Free Access
Table 5
All 6.7-GHz methanol maser features detected in W3(OH).
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | |
| Maser | Group | RAa | Deca | Peak flux | Vlsr | ΔvL | Pl | χ | ΔVib | TbΔΩb | ΔVZc | θd | |
| offset | offset | density(#I) | |||||||||||
| (mas) | (mas) | (Jy/beam) | (km s-1) | (km s-1) | (%) | (°) | (km s-1) | (log K sr) | (m/s) | (°) | |||
|
|
|||||||||||||
| W3OH.01 | III | –834.479 | 148.296 | 11.58 ± 0.02 | –46.55 | 0.20 | 1.2 ± 0.4 | −2 ± 10 |
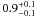
|
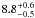
|
− |
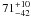
|
|
| W3OH.02 | III | –817.800 | 153.654 | 2.26 ± 0.01 | –47.07 | 0.18 | − | − | − | − | − | − | |
| W3OH.03 | III | –802.674 | 79.334 | 27.78 ± 0.28 | –45.76 | 0.22 | 3.2 ± 0.8 | 12 ± 69 |
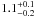
|
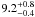
|
− |
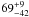
|
|
| W3OH.04 | VI | –383.492 | –1764.656 | 7.78 ± 0.04 | –42.24 | 0.19 | − | − | − | − | − | − | |
| W3OH.05e | – | -162.497 | –691.895 | 33.61 ± 0.55 | –44.00 | 0.18 | 5.6 ± 1.7 | −69 ± 5 |

|
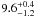
|
− |
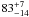
|
|
| W3OH.06 | VI | –122.187 | –1749.006 | 27.32 ± 0.05 | –42.33 | 0.21 | − | − | − | − | − | − | |
| W3OH.07 | VI | –153.206 | –1767.649 | 54.81 ± 0.34 | –42.60 | 0.20 | 1.9 ± 0.6 | 35 ± 14 |
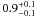
|
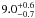
|
− |
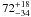
|
|
| W3OH.08 | VI | –149.964 | –1753.939 | 4.82 ± 0.04 | –42.24 | 0.19 | − | − | − | − | − | − | |
| W3OH.09 | VI | –132.122 | –1771.915 | 235.45 ± 0.33 | –42.60 | 0.19 | 2.5 ± 1.2 | 15 ± 33 |
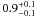
|
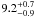
|
− |
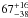
|
|
| W3OH.10e | VI | -131.591 | –1742.706 | 60.79 ± 0.34 | –42.60 | 0.18 | 5.3 ± 2.7 | −11 ± 7 |
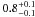
|
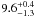
|
− |
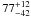
|
|
| W3OH.11 | VI | –123.541 | –1772.516 | 212.03 ± 0.33 | –42.60 | 0.22 | − | − | − | − | −3.5 ± 0.2 | − | |
| W3OH.12 | VI | –114.142 | –1773.373 | 111.69 ± 0.37 | –42.95 | 0.20 | − | − | − | − | − | − | |
| W3OH.13 | VI | –111.926 | –1747.892 | 22.93 ± 0.03 | –42.07 | 0.20 | 3.1 ± 0.3 | 5 ± 38 |
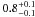
|
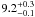
|
− |
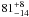
|
|
| W3OH.14e | IV | -59.565 | –1173.670 | 182.60 ± 0.64 | –43.65 | 0.26 | 5.9 ± 1.9 | −41 ± 44 |
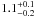
|
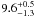
|
− |
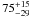
|
|
| W3OH.15 | II | –57.240 | –157.034 | 20.812 ± 0.59 | –44.79 | 0.33 | − | − | − | − | − | − | |
| W3OH.16 | IV | –46.725 | –1160.805 | 233.50 ± 0.38 | –42.95 | 0.32 | 3.9 ± 0.5 | −39 ± 86 |
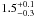
|
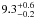
|
− |
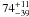
|
|
| W3OH.17e | IV | -42.741 | –1165.085 | 110.50 ± 0.29 | –43.12 | 0.25 | 5.6 ± 3.9 | −75 ± 41 |
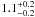
|
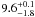
|
− |
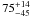
|
|
| W3OH.18e | IV | -40.408 | –1160.982 | 65.18 ± 0.49 | –43.12 | 0.29 | 5.0 ± 2.6 | 2 ± 85 |
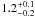
|
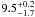
|
− |
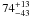
|
|
| W3OH.19 | II | –35.097 | –11.770 | 69.09 ± 0.62 | –44.79 | 0.25 | − | − | − | − | − | − | |
| W3OH.20 | II | –30.011 | –17.119 | 46.52 ± 0.72 | –44.70 | 0.25 | − | − | − | − | − | − | |
| W3OH.21 | IV | –29.630 | –1310.143 | 57.91 ± 0.52 | –43.91 | 0.31 | 1.5 ± 1.1 | 62 ± 49 |
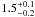
|
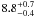
|
− |
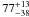
|
|
| W3OH.22e | II | 0 | 0 | 2051.30 ± 1.42 | –45.41 | 0.31 | 8.1 ± 0.7 | 4 ± 34 |
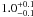
|
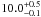
|
1.9 ± 0.1 |
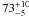
|
|
| W3OH.23 | II | 11.410 | –36.799 | 1099.80 ± 0.81 | –43.39 | 0.29 | 4.1 ± 1.2 | 2 ± 41 |
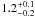
|
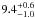
|
− |
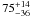
|
|
| W3OH.24 | II | 14.956 | –21.265 | 913.15 ± 0.92 | –44.53 | 0.37 | 4.4 ± 2.0 | −38 ± 41 |
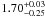
|
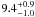
|
− |
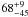
|
|
| W3OH.25 | IV | 15.969 | –1376.160 | 156.18 ± 0.65 | –43.74 | 0.21 | 1.5 ± 0.4 | 7 ± 23 |
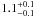
|
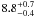
|
3.8 ± 0.5 |
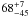
|
|
| W3OH.26 | II | 18.653 | –39.053 | 546.64 ± 0.63 | –43.65 | 0.21 | − | − | − | − | − | − | |
| W3OH.27 | II | 22.492 | –84.309 | 206.85 ± 0.36 | –43.03 | 0.18 | 1.8 ± 0.2 | 63 ± 35 |
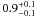
|
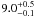
|
− |
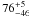
|
|
| W3OH.28 | II | 24.383 | –22.999 | 640.49 ± 0.70 | –44.70 | 0.29 | 4.1 ± 1.6 | −47 ± 19 |
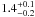
|
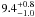
|
− |
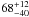
|
|
| W3OH.29 | II | 30.256 | –34.817 | 239.84 ± 0.56 | –44.09 | 0.27 | − | − | − | − | − | − | |
| W3OH.30 | II | 33.002 | –45.343 | 20.09 ± 0.63 | –43.56 | 0.26 | − | − | − | − | − | − | |
| W3OH.31 | II | 39.591 | –125.509 | 74.16 ± 0.50 | –43.30 | 0.19 | 1.2 ± 0.9 | 64 ± 29 |
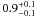
|
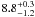
|
− |
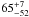
|
|
| W3OH.32 | II | 47.238 | –59.534 | 155.18 ± 0.36 | –43.03 | 0.18 | − | − | − | − | − | − | |
| W3OH.33 | II | 51.173 | –159.398 | 146.66 ± 0.36 | –43.03 | 0.26 | 4.1 ± 2.7 | 6 ± 42 |
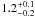
|
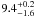
|
− |
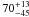
|
|
| W3OH.34 | II | 53.063 | –67.207 | 140.91 ± 0.98 | –44.53 | 0.40 | − | − | − | − | − | − | |
| W3OH.35 | II | 58.220 | –63.723 | 347.43 ± 0.37 | –42.86 | 0.19 | 3.2 ± 1.1 | 1 ± 37 |
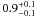
|
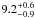
|
−1.5 ± 0.1 |
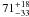
|
|
| W3OH.36 | II | 62.103 | –65.691 | 180.85 ± 0.70 | –44.35 | 0.37 | − | − | − | − | − | − | |
| W3OH.37 | II | 100.540 | –93.575 | 110.11 ± 0.28 | –42.51 | 0.31 | 3.2 ± 0.8 | 19 ± 53 |
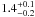
|
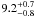
|
−1.7 ± 0.4 |
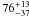
|
|
| W3OH.38 | II | 101.041 | 36.774 | 7.17 ± 0.02 | –46.37 | 0.25 | − | − | − | − | − | − | |
| W3OH.39 | II | 104.515 | –84.400 | 28.12 ± 0.05 | –42.33 | 0.27 | 2.7 ± 1.0 | −14 ± 6 |
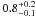
|
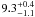
|
− |
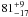
|
|
| W3OH.40 | I | 105.043 | 540.987 | 178.62 ± 0.45 | –45.14 | 0.22 | − | − | − | − | −3.4 ± 0.4 | − | |
| W3OH.41 | II | 106.053 | 32.572 | 19.21 ± 0.28 | –45.76 | 0.31 | 2.1 ± 0.3 | 63 ± 80 |
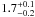
|
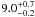
|
− |
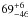
|
|
| W3OH.42 | II | 106.329 | 41.679 | 2.08 ± 0.01 | –46.99 | 0.44 | 4.4 ± 0.5 | 68 ± 5 | − | − | − | − | |
| W3OH.43 | II | 109.883 | 47.815 | 254.77 ± 0.69 | –44.35 | 0.24 | 2.6 ± 0.7 | 16 ± 27 |
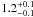
|
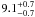
|
− |
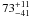
|
|
| W3OH.44 | I | 117.485 | 535.473 | 73.74 ± 0.44 | –45.05 | 0.17 | − | − | − | − | − | − | |
| W3OH.45 | I | 170.642 | 437.187 | 0.72 ± 0.01 | –47.07 | 0.18 | − | − | − | − | − | − | |
| W3OH.46 | V | 399.814 | –1735.970 | 4.32 ± 0.11 | –42.42 | 0.43 | − | − | − | − | − | − | |
| W3OH.47 | V | 423.769 | –1737.766 | 1.25 ± 0.02 | –41.89 | 0.28 | − | − | − | − | − | − | |
| W3OH.48 | V | 437.887 | –1731.754 | 19.97 ± 0.02 | –41.81 | 0.78 | 1.8 ± 0.3 | 4 ± 2 |
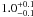
|
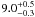
|
1.4 ± 0.2 |
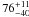
|
|
| W3OH.49 | V | 443.434 | –1710.760 | 15.71 ± 0.04 | –42.24 | 0.26 | − | − | − | − | − | − | |
| W3OH.50 | V | 450.607 | –1695.734 | 0.62 ± 0.02 | –41.72 | 0.27 | − | − | − | − | − | − | |
| W3OH.51 | V | 451.835 | –1673.563 | 4.46 ± 0.04 | –42.24 | 0.22 | 1.5 ± 0.2 | 22 ± 5 |
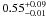
|
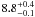
|
− |
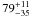
|
|
Notes.
(b)
The best-fitting results obtained by using a model based on the radiative transfer theory of methanol masers for Γ + Γν = 1 (Vlemmings et al. 2010; Surcis et al. 2011b). The errors were determined by analyzing the full probability distribution function.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.