Table A.1
Spectroscopic properties of the observed transitions.
| Transition | ncrit | Eu/kB |
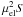
|
A | B | C |
| [cm-3] | [K] | [D2] | [MHz] | [MHz] | [MHz] | |
|
|
||||||
| SiO(5−4) | 2.3 × 106 | 31.3 | 48.14651 | ... | 21 711.96 | ... |
| DCN(3−2) | ~107 | 20.9 | 80.50709 | ... | 36 207.46 | ... |
| 13CO(2−1) | 8.9 × 103 | 15.9 | 0.02437 | ... | 55 101.01 | ... |
| C17O(2−1) | 9.5 × 103 | 16.2 | 0.02432 | ... | 56 179.99 | ... |
| CH3OH(5−1,5 − 4−1,4)-E | 9.5 × 105 | 40.4 | 3.88240 | 127 484.0 | 24 679.98 | 23 769.70 |
| CH3OH(50,5 − 40,4)-A+ | 1.1 × 106 | 34.8 | 4.04297 | − || − | − || − | − || − |
| CH3OH(54,∗ − 44,∗)-A+/−a | 3.5 × 105 | 115.2 | 1.45278 | − || − | − || − | − || − |
Notes. Column (2) gives the critical density of the transition,
ncrit = Aul/Cul,
where Aul is the spontaneous decay rate and
Cul is the collisional de-excitation rate. It was
calculated at T = 15 K using the collisional-rate data available
in the Leiden Atomic and Molecular Database (LAMDA; http://www.strw.leidenuniv.nl/~moldata/) (Schöier et al. 2005). For DCN, there is no molecular datafile
available, so we used the collisional rate coefficient of HCN to estimate
ncrit. Columns (3) and (4) give the upper-state
energy and the product 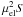 , where μel is the
permanent electric dipole moment, and S is the line strength.
Columns (5)–(7) list the rotational constants of the molecules. The data were
compiled from the Jet Propulsion Laboratory (JPL; Pickett et al. 1998) and CDMS (Müller
et al. 2005) databases.
, where μel is the
permanent electric dipole moment, and S is the line strength.
Columns (5)–(7) list the rotational constants of the molecules. The data were
compiled from the Jet Propulsion Laboratory (JPL; Pickett et al. 1998) and CDMS (Müller
et al. 2005) databases.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


