| Issue |
A&A
Volume 496, Number 2, March III 2009
|
|
|---|---|---|
| Page(s) | 365 - 374 | |
| Section | Cosmology (including clusters of galaxies) | |
| DOI | https://doi.org/10.1051/0004-6361:200811302 | |
| Published online | 14 January 2009 | |
HD and H2 formation in low-metallicity dusty gas clouds at high redshift
S. Cazaux - M. Spaans
Kapteyn Astronomical Institute, PO box 800, 9700 AV Groningen, The Netherlands
Received 6 November 2008 / Accepted 19 December 2008
Abstract
Context. HD and H2 molecules play important roles in the cooling of primordial and very metal-poor gas at high redshift.
Aims. Grain surface and gas phase formation of HD and H2 are investigated to assess the importance of trace amounts of dust,
![]() ,
in the production of HD and H2.
,
in the production of HD and H2.
Results. We consider carbonaceous and silicate grains and include both physisorption and chemisorption, tunneling, and realistic grain surface barriers. We find that, for a collapsing gas cloud environment with coupled chemical and thermal balance, dust abundances as small as 10-5 solar lead to a strong boost in the H2 formation rate due to surface reactions. As a result of this enhancement in H2, HD is formed more efficiently in the gas phase through the D+ + H2 reaction. Direct formation of HD on dust grains cannot compete well with this gas phase process for dust temperatures below 150 K. We also derive up-to-date analytic fitting formulae for the grain surface formation of H2 an HD, including the different binding energies of H and D.
Conclusions. Grain surface reactions are crucial to the availability of H2 and HD in very metal-poor environments. Above metallicities of 10-5 solar, the grain surface route dominates the formation of H2, which in turn drives the formation of HD in the gas phase. At dust temperatures above 150 K, laboratory experiments and theoretical modeling suggest that H2 formation on grains is suppressed while HD formation on grains is not.
Key words: ISM: dust, extinction - ISM: molecules - galaxies: high-redshift
1 Introduction
The chemistry occurring in primordial gas in the early universe
involves mainly hydrogen, deuterium, helium and lithium, as well as
their ionic forms (Glover & Abel 2008; Ripamonti 2007; Yoshida et al. 2006; Galli & Palla 1998). The formation of the first stars
crucially depends on the availability of specific molecular coolants
like H2 and HD. The molecule H2 allows gas to cool down to a few
hundred Kelvin, because its first rotationally excited state lies at
about 500 K. The molecule HD, which unlike H2 possesses a small
dipole moment, allows cooling to below 100 K, given that its first
excited state lies at about 150 K. Simulations that incorporate
non-equilibrium chemistry and cooling of primordial gas in the early
universe (e.g., Abel et al. 2000;
Bromm et al. 2002; Abel et al. 2007; Wise et al. 2007,
2008; Klessen et al. 2007; Jappsen et al. 2007) find that these molecules are instrumental in the collapse
of clouds in young galaxies and the formation of the first stars.
Once the first stars were formed and ionized the universe, the next
generation of stars could be formed from more ionized gas. It is generally
thought that the final masses of the first stars are a few dozen to a
hundred solar masses (Johnson & Bromm 2006; Yoshida et al. 2007).
In this, an increased electron abundance boosts the formation
of H2 and lowers the gas temperature to below 200 K, relative to less
ionized gas. Subsequently, HD can become the dominating coolant and be
more important than the cooling due to H2. Recent work by McGreer & Bryan (2008) on zero metallicity gas shows that HD cooling is dominant for halos with masses below 105.5 ![]() ,
yielding stars that are 6 times less massive. Also, HD cooling
in ionized halos is most effective for a density range between
102-106 cm-3, while above this range H2 cooling dominates
again. In all, it is important to determine the chemical composition of collapsing clouds with
density, in order to establish which coolant (H2, HD, other)
dominates and which mass of star results from gravitational collapse.
,
yielding stars that are 6 times less massive. Also, HD cooling
in ionized halos is most effective for a density range between
102-106 cm-3, while above this range H2 cooling dominates
again. In all, it is important to determine the chemical composition of collapsing clouds with
density, in order to establish which coolant (H2, HD, other)
dominates and which mass of star results from gravitational collapse.
The molecules H2 and HD can be formed in the gas phase at zero
metallicity. For HD, the dominant reactions are
D+ + H2
![]() HD + H+ (exothermic) for its formation, and
H+ + HD
HD + H+ (exothermic) for its formation, and
H+ + HD
![]() H2 + D+ (-962 K) for its destruction (Glover &
Abel 2008). For H2, one has H- + H
H2 + D+ (-962 K) for its destruction (Glover &
Abel 2008). For H2, one has H- + H
![]() H2 + e.
Additional relevant reactions are H- + D
H2 + e.
Additional relevant reactions are H- + D
![]() HD + e,
D- + H
HD + e,
D- + H
![]() HD + e, and D+ + H2
HD + e, and D+ + H2
![]() HD + H+.
These reactions typically proceed during free-fall collapse, i.e.,
in a time-dependent environment. Since the gas starts out at temperatures
of about 103 K or more prior to collapse, HD destruction is efficient.
As the H2 abundance increases with time, so does the cooling rate and
the temperature (and the H+ abundance) drops, stimulating the
presence of HD.
However, once some pollution by metals has occurred, i.e., after the
very first stars have exploded as supernovae (SNe), dust grains can also be
present in the ambient interstellar medium (ISM), see work by
Todini & Ferrara (2001) and Bianchi & Schneider (2007). The presence
of dust at high redshift, as observed toward a QSO at
HD + H+.
These reactions typically proceed during free-fall collapse, i.e.,
in a time-dependent environment. Since the gas starts out at temperatures
of about 103 K or more prior to collapse, HD destruction is efficient.
As the H2 abundance increases with time, so does the cooling rate and
the temperature (and the H+ abundance) drops, stimulating the
presence of HD.
However, once some pollution by metals has occurred, i.e., after the
very first stars have exploded as supernovae (SNe), dust grains can also be
present in the ambient interstellar medium (ISM), see work by
Todini & Ferrara (2001) and Bianchi & Schneider (2007). The presence
of dust at high redshift, as observed toward a QSO at
![]() (Maiolino et al. 2004), requires efficient condensation of grains in
SN ejecta. Models of dust formation in ejecta of SNe (Bianchi &
Schneider 2007; Todini & Ferrara 2001) present the grain size
distribution of silicates, amorphous carbon (AC), magnetite and
corundum; and show that the largest grains are the AC ones, with sizes
around 300 Å, whereas the other grain types have smaller radii,
around 10-20 Å. Once the first grains are produced by SNe, they will
influence the next generation of stars as some species will start to
form on dust grains. Consequently, additional pathways for H2 and
HD formation open up.
(Maiolino et al. 2004), requires efficient condensation of grains in
SN ejecta. Models of dust formation in ejecta of SNe (Bianchi &
Schneider 2007; Todini & Ferrara 2001) present the grain size
distribution of silicates, amorphous carbon (AC), magnetite and
corundum; and show that the largest grains are the AC ones, with sizes
around 300 Å, whereas the other grain types have smaller radii,
around 10-20 Å. Once the first grains are produced by SNe, they will
influence the next generation of stars as some species will start to
form on dust grains. Consequently, additional pathways for H2 and
HD formation open up.
Efforts that aim to include both gas and grain surface reactions
through an equation of state analysis can be found in Omukai et al. (2005) and Spaans & Silk (2005). In this, the pressure Pis usually assumed to follow the polytropic form
![]() ,
for the mass density
,
for the mass density ![]() and polytropic exponent
and polytropic exponent ![]() .
More detailed hydrodynamical
simulations of gas phase chemistry only can be found in Smith et al.
(2008), while first steps to include some aspects of grain chemistry
can be found in Glover & Jappsen (2007). All these works show that
the interplay between gravity and thermodynamics acts to a large
degree through the amount of fragmentation, through the Jeans
mass, that a collapsing gas cloud
experiences, thus setting the typical masses of stars. In this, the
occurrence of fragmentation follows from whether the gas temperature
rises or decreases under compression. I.e., whether
.
More detailed hydrodynamical
simulations of gas phase chemistry only can be found in Smith et al.
(2008), while first steps to include some aspects of grain chemistry
can be found in Glover & Jappsen (2007). All these works show that
the interplay between gravity and thermodynamics acts to a large
degree through the amount of fragmentation, through the Jeans
mass, that a collapsing gas cloud
experiences, thus setting the typical masses of stars. In this, the
occurrence of fragmentation follows from whether the gas temperature
rises or decreases under compression. I.e., whether ![]() is
larger of smaller than unity. This change in temperature under compression
can be a strong function of density for
0.1<n<1017 cm-3, and
depends on the ambient metallicity (Omukai et al. 2005;
Spaans & Silk 2005). The aim of this work is then to investigate
the importance of HD and H2 formation on grain surfaces and their
influence on gas thermodynamics, and to provide analytic fits for
H2 and HD formation on grains that are easy to implement in
cosmological simulations of early structure formation.
is
larger of smaller than unity. This change in temperature under compression
can be a strong function of density for
0.1<n<1017 cm-3, and
depends on the ambient metallicity (Omukai et al. 2005;
Spaans & Silk 2005). The aim of this work is then to investigate
the importance of HD and H2 formation on grain surfaces and their
influence on gas thermodynamics, and to provide analytic fits for
H2 and HD formation on grains that are easy to implement in
cosmological simulations of early structure formation.
2 Grain surface chemistry
2.1 Formation efficiencies of H2 and HD on dust surfaces
In a previous paper (Cazaux et al. 2008), we discussed the formation of H2 and HD on surfaces that are typical of the ISM. We found that the formation of molecules depends on the binding energy of atoms with the surface and on the barrier that atoms from the gas phase have to cross in to become strongly bound to the surface. Indeed, there are two interactions between the atoms and the surface: a weak one, called physisorption (Van der Waals interaction), and a strong one, called chemisorption (covalent bond), as represented in Fig. 1. Atoms on the grain surface can move from site to site by tunneling effects and thermal hopping. Atoms from the gas phase can access easily the physisorbed sites and become physisorbed atoms. These weakly bound atoms can scout the surface at very low dust temperatures, and can meet each other to form molecules. Once the dust temperature becomes higher, the physisorbed atoms evaporate and the formation of molecules is insured by the contribution of strongly bound (chemisorbed) atoms. Depending on the magnitude of the barrier that needs to be crossed to access the chemisorbed sites, a fraction of physisorbed atoms can enter the chemisorbed sites and meet an already chemisorbed atom to form molecules, but, if the barrier is very high, atoms from the gas phase, which have higher energy, cross the barrier to enter directly into chemisorbed sites and form molecules. These processes allow molecules to form for a wide range of dust grain temperatures (Cazaux et al. 2008; Cazaux & Tielens 2002; Cazaux & Spaans 2004).
We have developed a rate equations method to describe the chemistry
occurring on interstellar dust grains. This method follows the
populations of the different species on the grain (physisorbed H, D,
H2 and HD and chemisorbed H and D). The different processes that can
occur in this model are the following: 1) atoms from the gas phase
accrete into a physisorbed or chemisorbed site; 2) physisorbed atoms
go to another physisorbed site, or to a chemisorbed site, or evaporate; 3)
chemisorbed atoms evaporate. The mechanisms to form molecules are
either through the Langmuir-Hinshelwood kinetic (an atom on the
surface moves into an already occupied site) or the Eley-Rideal
kinetic (an atom from the gas phase arrives in an occupied site).
With this method, we obtain the efficiencies of the formation of H2 and HD on interstellar dust grains (note that D2 has been treated
in Cazaux et al. (2008), and is not studied here). These efficiencies,
as function of dust grain temperature, are reported in Fig. 2. The formation of H2 and HD is very efficient on all
types of grains at low temperatures (![]() 20 K), because it involves
physisorbed atoms. At higher dust temperatures, the chemisorbed atoms
become relevant. Depending on the barrier against chemisorption, the
physisorbed atoms may be able to enter chemisorbed sites, as is the
case for an amorphous carbon surface (no barrier), or the physisorbed
atoms evaporate before entering the chemisorbed sites, as is the case
for silicates and graphitic surfaces (high barrier). In the latter
case, the formation of molecules is insured by atoms that come from
the gas phase and enter directly into chemisorbed sites. Consequently,
for intermediate dust temperatures (
20 K), because it involves
physisorbed atoms. At higher dust temperatures, the chemisorbed atoms
become relevant. Depending on the barrier against chemisorption, the
physisorbed atoms may be able to enter chemisorbed sites, as is the
case for an amorphous carbon surface (no barrier), or the physisorbed
atoms evaporate before entering the chemisorbed sites, as is the case
for silicates and graphitic surfaces (high barrier). In the latter
case, the formation of molecules is insured by atoms that come from
the gas phase and enter directly into chemisorbed sites. Consequently,
for intermediate dust temperatures (![]() 20 K), the efficiency
decreases as the barrier against chemisorption increases.
20 K), the efficiency
decreases as the barrier against chemisorption increases.
![\begin{figure}
\par\includegraphics[width=8cm,clip]{1302fig1.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg17.gif) |
Figure 1:
Interactions between an atom and a surface. Two types of surfaces
are
represented, the one with a high barrier against chemisorption (where
|
| Open with DEXTER | |
![\begin{figure}
\par\mbox{\includegraphics[width=5.3cm]{1302fig2.eps}\includegrap...
...=5.3cm]{1302fig3.eps}\includegraphics[width=5.3cm]{1302fig4.eps} }\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg18.gif) |
Figure 2: H2 and HD formation efficiencies on amorphous carbon ( left), silicates ( middle) and PAHs ( right) as a function of the grain temperature. These efficiencies have been calculated using a rate equations method. |
| Open with DEXTER | |
| |
Figure 3: H2 (solid) and HD (dashed-dot) formation efficiencies on amorphous carbon ( left), silicates ( middle) and PAHs ( right) as a function of the grain temperature. These efficiencies have been calculated using Monte Carlo simulations. For the case of PAHs, the properties of para-sites have been included, resulting in a much higher efficiency. |
| Open with DEXTER | |
In the ISM, dust grains are mainly carbonaceous particles or silicates, with various sizes, and a large fraction of the available surface for chemistry is in the form of very small grains or polycyclic aromatic hydrocarbons (PAHs; Weintgarner & Draine 2001). Small carbon grains exist mostly in the form of PAHs, while big carbon grains occur as amorphous carbon. These two types of grains have different surface properties, as discussed in Cazaux et al. (2008). PAHs have surfaces similar to graphite, that consist in hexagonally arranged carbon atoms. Atoms that are coming on these surfaces can be placed in these hexagonal structures in different configurations. Ortho refers to two neighboring atoms, meta refers to two atoms which are separated, but not opposed and para refers to two opposed atoms. Recent discoveries show that the properties of PAHs and graphite surfaces depend on the presence of atoms on the surfaces. Indeed, once an atom becomes chemisorbed on the surface, it has to cross a high barrier of 0.2 eV (Hornekær et al. 2006; Sha & Jackson 2002; Jeloaica & Sidis 1999). A second atom can become chemisorbed in a para-site without a barrier (Hornekær et al. 2006; Rougeau et al. 2006) and a third atom will form a molecule without a barrier (Bachellerie et al. 2007). Because the formation of molecules on PAHs cannot be described by rate equations, since the surface characteristics change when atoms are present on the surface, we developed a Monte Carlo method to follow the formation of H2 and HD on these very small grains. Also, for silicates and amorphous carbon grains, which are bigger grains, this Monte Carlo method can be applied and compared to rate equations methods. In our model, we represent the grain by a square grid, with at each point a physisorbed or a chemisorbed site. A list of events is first calculated to determine the accretion of H and D on the grain. These times depend on the flux of H or D that arrives on the grain, and we select randomly a time for each event following a Poisson distribution. Then, once the atoms have arrived on the grain, the list is updated taking into account the different events that can occur.
The results of our Monte Carlo simulations for graphitic surfaces
(PAHs), with the inclusion of the para-site properties, are reported
in Fig. 3, right panel. The efficiencies of H2 and HD are
enhanced by a few orders of magnitude in comparison to a graphitic
surface that does not change its properties in the presence of atoms
on its surface. We consider here a very small grid of 30 Å length,
and also present, for comparison, the efficiencies of H2 and HD on
amorphous carbon and silicates. For these surfaces, the results are
similar to the ones computed with rate equations. This is due to the
fact that, as discussed in Cazaux et al. (2008), HD formation changes
with grain size only for high D/H ratios, and only at very low
temperatures, when physisorbed atoms are involved in the formation of
molecules. Only D2 formation is very sensitive to grain size
changes. So because we focus our study here on the formation of H2 and HD, the changes in efficiency with grain size can be neglected as
long as the D/H ratio remains small. As a general conclusion, the H2 and HD formation efficiencies are similar on graphitic surfaces (with
para-site properties) and on amorphous carbon grains. The efficiencies
on silicate surfaces, on the other hand, are very different because of
the high barrier against chemisorption. The efficiencies for the
formation of molecule are the rate of molecules that form on the
surface divided by the incoming flux of atoms. The flux of incoming
atoms depends on the velocity of the atoms in the gas phase
![]() ,
the sticking coefficient
,
the sticking coefficient
![]() ,
the number density of the atoms
,
the number density of the atoms
![]() ,
and the cross section of the dust grain as
,
and the cross section of the dust grain as
![]() ,
where (at) can be H or D. The efficiencies for the
formation of H2 and HD are presented in Fig. 3 and can be
written as follows:
,
where (at) can be H or D. The efficiencies for the
formation of H2 and HD are presented in Fig. 3 and can be
written as follows:
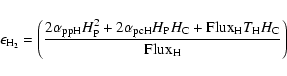 |
(1) |
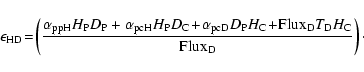 |
(2) |
These equations show the principal mechanisms for the formation of molecules at different grain temperatures: 1) at low surface temperatures Td, an H (D) atom moves to a filled physisorbed site with a mobility
Under steady state conditions,
![]() ,
,
![]() ,
,
![]() and
and
![]() .
.
The formation of H2 and HD, due to the association of physisorbed and
chemisorbed atoms at low surface temperature, and to the association of
gas phase atoms arriving in chemisorbed sites at high surface
temperature, can be approximated as:
| |
= | ||
| = | 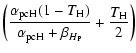 |
(3) | |
| = | |||
| = | 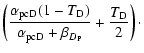 |
(4) |
The different mobilities of the atoms to go from a physisorbed site to a chemisorbed site (see Fig. 1 for the meaning of the different parameters) can be approximated as follows:
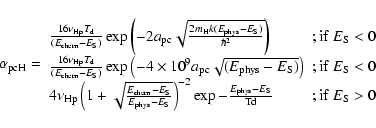
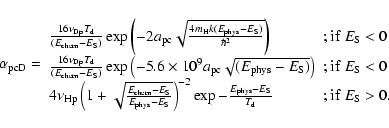
The first two expressions for
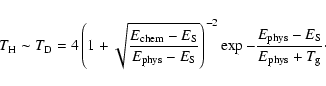 |
(5) |
The different rates for an H and an D atom to evaporate from a physisorbed site are:
![\begin{figure}
\par\includegraphics[width=8.5cm,clip]{1302fig8.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg62.gif) |
Figure 4:
Probability for an atom in the gas phase with a temperature
|
| Open with DEXTER | |
For carbonaceous grains (PAHs and amorphous carbon), the formation of
H2 and HD is insured by physisorbed atoms populating chemisorbed
sites (for temperatures ![]() 20 K). In this sense, the formation with
direct chemisorption from the gas phase is negligible. The term
20 K). In this sense, the formation with
direct chemisorption from the gas phase is negligible. The term
![]() can therefore be ignored and the H2 and HD formation
efficiencies can be approximated as follows:
can therefore be ignored and the H2 and HD formation
efficiencies can be approximated as follows:
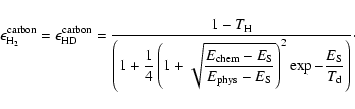 |
(6) |
For silicate grains, the efficiencies are different due to the fact that chemisorbed sites are populated by the tunneling effect, and therefore are less easy to access. Also, because
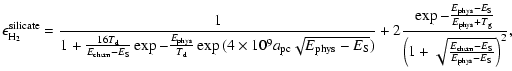 |
(7) |
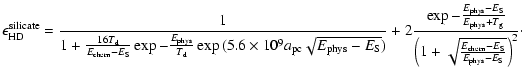 |
(8) |
2.2 Formation rates of H2 and HD on dust surfaces
In astrophysical environments, H2 and HD formation rates (in
cm-3 s-1) on dust grains are written as:
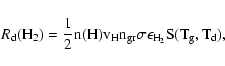 |
(9) |
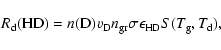 |
(10) |
where
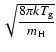 and
and
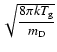 ,
,
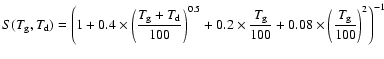 .
The sticking coefficient is equal to unity for low gas and dust
temperatures, and decreases strongly as gas temperature increases. The
mean cross section for collisions between grains and atoms per H atom,
.
The sticking coefficient is equal to unity for low gas and dust
temperatures, and decreases strongly as gas temperature increases. The
mean cross section for collisions between grains and atoms per H atom,
| (11) |
| (12) |
In the high redshift universe, grain size distributions are different than in our Milky Way, and at lower metallicity, grains are supposed to be smaller. Models of grain size distributions have been made by Todini & Ferrara (2001) for type II SN progenitors with different metallicities. In this study, carbon grains, produced in the ejecta of the supernovae, have a grain size distribution that slightly changes with the metallicity of the progenitor, while silicate grain size distributions are strongly affected by their metallicities. Bianchi & Schneider (2007) show that dust grains produced in the supernova ejecta are also affected by the reverse shock that destroys a big fraction of dust grains and creates smaller grains. The result of the reverse shock is a constant grain size distribution until very small grain sizes, and therefore an increase of the total cross section of dust grains.
Because our goal is to model the formation of HD and H2 at high
redshift, we assume that at solar metallicity, the total dust grain
cross section is equal to the ISM cross section derived by Weingartner
& Draine (2001) of
![]() cm-2, and that the dust
abundance scales linearly with the overall metallicity, ignoring
corrections due to non-solar elemental abundance ratios. In this
sense, by using a linear scale we do not take into account the fact
that grains might be smaller at higher redshift, which could increase
the total cross section considerably. For exploratory purposes we
therefore consider linear scaling, keeping in mind that the cross
sections derived as a function of metallicity are lower limits, so
that the H2 and HD formation rate can be written as:
cm-2, and that the dust
abundance scales linearly with the overall metallicity, ignoring
corrections due to non-solar elemental abundance ratios. In this
sense, by using a linear scale we do not take into account the fact
that grains might be smaller at higher redshift, which could increase
the total cross section considerably. For exploratory purposes we
therefore consider linear scaling, keeping in mind that the cross
sections derived as a function of metallicity are lower limits, so
that the H2 and HD formation rate can be written as:
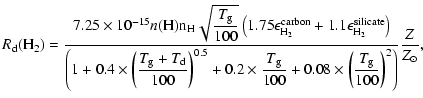 |
(13) |
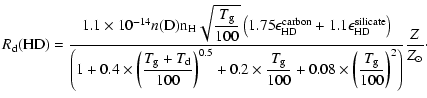 |
(14) |
The more general expressions (11) and (12) should be used to incorporate deviations from the Galactic dust grain size distribution.
3 Chemical model and results
3.1 Gas phase
Gas phase chemistry of HD in the early Universe has been discussed in great detail by Glover & Abel (2008, and references therein). These authors study the effects of the uncertainties of the different rate coefficients on H2 and HD chemistry and cooling. We here consider only those reactions that are needed to form and destroy H2 and HD, such that the impact of grain surface reactions can be assessed. The different reactions considered in our calculations are summarized in Table 2. The formation rates of H2 and HD through the H- + H route and the D- + H and H- + D routes have uncertainties, as discussed by Glover & Abel (2008). We list in the table the low and high values for these rates and consider in our calculations only the high values in to derive conservative estimates for the contributions of grain surface reactions.
3.2 Grain and gas coupling
The gas phase and grain surface chemical models described above are coupled in to follow the relative importance of grain and gas chemistry in the formation of H2 and HD. The abundance of each species, on grains and in the gas phase, is calculated through a system of rate equations. These equations are solved using the DVODE solver (Brown et al. 1989) with fixed-leading coefficient implementation. For the grain surface chemistry, we follow the evolution of physisorbed H and D, H2, HD and D2, chemisorbed H and D and for the gas phase chemistry, the evolution of H, H+, H-, D, D+, D-, H2, HD, D2, and H2+. The formation of D2, as discussed in Cazaux et al. (2008), is never relevant.
In the case of the grain surface chemistry, the incoming fluxes of H and
D atoms from the gas phase are in MLyrs/s (monolayers/s). These
fluxes are calculated as follows:
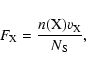 |
(15) |
where
To extend the rate equations that describe the chemistry in the gas phase, we convert the densities of the species that are released into the gas phase by dust grains into atoms cm-3 s-1. This allows us to compare grain surface and gas phase chemistries on the level of rates.
Table 2: Reactions and rate coefficients adopted in the chemical model.
3.3 Cloud collapse and thermodynamics
We assume our model clouds to have a uniform metallicity and to
undergo a gravitational collapse at the free-fall rate. Metal
enrichments of 10-5 ![]() ,
10-4
,
10-4 ![]() and
10-3
and
10-3 ![]() ,
are considered, motivated by Bromm & Loeb
(2003). The simulations start with a density of 1 cm-3 at
1000 K, include dust-grain thermal coupling by collisions and are exposed
to a modest cosmic ray ionization rate of 10-18 s-1,
appropriate for a situation where prior massive star formation and
metal enrichment has occurred. It is assumed that the cloud sees a
mean Lyman Werner UV background of 40 in units of
10-21 erg s-1 cm-2 sr-1 Hz-1, modest enough to allow H2self-shielding (Dijkstra et al. 2008). The helium and deuterium
abundances are 0.0825 and
,
are considered, motivated by Bromm & Loeb
(2003). The simulations start with a density of 1 cm-3 at
1000 K, include dust-grain thermal coupling by collisions and are exposed
to a modest cosmic ray ionization rate of 10-18 s-1,
appropriate for a situation where prior massive star formation and
metal enrichment has occurred. It is assumed that the cloud sees a
mean Lyman Werner UV background of 40 in units of
10-21 erg s-1 cm-2 sr-1 Hz-1, modest enough to allow H2self-shielding (Dijkstra et al. 2008). The helium and deuterium
abundances are 0.0825 and
![]() ,
respectively. In
general, we follow the set-up as presented in Glover & Savin
(2008). We adopt a fiducial redshift of z=10, which yields a
temperature of 30 K for the cosmic microwave background (CMB). The
dust temperature is set equal to the CMB temperature. The gas density
evolution follows:
,
respectively. In
general, we follow the set-up as presented in Glover & Savin
(2008). We adopt a fiducial redshift of z=10, which yields a
temperature of 30 K for the cosmic microwave background (CMB). The
dust temperature is set equal to the CMB temperature. The gas density
evolution follows:
| (16) |
where
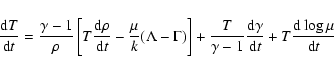 |
(17) |
where
![\begin{figure}
\par\includegraphics[width=9cm,clip]{1302fig9.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg142.gif) |
Figure 5: Typical temperature profiles of collapsing gas clouds with different metallicities. |
| Open with DEXTER | |
![\begin{figure}
\par\includegraphics[width=0.32\textwidth]{1302fig10.eps}\include...
...1302fig14.eps}\includegraphics[width=0.32\textwidth]{1302fig15.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg146.gif) |
Figure 6:
Left panels: formation rates of H2 (dotted and dashed
lines) and HD (solid lines) via dust grain and gas phase routes.
Right panels: fractional abundances of the species present in the collapsing
could. Different metallicities are considered:
|
| Open with DEXTER | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{1302fig16.eps}
\par\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg147.gif) |
Figure 7: H2 and HD formation efficiencies on graphite (PAHs) surfaces in the high dust temperature limit. The impact of the higher binding energy of D is clearly visible. |
| Open with DEXTER | |
3.4 Chemical results
The fractional abundances of the different species, as well as the rate of formation of H2 and HD are presented in Fig. 6. Grain surface chemistry plays an important role in the formation of H2 even for a collapsing cloud with a very low metallicity of 10-5-10-4 solar, while the H- route mostly dominates in diffuse environments, below 103 cm-3 (see also Cazaux & Spaans 2004). The formation of HD, on the other hand, is always dominated by gas phase reactions. Indeed, HD forms mostly through the association of D+ and H2. Once the dust grains boost the formation of H2, the H2 abundance increases, and favors the gas phase formation of HD even more. The HD grain surface route scales with metallicity, but also with the amount of neutral hydrogen and deuterium available in the gas phase. Therefore, as the metallicity increases, H2 formation is boosted by dust grains and H is converted into H2. This leads to a higher amount of H2 to form HD through gas phase reactions, and a lower amount of H to form HD through grain surface reactions.
The abundances show that at very low metallicities
(
![]() ), neutral hydrogen and deuterium are not
completely converted into molecular form when the cloud reaches
), neutral hydrogen and deuterium are not
completely converted into molecular form when the cloud reaches
![]() cm-3. This result is in agreement with the
calculations of Glover & Savin (2008). As the medium contains more
metals, the H/H2 front appears before
cm-3. This result is in agreement with the
calculations of Glover & Savin (2008). As the medium contains more
metals, the H/H2 front appears before
![]() cm-3, and the
D/HD conversion front occurs at earlier stages of the collapse. Once
H2 is available in the medium, the D+ + H2 route is so efficient,
despite the dropping D+ abundance, that all deuterium is converted
quickly into HD. This D/HD conversion occurs at densities
<103 cm-3 for metallicities >
cm-3, and the
D/HD conversion front occurs at earlier stages of the collapse. Once
H2 is available in the medium, the D+ + H2 route is so efficient,
despite the dropping D+ abundance, that all deuterium is converted
quickly into HD. This D/HD conversion occurs at densities
<103 cm-3 for metallicities >
![]() .
In this range, the HD
level populations are not yet thermalized by collisions. Hence, the
associated cooling rates scale
.
In this range, the HD
level populations are not yet thermalized by collisions. Hence, the
associated cooling rates scale ![]()
![]() ,
and help to cool the
medium down.
,
and help to cool the
medium down.
4 Conclusions and discussion
In general, we find that grain surface reactions make a significant
contribution to the formation of H2, even at metallicities as low
as
![]() .
It seems that HD formation is driven by gas
phase chemistry routes through the association of D+ and H2,
helped by the fact that the H2 abundance is strongly boosted by
grain surface reactions for all considered metallicities.
.
It seems that HD formation is driven by gas
phase chemistry routes through the association of D+ and H2,
helped by the fact that the H2 abundance is strongly boosted by
grain surface reactions for all considered metallicities.
In our simulations, we have fixed the grain temperature at the
CMB temperature. This might be true if the dust grains are able to cool
efficiently irrespective of their size, and if dust-gas coupling is
modest. The latter coupling dominates for densities obeying
![]() cm-3, with Z in solar units (Schneider et al. 2006).
For the metallicities considered here,
cm-3, with Z in solar units (Schneider et al. 2006).
For the metallicities considered here, ![]() is larger than the critical
densities of the dominant HD and H2 cooling lines. Hence, the gas is
not able to heat up the dust in this case.
is larger than the critical
densities of the dominant HD and H2 cooling lines. Hence, the gas is
not able to heat up the dust in this case.
However, dust grains in high redshift environments are likely to be in
the form of very small graphitic grains (PAHs), which have quite
modest heat capacities. Consequently, they can enjoy large excursions
in temperature, up to a few hundred Kelvin, when exposed to a soft
(non-dissociative UV-visual) background radiation field (Draine & Li
2001). Interestingly, experiments on graphite by Zecho et al. (2002)
show that there is an important isotopic effect between the formation
of H2 and HD. In their experiments, H2 formation is effective
until a lower surface temperature than HD formation. These authors
suggest that this effect could be the result of a higher binding
energy of deuterium compared to hydrogen on graphite. If we assume
these binding energies for the formation of H2 and HD, then we see
that the efficiency for the formation of H2 declines more rapidly
with increasing dust temperature than the corresponding rate of HD,
see Fig. 7. Therefore, if dust grains are warm
(![]() 150 K), then H2 formation can be suppressed, while HD formation
remains fast. This would lead to a mode where HD is formed more
efficiently on grain surfaces than in the gas phase.
150 K), then H2 formation can be suppressed, while HD formation
remains fast. This would lead to a mode where HD is formed more
efficiently on grain surfaces than in the gas phase.
Acknowledgements
We would like to thank the referee, Paola Caselli, for very constructive comments and careful reading of the manuscript that improved considerably the quality of this paper.
Appendix: Mobility of H and D atoms
In a previous paper, Cazaux & Tielens (2004) calculated the different
mobilities for an atom to go from a site i (physisorbed or chemisorbed)
to a site j (physisorbed or chemisorbed). These mobilities depend on the
transmission coefficients to cross the barrier as:
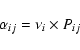 |
(18) |
where
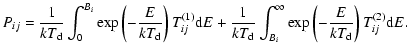 |
(19) |
The first term of this expression shows the probability to cross the barrier through tunneling effects, meaning that the energy of the atom is lower than the barrier Bi, while the second term shows the probability through thermal hopping, meaning that the energy of the atom is higher than Bi. Tij(1) and Tij(2) are the transmission coefficients for tunneling and thermal hopping, respectively. In this work, we are interested in the mobility to go from a physisorbed site to a chemisorbed site. As shown in Cazaux & Tielens (2004), the transmission coefficients
![$\displaystyle \left[T_{\rm pc}^{(1)}\right]^{-1}=\frac{1}{4}\left(1+\sqrt{\frac{E-(E_{\rm phys}-E_{\rm chem})}{E}}\right)^2$](/articles/aa/full_html/2009/11/aa11302-08/img156.gif) |
|||
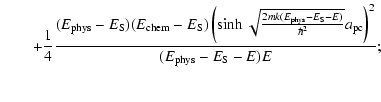 |
|||
| (20) |
![$\displaystyle \left[T_{\rm pc}^{(2)}\right]^{-1} = \frac{1}{4}\left(1+\sqrt{\frac{E-(E_{\rm phys}-E_{\rm chem})}{E}}\right)^2$](/articles/aa/full_html/2009/11/aa11302-08/img159.gif) |
|||
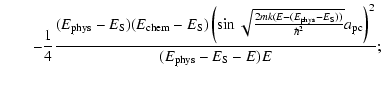 |
|||
| (21) |
The probabilities and mobilities of H and D atoms are then calculated using Eqs. (18) and (19). Because of the weight of the Boltzmann distribution, the energies of the atoms that tunnel are mostly very small, on the order of the temperature of the dust. Therefore, only the second term of Eq. (20) is important. For thermal hopping, on the other hand, the energies of the atoms are higher than the energy of the barrier, and the first term of Eq. (20) dominates. In this way, we can derive the approximations used in this paper.
References
- Abel, T., Bryan, G. L., & Norman, M. L. 2000, ApJ, 540, 39 [NASA ADS] [CrossRef] (In the text)
- Abel, T., Wise, J. H., & Bryan, G. L. 2007, ApJ, 659, L87 [NASA ADS] [CrossRef] (In the text)
- Bachellerie, D., Sizun, M., Teillet-Billy, D., Rougeau, N., & Sidis, V. 2007, Chem. Phys. Lett., 448, 223 [NASA ADS] [CrossRef] (In the text)
- Bianchi, S., & Schneider, R. 2007, MNRAS, 378, 973 [NASA ADS] [CrossRef] (In the text)
- Bromm, V., & Loeb, A. 2003, Nature, 425, 812 [NASA ADS] [CrossRef] (In the text)
- Bromm, V., Coppi, P. S., & Larson, R. B. 2002, ApJ, 564, 23 [NASA ADS] [CrossRef] (In the text)
- Brown, P. N., Byrne, G. D., & Hindmarsh, A. C. 1989, SIAM J. Sci. Stat. Comput., 10, 1038 [CrossRef] (In the text)
- Burke, J. R., & Hollenbach, D. J. 1983, ApJ, 265, 223 [NASA ADS] [CrossRef] (In the text)
- Cazaux, S., & Tielens, A. G. G. M. 2002, ApJ, 575, L29 [NASA ADS] [CrossRef] (In the text)
- Cazaux, S., & Spaans, M. 2004, ApJ, 611, 40 [NASA ADS] [CrossRef] (In the text)
- Cazaux, S., Caselli, P., Cobut, V., & Le Bourlot, J. 2008, A&A, 483, 495 [NASA ADS] [CrossRef] [EDP Sciences] (In the text)
- Dijkstra, M., Haiman, Z., Mesinger, A., & Wyithe, J. S. B. 2008, MNRAS, 391, 1961 [NASA ADS] [CrossRef] (In the text)
- Draine, B. T., & Li, A. 2001, ApJ, 551, 807 [NASA ADS] [CrossRef] (In the text)
- Galli, D., & Palla, F. 1998, A&A, 335, 403 [NASA ADS] (In the text)
- Glover, S. C. O., & Abel, T. 2008, ArXiv e-prints, 803 [arXiv:0803.1768]
- Glover, S. C. O., & Jappsen, A.-K. 2007, ApJ, 666, 1 [NASA ADS] [CrossRef] (In the text)
- Glover, S. C. O., & Savin, D. W. 2008 [arXiv:0809.0780] (In the text)
- Hornekær, L., Sljivancanin, Z., Xu, W., et al. 2006, Phys. Rev. Lett., 96, 156104 [NASA ADS] [CrossRef]. (In the text)
- Jappsen, A.-K., Glover, S. C. O., Klessen, R. S., & Mac Low, M.-M. 2007, ApJ, 660, 1332 [NASA ADS] [CrossRef] (In the text)
- Jeloaica, L., & Sidis, V. 1999, Chem. Phys. Lett., 300, 157 [NASA ADS] [CrossRef] (In the text)
- Johnson, J. L., & Bromm, V. 2006, MNRAS, 366, 247 [NASA ADS] (In the text)
- Klessen, R. S., Spaans, M., & Jappsen, A.-K. 2007, MNRAS, 374, L29 [NASA ADS] [CrossRef] (In the text)
- Maiolino, R., Schneider, R., Oliva, E., et al. 2004, Nature, 431, 533 [NASA ADS] [CrossRef] (In the text)
- Mathis, J. S., Rumpl, W., & Nordsieck, K. H. 1977, ApJ, 217, 425 [NASA ADS] [CrossRef] (In the text)
- McGreer, I. D., & Bryan, G. L. 2008, ApJ, 685, 8 [NASA ADS] [CrossRef] (In the text)
- Meijerink, R., & Spaans, M. 2005, A&A, 436, 397 [NASA ADS] [CrossRef] [EDP Sciences] (In the text)
- Nozawa, T., Kozasa, T., Umeda, H., Maeda, K., & Nomoto, K. 2003, ApJ, 598, 785 [NASA ADS] [CrossRef]
- Omukai, K., Tsuribe, T., Schneider, R., & Ferrara, A. 2005, ApJ, 626, 627 [NASA ADS] [CrossRef] (In the text)
- Ripamonti, E. 2007, MNRAS, 376, 709 [NASA ADS] [CrossRef] (In the text)
- Rougeau, N., Teillet-Billy, D., & Sidis, V. 2006, Chem. Phys. Lett., 431, 135 [NASA ADS] [CrossRef] (In the text)
- Sha, X., & Jackson, B. 2002, Surface Science, Issue 3, 10 January, 496, 318 (In the text)
- Smith, B., Sigurdsson, S., & Abel, T. 2008, MNRAS, 385, 1443 [NASA ADS] [CrossRef] (In the text)
- Schneider, R., Omukai, K., Inoue, A. K., & Ferrara, A. 2006, MNRAS, 369, 1437 [NASA ADS] [CrossRef] (In the text)
- Spaans, M., & Silk, J. 2005, ApJ, 626, 644 [NASA ADS] [CrossRef] (In the text)
- Todini, P., & Ferrara, A. 2001, MNRAS, 325, 726 [NASA ADS] [CrossRef] (In the text)
- Walmsley, C. M., Flower, D. R., & Pineau des Forêts, G. 2004, A&A, 418, 1035 [NASA ADS] [CrossRef] [EDP Sciences]
- Weingartner, J. C., & Draine, B. T. 2001, ApJ, 548, 296 [NASA ADS] [CrossRef] (In the text)
- Wise, J. H., & Abel, T. 2007, ApJ, 671, 1559 [NASA ADS] [CrossRef] (In the text)
- Wise, J. H., & Abel, T. 2008, ApJ, 684, 1 [NASA ADS] [CrossRef] (In the text)
- Yoshida, N., Omukai, K., Hernquist, L., & Abel, T. 2006, ApJ, 652, 6 [NASA ADS] [CrossRef] (In the text)
- Yoshida, N., Omukai, K., & Hernquist, L. 2007, ApJ, 667, L117 [NASA ADS] [CrossRef] (In the text)
- Zecho, T., Guttler, A., Sha, X., Jackson, B., & Kuppers, J. 2002, J. Chem. Phys., 117, 8486 [NASA ADS] [CrossRef] (In the text)
All Tables
Table 2: Reactions and rate coefficients adopted in the chemical model.
All Figures
![\begin{figure}
\par\includegraphics[width=8cm,clip]{1302fig1.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg17.gif) |
Figure 1:
Interactions between an atom and a surface. Two types of surfaces
are
represented, the one with a high barrier against chemisorption (where
|
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\mbox{\includegraphics[width=5.3cm]{1302fig2.eps}\includegrap...
...=5.3cm]{1302fig3.eps}\includegraphics[width=5.3cm]{1302fig4.eps} }\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg18.gif) |
Figure 2: H2 and HD formation efficiencies on amorphous carbon ( left), silicates ( middle) and PAHs ( right) as a function of the grain temperature. These efficiencies have been calculated using a rate equations method. |
| Open with DEXTER | |
| In the text | |
| |
Figure 3: H2 (solid) and HD (dashed-dot) formation efficiencies on amorphous carbon ( left), silicates ( middle) and PAHs ( right) as a function of the grain temperature. These efficiencies have been calculated using Monte Carlo simulations. For the case of PAHs, the properties of para-sites have been included, resulting in a much higher efficiency. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=8.5cm,clip]{1302fig8.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg62.gif) |
Figure 4:
Probability for an atom in the gas phase with a temperature
|
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{1302fig9.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg142.gif) |
Figure 5: Typical temperature profiles of collapsing gas clouds with different metallicities. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=0.32\textwidth]{1302fig10.eps}\include...
...1302fig14.eps}\includegraphics[width=0.32\textwidth]{1302fig15.eps}
\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg146.gif) |
Figure 6:
Left panels: formation rates of H2 (dotted and dashed
lines) and HD (solid lines) via dust grain and gas phase routes.
Right panels: fractional abundances of the species present in the collapsing
could. Different metallicities are considered:
|
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{1302fig16.eps}
\par\end{figure}](/articles/aa/full_html/2009/11/aa11302-08/Timg147.gif) |
Figure 7: H2 and HD formation efficiencies on graphite (PAHs) surfaces in the high dust temperature limit. The impact of the higher binding energy of D is clearly visible. |
| Open with DEXTER | |
| In the text | |
Copyright ESO 2009
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


