in Fig. 1, which represents a simulated spectrum of 13CH3CH2CN. The most intense transitions of the b-type spectrum are found at 1 THz for a rotational temperature of 300 K, and in this portion of spectrum, the lines could be relevant for astrophysical detection. Indeed, even at the ISM temperature of 150 K, the intensity of these lines is sufficiently high to permit the detection up to 850 GHz with the ALMA telescope, which will be fully operational in the next years.
Acknowledgments
This work was supported by the Centre National d’Études Spatiales (CNES) and the Action sur Projets de l’INSU, “Physique et Chimie du Milieu Interstellaire”. C.R. gratefully acknowledges the ANR-08-BLAN-0225 for the post-doc fellowship.
References
- Belsley, D. A. 1991, Conditioning Diagnostics (New York: Wiley) [Google Scholar]
- Blake, G. A., Sutton, E. C., Masson, C. R., & Philips, T. H. 1987, ApJ, 315, 621 [NASA ADS] [CrossRef] [Google Scholar]
- Blake, G. A., Mundy, L. G., Carlstrom, J. E., et al. 1996, ApJ, 472, L49 [NASA ADS] [CrossRef] [Google Scholar]
- Brauer, C. S., Pearson, J. C., Drouin, B. J., & Yu, S. 2009, ApJS, 184, 133 [NASA ADS] [CrossRef] [Google Scholar]
- Comito, C., Schilke, P., Phillips, T. G., et al. 2005, ApJS, 156, 127 [NASA ADS] [CrossRef] [Google Scholar]
- Demaison, J., Cosléou, J., Bocquet, R., & Lesarri, A. G. 1994, J. Mol. Spectr., 167, 400 [NASA ADS] [CrossRef] [Google Scholar]
- Demyk, K., Mäder, H., Tercero, B., et al. 2007, A&A, 466, 255 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Heise, H. M., Lutz, H., & Dreizler, H. 1973, Z. Naturforsh., 29a, 1345 [Google Scholar]
- Johnson, D. R., Lovas, F. J., Gottlieb, C. A., et al. 1977, ApJ, 218, 370 [NASA ADS] [CrossRef] [Google Scholar]
- Kisiel, Z. 2001, Spectroscopy from Space (Dordrecht: Kluwer Academic Publishers), see also PROSPE – Programs for ROtational SPEctroscopy, http://info.ifpan.edu.pl/~kisiel/prospe.htm [Google Scholar]
- Motiyenko, R. A., Margulès, L., Alekseev, E. A., Guillemin, J. C., & Demaison, J. 2010, J. Mol. Spectr., 264, 94 [NASA ADS] [CrossRef] [Google Scholar]
- Schilke, P., Benford, D. J., Hunter, T. R., et al. 2001, ApJS, 132, 281 [NASA ADS] [CrossRef] [Google Scholar]
- Watson, J. K. G. 1977, Vibrational Spectra and Structure, 6, 1 (Amsterdam: Elsevier) [Google Scholar]
- White, G. J., Araki, M., Greaves, J. S., Ohishi, M., & Higginbottom, N. S. 2003, A&A, 407, 589 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
Appendix A: NMR of the three isotopologues
A.1. propanenitrile-3-13C(13CH3CH2CN)
Yield: 89%. 1H NMR (CDCl3, 400 MHz) δ 1.29 (dt, 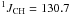 Hz,
Hz, 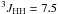 Hz, CH3); 2.34 (dq,
Hz, CH3); 2.34 (dq, 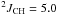 Hz,
Hz, 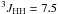 Hz, CH2). 13C NMR (CDCl3, 100 MHz) δ 10.6 (q,
Hz, CH2). 13C NMR (CDCl3, 100 MHz) δ 10.6 (q, 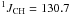 Hz, CH3); 10.8 (t,
Hz, CH3); 10.8 (t, 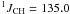 Hz,
Hz,  Hz (d), CH2); 120.8 (CN).
Hz (d), CH2); 120.8 (CN).
A.2. Propanenitrile-2-13C (CH313CH2CN)
Yield: 89%. 1H NMR (CDCl3, 400 MHz) δ 1.29 (dt, 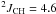 Hz,
Hz, 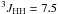 Hz, CH3); 2.34 (dq,
Hz, CH3); 2.34 (dq, 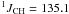 Hz,
Hz, 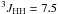 Hz, CH2). 13C NMR (CDCl3, 100 MHz) δ 10.6 (q,
Hz, CH2). 13C NMR (CDCl3, 100 MHz) δ 10.6 (q,  Hz,
Hz,  Hz (d), CH3); 10.8 (t,
Hz (d), CH3); 10.8 (t, 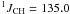 Hz, CH2); 120.8 (
Hz, CH2); 120.8 (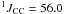 Hz (d), CN).
Hz (d), CN).
A.3. Propanenitrile-1-13C (CH3CH213CN)
Yield: 93%. 1H NMR (CDCl3, 400 MHz) δ 1.29 (dt, 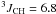 Hz,
Hz, 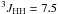 Hz, CH3); 2.34 (dq,
Hz, CH3); 2.34 (dq, 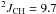 Hz,
Hz, 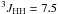 Hz, CH2). 13C NMR (CDCl3, 100 MHz) δ 10.6 (q,
Hz, CH2). 13C NMR (CDCl3, 100 MHz) δ 10.6 (q,  Hz,
Hz, 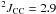 Hz (d), CH3); 10.9 (t,
Hz (d), CH3); 10.9 (t, 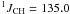 Hz,
Hz,  Hz (d), CH2); 120.8 (CN).
Hz (d), CH2); 120.8 (CN).
Appendix B: Example of transitions measured in the spectrum for the three isotopologues
Example of transitions measured in the spectrum of 13CH3CH2CN.
Example of transitions measured in the spectrum of CH313CH2CN.
Example of transitions measured in the spectrum of CH3CH213CN.
All Tables
Spectroscopic constants of the ground-vibrational state of 13C–CH3CH2CN, A-reduction.
Spectroscopic constants of the ground-vibrational state of 13C–CH3CH2CN, S-reduction.
Comparison between observed frequency and preliminary calculation of CH313CH2CN for several J and Ka values.
All Figures
 |
Fig. 1 Stick spectrum of 13CH3CH2CN at 150 K (above) and 300 K (below) from 0 to 2 THz. The a-type spectrum is represented in grey while the b-type spectrum is shown in black. This simulation is based upon the spectroscopic constants determined in this work and was provided by the ASROT program (Kisiel 2001). |
| In the text | |
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


