Table 1
Details of MCLZ and Demkov calculations.
| Exit | ΔE | Rx | ΔU(Rx) | Molecular | |
| Channelsa | (a.u.) | (a.u.) | (a.u.) | Symmetriesb | Type |
|
|
|||||
Ge0(3P) + H+(1S) ⇌ Ge+ + H(2S) + ΔE ( , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s2 4p 2P° | 0.2041 | 7.258 | 0.2089 |

|
I |
| 4s2 4p 2P° | 0.2041 | 7.406 | 0.1943 | 3Π | I |
|
|
|||||
Ge2+(1S) + H(2S) → Ge+ + H+(1S) + ΔE (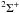 ) ) |
|||||
|
|
|||||
| 4s2 4p 2P° | 0.0805 | 12.48 | 5.922E-03 |
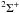
|
I |
|
|
|||||
Ge3+(2S) + H(2S) → Ge2+ + H+(1S) + ΔE ( , ,  ) ) |
|||||
|
|
|||||
| 4s 4p 3P° | 0.4633 | 4.648 | 0.2070 |

|
I |
| 4s 4p 1P° | 0.3333 | 6.240 | 7.592E-02 |

|
I |
|
|
|||||
Ge4+(1S) + H(2S) → Ge3+ + H+(1S) + ΔE (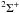 ) ) |
|||||
|
|
|||||
| 4p 2P° | 0.8013 | 4.138 | 0.2732 |
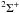
|
I |
| 4d 2D | 0.3111 | 9.768 | 5.463E-03 |
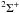
|
I |
| 5s 2S | 0.2724 | 11.11 | 1.847E-03 |
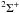
|
I |
|
|
|||||
Ge5+(2D) + H(2S) → Ge4+ + H+(1S) + ΔE ( , 1Π, 1Δ, , 1Π, 1Δ,  , 3Π, 3Δ) , 3Π, 3Δ) |
|||||
|
|
|||||
| 3d9 4d 3S | 0.7481 | 5.723 | 0.1071 |

|
I |
| 3d9 4d 1P | 0.7219 | 5.901 | 9.530E-02 | 1Π | I |
| 3d9 4d 3G | 0.7202 | 5.913 | 9.454E-02 |  , 3Π, 3Δ , 3Π, 3Δ |
I |
| 3d9 4d 3P | 0.7161 | 5.942 | 9.274E-02 | 3Π | I |
| 3d9 4d 3D | 0.7095 | 5.990 | 8.983E-02 |  , 3Π, 3Δ , 3Π, 3Δ |
I |
| 3d9 4d 1F | 0.6993 | 6.065 | 8.544E-02 | 1Π, 1Δ | I |
| 3d9 4d 1D | 0.6905 | 6.132 | 8.168E-02 |  , 1Π, 1Δ , 1Π, 1Δ |
I |
| 3d9 4d 3F | 0.6903 | 6.134 | 8.157E-02 | 3Π, 3Δ | I |
| 3d9 5s 3D | 0.5888 | 7.062 | 4.274E-02 |  , 3Π, 3Δ , 3Π, 3Δ |
I |
| 3d9 4d 1S | 0.5763 | 7.200 | 3.870E-02 |

|
I |
| 3d9 5s 3P | 0.5677 | 7.299 | 3.603E-02 | 3Π | I |
| 3d9 5p 3P° | 0.4370 | 9.313 | 7.827E-03 |  , 3Π , 3Π |
I |
| 3d9 5p 3F° | 0.4313 | 9.430 | 7.139E-03 |  , 3Π, 3Δ , 3Π, 3Δ |
I |
| 3d9 5p 1D° | 0.4277 | 9.506 | 6.724E-03 | 1Π, 1Δ | I |
| 3d9 5p 3D° | 0.4149 | 9.786 | 5.385E-03 | 3Π, 3Δ | I |
| 3d9 5p 1P° | 0.4136 | 9.815 | 5.262E-03 |  , 1Π , 1Π |
I |
| 3d9 5p 1F° | 0.4107 | 9.882 | 4.989E-03 |  , 1Π, 1Δ , 1Π, 1Δ |
I |
|
|
|||||
Se0(3P) + H+(1S) ⇌ Se+ + H(2S) + ΔE ( , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s2 4p34S° | 0.1413 | 7.006 | 0.1304 |

|
I |
| 4s2 4p32D° | 0.0796 | 7.860 | 7.958E-02 | 3Π | I |
| 4s2 4p32D° | 0.0796 | 8.020 | 7.240E-02 |

|
I |
| 4s2 4p32P° | 0.0338 | 9.251 | 3.396E-02 | 3Π | I |
|
|
|||||
Se2+(3P) + H(2S) → Se+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 4s2 4p32P° | 0.1714 | 6.040 | 8.688E-02 | 2Π | I |
|
|
|||||
Se3+(2P°) + H(2S) → Se2+ + H+(1S) + ΔE ( , 1Π, , 1Π,  , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s2 4p21S | 0.5034 | 4.256 | 0.2568 |

|
I |
| 4s 4p33D° | 0.2037 | 9.921 | 4.588E-05 | 3Π | II |
|
|
|||||
Se4+(1S) + H(2S) → Se3+ + H+(1S) + ΔE (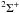 ) ) |
|||||
|
|
|||||
| 4s 4p22D | 0.6023 | 5.328 | 8.177E-03 |
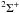
|
II |
| 4s 4p22S | 0.4917 | 6.374 | 2.706E-03 |
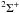
|
II |
| 4s2 4d 2D | 0.3793 | 8.088 | 2.010E-02 |
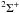
|
I |
| 4s2 5s 2S | 0.3620 | 8.452 | 1.525E-02 |
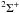
|
I |
| 4s2 5p 2P° | 0.2095 | 14.38 | 1.178E-04 |
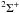
|
I |
|
|
|||||
Se5+(2S) + H(2S) → Se4+ + H+(1S) + ΔE ( , ,  ) ) |
|||||
|
|
|||||
| 4s 4d 1D | 1.0394 | 4.342 | 0.2453 |

|
I |
| 4s 4d 3D | 0.8359 | 5.209 | 0.1483 |

|
I |
| 4s 4d 3D | 0.7012 | 6.051 | 8.625E-02 |

|
I |
| 4s 4f 1F° | 0.3639 | 11.11 | 1.854E-03 |

|
I |
|
|
|||||
Br0(2P°) + H+(1S) ⇌ Br+ + H(2S) + ΔE (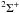 , 2Π) , 2Π) |
|||||
|
|
|||||
| 4s2 4p43P | 0.0589 | 7.241 | 6.159E-02 | 2Π | I |
| 4s2 4p41D | 0.0105 | 9.612 | 1.191E-02 | 2Π | I |
| 4s2 4p41D | 0.0105 | 10.05 | 8.681E-03 |
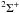
|
I |
|
|
|||||
Br2+(4S°) + H(2S) → Br+ + H+(1S) + ΔE ( , , 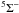 ) ) |
|||||
|
|
|||||
| 4s2 4p43P | 0.2947 | 3.576 | 0.3579 |

|
I |
|
|
|||||
Br3+(3P) + H(2S) → Br2+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 4s 4p42D | 0.4068 | 5.219 | 9.140E-03 | 2Π | II |
| 4s2 4p2 5s 4P | 0.1462 | 13.73 | 2.046E-04 |  , 4Π , 4Π |
I |
| 4s2 4p2 5s 2P | 0.1346 | 14.91 | 7.473E-05 |  , 2Π , 2Π |
I |
|
|
|||||
Br4+(2P°) + H(2S) → Br3+ + H+(1S) + ΔE ( , 1Π, , 1Π,  , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s 4p33D° | 0.5728 | 5.570 | 6.369E-03 | 3Π | II |
| 4s 4p33P° | 0.5091 | 6.176 | 3.352E-03 |  , 3Π , 3Π |
II |
| 4s 4p31D° | 0.5002 | 6.277 | 3.006E-03 | 1Π | II |
| 4s2 4p 4d 3F° | 0.4212 | 7.336 | 3.507E-02 |  , 3Π , 3Π |
I |
| 4s 4p31P° | 0.4157 | 7.426 | 8.421E-04 |  , 1Π , 1Π |
II |
| 4s2 4p 4d 3D° | 0.3800 | 8.074 | 2.031E-02 | 3Π | I |
| 4s2 4p 4d 3P° | 0.3468 | 8.803 | 1.165E-02 |  , 3Π , 3Π |
I |
| 4s2 4p 4d 1F° | 0.3448 | 8.852 | 1.121E-02 |  , 1Π , 1Π |
I |
| 4s2 4p 4d 1P° | 0.3301 | 9.227 | 8.373E-03 |  , 1Π , 1Π |
I |
| 4s2 4p 4d 1D° | 0.3245 | 9.380 | 7.426E-03 | 1Π | I |
| 4s2 4p 5s 3P° | 0.3180 | 9.564 | 6.422E-03 |  , 3Π , 3Π |
I |
| 4s2 4p 5s 1P° | 0.3035 | 10.04 | 4.526E-03 |  , 1Π , 1Π |
I |
| 4s2 4p 5p 3D | 0.1619 | 18.57 | 2.983E-06 |  , 3Π , 3Π |
I |
|
|
|||||
Br5+(1S) + H(2S) → Br4+ + H+(1S) + ΔE (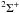 ) ) |
|||||
|
|
|||||
| 4s 4p22D | 1.1321 | 4.040 | 2.853E-02 |
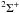
|
II |
| 4s2 4d 2D | 0.8360 | 5.209 | 0.1483 |
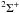
|
I |
| 4s2 5s 2S | 0.7214 | 5.904 | 9.511E-02 |
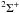
|
I |
|
|
|||||
Kr+(2P°) + H(2S) ⇌ Kr + H+(1S) + ΔE ( , 1Π, , 1Π,  , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s2 4p61S | 0.0147 | 8.394 | 1.595E-02 |

|
I |
|
|
|||||
Kr2+(3P) + H(2S) → Kr+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 4s2 4p52P° | 0.3873 | 2.677 | 0.4928 | 2Π | I |
|
|
|||||
Kr3+(4S°) + H(2S) → Kr2+ + H+(1S) + ΔE ( , , 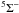 ) ) |
|||||
|
|
|||||
| 4s2 4p43P | 0.8485 | 2.577 | 0.5047 |

|
I |
| 4s2 4p3 4d 5D° | 0.2269 | 8.940 | 1.047E-02 |
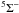
|
I |
| 4s2 4p3 5s 5S° | 0.1942 | 10.39 | 3.313E-03 |
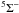
|
I |
| 4s2 4p3 4d 3D° | 0.1816 | 11.10 | 1.870E-03 |

|
I |
| 4s2 4p3 5s 3S° | 0.1675 | 12.01 | 8.767E-04 |

|
I |
| 4s2 4p3 4d 3G° | 0.1264 | 15.86 | 3.248E-05 |

|
I |
| 4s2 4p3 5s 3D° | 0.1099 | 18.23 | 4.025E-06 |

|
I |
|
|
|||||
Kr4+(3P) + H(2S) → Kr3+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 4s 4p44P | 0.8787 | 3.834 | 3.429E-02 |  , 4Π , 4Π |
II |
| 4s2 4p2 4d 2Pa | 0.6847 | 4.775 | 0.1924 |  , 2Π , 2Π |
I |
| 4s2 4p2 4d 4F | 0.6265 | 5.155 | 0.1533 |  , 4Π , 4Π |
I |
| 4s2 4p2 4d 4D | 0.6030 | 5.328 | 0.1378 | 4Π | I |
| 4s2 4p2 4d 2Fa | 0.5937 | 5.400 | 0.1317 |  , 2Π , 2Π |
I |
| 4s 4p42P | 0.5532 | 5.744 | 5.308E-03 |  , 2Π , 2Π |
II |
| 4s2 4p2 4d 4P | 0.5079 | 6.193 | 7.838E-02 |  , 4Π , 4Π |
I |
| 4s2 4p2 5s 4P | 0.4901 | 6.394 | 6.834E-02 |  , 4Π , 4Π |
I |
| 4s2 4p2 4d 2Da | 0.4719 | 6.615 | 5.864E-02 | 2Π | I |
| 4s2 4p2 5s 2P | 0.4691 | 6.650 | 5.723E-02 |  , 2Π , 2Π |
I |
| 4s2 4p2 4d 2Db | 0.4386 | 7.069 | 4.253E-02 | 2Π | I |
| 4s2 4p2 4d 2Pb | 0.4257 | 7.266 | 3.690E-02 |  , 2Π , 2Π |
I |
| 4s2 4p2 4d 2Fb | 0.4240 | 7.293 | 3.618E-02 |  , 2Π , 2Π |
I |
| 4s2 4p2 5s 2D | 0.4124 | 7.482 | 3.152E-02 | 2Π | I |
| 4s2 4p2 4d 2Dc | 0.3710 | 8.259 | 1.766E-02 | 2Π | I |
| 4s2 4p2 5p 2S° | 0.3595 | 8.508 | 1.461E-02 |

|
I |
| 4s2 4p2 5p 4D° | 0.3289 | 9.260 | 8.159E-03 |  , 4Π , 4Π |
I |
| 4s2 4p2 5p 4P° | 0.3203 | 9.498 | 6.766E-03 | 4Π | I |
| 4s2 4p2 5p 4S° | 0.3058 | 9.931 | 4.797E-03 |

|
I |
4s2 4p2 5p 2D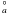 |
0.3036 | 10.00 | 4.540E-03 |  , 2Π , 2Π |
I |
4s2 4p2 5p 2P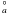 |
0.2884 | 10.51 | 3.011E-03 | 2Π | I |
| 4s2 4p2 5p 2F° | 0.2543 | 11.88 | 9.761E-04 | 2Π | I |
4s2 4p2 5p 2D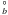 |
0.2512 | 12.03 | 8.658E-04 |  , 2Π , 2Π |
I |
4s2 4p2 5p 2P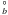 |
0.2235 | 13.49 | 2.522E-04 | 2Π | I |
|
|
|||||
Kr5+(2P°) + H(2S) → Kr4+ + H+(1S) + ΔE ( , 1Π, , 1Π,  , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s2 4p 4d 3P° | 0.9083 | 4.841 | 0.1851 |  , 3Π , 3Π |
I |
| 4s2 4p 4d 3D° | 0.8781 | 4.998 | 0.1687 | 3Π | I |
| 4s2 4p 4d 1F° | 0.8110 | 5.344 | 0.1364 |  , 1Π , 1Π |
I |
| 4s2 4p 4d 1P° | 0.7946 | 5.437 | 0.1287 |  , 1Π , 1Π |
I |
| 4s2 4p 5s 3P° | 0.7633 | 5.619 | 0.1146 |  , 3Π , 3Π |
I |
| 4s2 4p 5s 1P° | 0.7342 | 5.816 | 0.1008 |  , 1Π , 1Π |
I |
| 4s2 4p 5p 1P | 0.5858 | 7.094 | 4.177E-02 | 1Π | I |
| 4s2 4p 5p 3D | 0.5761 | 7.202 | 3.865E-02 |  , 3Π , 3Π |
I |
| 4s2 4p 5p 3P | 0.5554 | 7.446 | 3.237E-02 | 3Π | I |
| 4s2 4p 5p 3S | 0.5395 | 7.646 | 2.794E-02 |

|
I |
| 4s2 4p 5p 1D | 0.5264 | 7.822 | 2.452E-02 |  , 1Π , 1Π |
I |
| 4s2 4p 5p 1S | 0.4759 | 8.592 | 1.370E-02 |

|
I |
|
|
|||||
Rb0(2S) + H+(1S) ⇌ Rb+ + H(2S) + ΔE (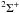 ) ) |
|||||
|
|
|||||
| 4s2 4p61S | 0.3462 | 10.46 | 0.3332 |
|
I |
|
|
|||||
Rb2+(2P°) + H(2S) → Rb+ + H+(1S) + ΔE ( , 1Π, , 1Π,  , 3Π) , 3Π) |
|||||
|
|
|||||
| 4s2 4p61S | 0.5031 | 2.175 | 0.5374 |

|
I |
|
|
|||||
Rb3+(3P) + H(2S) → Rb2+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 4s2 4p4 4d 4D | 0.2275 | 8.917 | 1.066E-02 | 4Π | I |
| 4s2 4p4 4d 4F | 0.1798 | 11.20 | 1.710E-03 |  , 4Π , 4Π |
I |
| 4s2 4p4 5s 2D | 0.1629 | 12.34 | 6.637E-04 | 2Π | I |
| 4s2 4p4 4d 2P | 0.1593 | 12.62 | 5.269E-04 |  , 2Π , 2Π |
I |
| 4s2 4p4 4d 4P | 0.1559 | 12.89 | 4.192E-04 |  , 4Π , 4Π |
I |
| 4s2 4p4 4d 2D | 0.1463 | 13.73 | 2.062E-04 | 2Π | I |
| 4s2 4p4 4d 2F | 0.1428 | 14.06 | 1.552E-04 |  , 2Π , 2Π |
I |
| 4s2 4p4 5s 2P | 0.1261 | 15.90 | 3.142E-05 |  , 2Π , 2Π |
I |
| 4s2 4p4 4d 2D | 0.1219 | 16.45 | 1.950E-05 | 2Π | I |
|
|
|||||
Rb4+(4S°) + H(2S) → Rb3+ + H+(1S) + ΔE ( , , 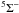 ) ) |
|||||
|
|
|||||
| 4s2 4p3 4d 5D° | 0.6392 | 5.043 | 0.1642 |
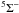
|
I |
4s2 4p3 4d 3D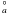 |
0.5865 | 5.458 | 0.1270 |

|
I |
| 4s2 4p3 4d 3F° | 0.5553 | 5.724 | 0.1070 |

|
I |
| 4s2 4p3 4d 3G° | 0.5237 | 6.027 | 8.764E-02 |

|
I |
| 4s2 4p3 5s 5S° | 0.4923 | 6.364 | 6.976E-02 |
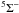
|
I |
| 4s2 4p3 5s 3S° | 0.4619 | 6.744 | 5.357E-02 |

|
I |
4s2 4p3 4d 3D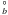 |
0.4225 | 7.316 | 3.558E-02 |

|
I |
| 4s2 4p3 5s 3D° | 0.3955 | 7.778 | 2.534E-02 |

|
I |
| 4s2 4p3 4d 3S° | 0.3935 | 7.815 | 2.465E-02 |

|
I |
4s2 4p3 4d 3D |
0.3931 | 7.822 | 2.452E-02 |

|
I |
| 4s2 4p3 5p 5P | 0.3123 | 9.732 | 5.622E-03 |
|
I |
| 4s2 4p3 5p 3Pa | 0.2901 | 10.45 | 3.161E-03 |

|
I |
| 4s2 4p3 5p 3F | 0.2189 | 13.77 | 1.988E-04 |

|
I |
| 4s2 4p3 5p 3Pb | 0.1942 | 15.50 | 4.465E-05 |

|
I |
|
|
|||||
Rb5+(3P) + H(2S) → Rb4+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 4s 4p44P | 1.3832 | 3.388 | 4.999E-02 |  , 4Π , 4Π |
II |
| 4s 4p42D | 1.2509 | 3.706 | 3.833E-02 | 2Π | II |
| 4s 4p42P | 1.1465 | 3.996 | 2.969E-02 |  , 2Π , 2Π |
II |
| 4s2 4p2 5s 4P | 0.7897 | 5.465 | 0.1264 |  , 4Π , 4Π |
I |
| 4s2 4p2 5s 2P | 0.7817 | 5.513 | 0.1226 |  , 2Π , 2Π |
I |
| 4s2 4p2 5s 2D | 0.7110 | 5.979 | 9.049E-02 | 2Π | I |
|
|
|||||
Xe0(1S) + H+(1S) ⇌ Xe+ + H(2S) + ΔE ( ) ) |
|||||
|
|
|||||
| 5s2 5p52P° | 0.0380 | 7.933 | 3.515E-02 |

|
I |
|
|
|||||
Xe2+(3P) + H(2S) → Xe+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 5s2 5p52P° | 0.2551 | 4.078 | 0.2817 |  , 2Π , 2Π |
I |
|
|
|||||
Xe3+(4S°) + H(2S) → Xe2+ + H+(1S) + ΔE ( , ,  ) ) |
|||||
|
|
|||||
| 5s2 5p3 5d 5D° | 0.1307 | 15.35 | 5.097E-05 |
|
I |
|
|
|||||
Xe4+(3P) + H(2S) → Xe3+ + H+(1S) + ΔE ( , 2Π, , 2Π,  , 4Π) , 4Π) |
|||||
|
|
|||||
| 5s 5p44P | 0.5315 | 5.947 | 4.283E-03 |  , 4Π , 4Π |
II |
| 5s 5p42D | 0.4386 | 7.068 | 1.259E-03 | 2Π | II |
| 5s2 5p2 5d 2Pa | 0.3920 | 7.843 | 2.414E-02 |  , 2Π , 2Π |
I |
| 5s2 5p2 5d 4F | 0.3608 | 8.479 | 1.494E-02 |  , 4Π , 4Π |
I |
| 5s2 5p2 5d 2Fa | 0.3494 | 8.741 | 1.222E-02 |  , 2Π , 2Π |
I |
| 5s2 5p2 5d 4D | 0.3172 | 9.587 | 6.306E-03 | 4Π | I |
| 5s2 5p2 5d 4P | 0.2713 | 11.15 | 1.781E-03 |  , 4Π , 4Π |
I |
| 5s2 5p2 5d 2G | 0.2647 | 11.43 | 1.425E-03 | 2Π | I |
| 5s2 5p2 6s 4P | 0.2450 | 12.32 | 6.749E-04 |  , 4Π , 4Π |
I |
| 5s2 5p2 5d 2Da | 0.2437 | 12.39 | 6.397E-04 | 2Π | I |
| 5s2 5p2 6s 2P | 0.2237 | 13.48 | 2.548E-04 |  , 2Π , 2Π |
I |
| 5s2 5p2 5d 2Fb | 0.1969 | 15.29 | 5.361E-05 |  , 2Π , 2Π |
I |
| 5s2 5p2 5d 2Db | 0.1935 | 15.55 | 4.253E-05 | 2Π | I |
| 5s2 5p2 5d 2Pb | 0.1867 | 16.12 | 2.605E-05 |  , 2Π , 2Π |
I |
| 5s2 5p2 4f 4G° | 0.1645 | 18.27 | 3.870E-06 |  , 4Π , 4Π |
I |
|
|
|||||
Xe5+(2P°) + H(2S) → Xe4+ + H+(1S) + ΔE ( , 1Π, , 1Π,  , 3Π) , 3Π) |
|||||
|
|
|||||
| 5s2 5p 4f 3D | 0.9346 | 4.745 | 0.1958 |  , 3Π , 3Π |
I |
| 5s2 5p 4f 1D | 0.8902 | 4.942 | 0.1744 |  , 1Π , 1Π |
I |
| 5s2 5p 6p 3D | 0.7893 | 5.468 | 0.1262 |  , 3Π , 3Π |
I |
| 5s2 5p 6p 3P | 0.7835 | 5.502 | 0.1235 | 3Π | I |
| 5s2 5p 6p 1P | 0.7696 | 5.586 | 0.1170 | 1Π | I |
| 5s2 5p 6p 3S | 0.7487 | 5.719 | 0.1074 |

|
I |
| 5s2 5p 6p 1D | 0.7361 | 5.803 | 0.1017 |  , 1Π , 1Π |
I |
| 5s2 5p 6p 1S | 0.6947 | 6.100 | 8.346E-02 |

|
I |
Notes. The table includes exit channels, molecular symmetries, energy defects ΔE, avoided crossing or interaction distances Rx, and the energy separations of the adiabatic potentials ΔU(Rx). The molecular symmetries of each entrance channel is given in parentheses after the reaction.
Exit channels refer to states in the resulting ion after CT with H or H+. Only information about electrons in the valence principal quantum number shell is given. Some terms are given a lettered sub-index (e.g., 2Da) if multiple terms with the same configuration and orbital and spin angular momenta are exoergic exit channels. Note that exit channels with rate coefficients smaller than 10-14 cm3 s-1 (the canonical radiative CT rate) in the temperature range 102–106 K are not listed, unless no exoergic exit channels with larger rate coefficients exist.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


