| Issue |
A&A
Volume 464, Number 1, March II 2007
AMBER: Instrument description and first astrophysical results
|
|
|---|---|---|
| Page(s) | 253 - 257 | |
| Section | Interstellar and circumstellar matter | |
| DOI | https://doi.org/10.1051/0004-6361:20066631 | |
| Published online | 19 December 2006 | |
Carbamic acid produced by the UV/EUV irradiation of interstellar ice analogs
1
Department of Physics, National Central University, No. 300, Jhongda Rd, Jhongli City, Taoyuan County 32054, Taiwan e-mail: s9222002@cc.ncu.edu.tw; mnuevo@astro.ncu.edu.tw
2
Graduate Institute of Astronomy, National Central University, No. 300, Jhongda Rd, Jhongli City, Taoyuan County 32049, Taiwan
3
National Synchrotron Radiation Research Center, Hsinchu 30076, Taiwan
4
Space Sciences Center and Department of Physics and Astronomy, University of Southern California, Los Angeles, CA 90089-1341, USA
Received:
24
October
2006
Accepted:
14
December
2006
Context.Carbamic acid (NH2COOH) is the smallest amino acid, smaller than the
smallest proteinaceous amino acid glycine. This compound has never been
observed in the interstellar medium (ISM). Previous experiments where ice
mixtures containing H2O, CO2 and NH3 were subjected to 1-MeV
proton bombardment showed that carbamic acid is formed
in a stable zwitterionic (NH COO-) form.
COO-) form.
Aims.In the present work, we have carried out irradiations of ice mixtures containing H2O, 12CO2/13CO2 and NH3 with ultraviolet (UV)/extreme ultraviolet (EUV) photons provided by a synchrotron source in the 4–20 eV range, and compared the results with those obtained for energetic protons.
Methods.Infrared (IR) spectroscopy and mass spectrometry were used to identify the formed photo-products and monitor their evolution in the ices at 15 K and during the warming up to room temperature in the formed residues.
Results.We identified the IR absorption features of HNCO, OCN-, CO, NH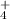 and NH2CHO at low temperature in the ices, and features assigned to
carbamic acid in the residues around 250 K. Finally, we conclude that
under our experimental conditions, unlike what was obtained after
bombardment with energetic protons, carbamic acid may be formed in the
neutral form, and propose some photochemical pathways leading to its
formation.
and NH2CHO at low temperature in the ices, and features assigned to
carbamic acid in the residues around 250 K. Finally, we conclude that
under our experimental conditions, unlike what was obtained after
bombardment with energetic protons, carbamic acid may be formed in the
neutral form, and propose some photochemical pathways leading to its
formation.
Key words: astrochemistry / molecular processes / methods: laboratory / techniques: spectroscopic / ISM: molecules / ultraviolet: ISM
© ESO, 2007
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


