| Issue |
A&A
Volume 406, Number 2, August I 2003
|
|
|---|---|---|
| Page(s) | 385 - 391 | |
| Section | Astrophysical processes | |
| DOI | https://doi.org/10.1051/0004-6361:20030773 | |
| Published online | 17 November 2003 | |
Elementary reactions of the phenyl radical, C H
H , with C
, with C H
H isomers, and of benzene, C
isomers, and of benzene, C H
H , with atomic carbon in extraterrestrial
environments
, with atomic carbon in extraterrestrial
environments
1
Department of Chemistry, University of Hawai'i at Manoa, Honolulu, HI 95622, USA
2
Department of Chemistry, University of Leuven, Celestijnenlaan 200F, 3001 Heverlee, Belgium
3
Lehrstuhl für Organische Chemie 2, Ruhr-Universität Bochum, 44780 Bochum, Germany
4
Center for Computational Quantum Chemistry, The University of Georgia, Athens, GA, USA
5
Institut für Organische Chemie, Universität Erlangen-Nürnberg, 91052 Erlangen, Germany
Corresponding author: R. I. Kaiser, kaiser@gold.chem.hawaii.edu
Received:
19
February
2003
Accepted:
15
May
2003
Binary collisions of ground state carbon atoms, C(3Pj), with
benzene, C6H6(X1A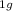 ), and of phenyl radicals, C6H5(X2A1),
with methylacetylene, CH3CCH(X1A1), were investigated in
crossed beam experiments, ab initio calculations, and via RRKM
theory to elucidate the underlying mechanisms of elementary reactions
relevant to the formation of polycyclic aromatic hydrocarbons
(PAHs) in extraterrestrial environments. The reactions of phenyl
radicals with allene, H2CCCH2, and with cyclopropene, cyc-C3H4,
as well as the reaction of benzyl radicals, C6H5CH2, with
acetylene, HCCH, were also investigated theoretically. The C(3Pj)
atom reacts with benzene via complex formation to a cyclic, seven
membered C7H5 doublet radical plus atomic hydrogen. Since
this pathway has neither an entrance nor an exit barrier and
is exoergic, the benzene molecule can be destroyed by carbon
atoms even in the coldest molecular clouds. On the other hand,
the reaction of phenyl radicals with methylacetylene has an entrance
barrier; at high collision energies, the dynamics are at the
boundary between an osculating complex and a direct pathway.
Statistical calculations on the phenyl plus methylacetylene reaction
demonstrate dramatic energy/temperature dependencies: at lower
temperatures, the bicyclic indene isomer is the sole reaction
product. But as the temperature increases to 2000 K, formation
of indene diminishes in favor of substituted acetylenes and allenes,
such as PhCCH, PhCCCH3, PhCHCCH2, and PhCH2CCH. Also, direct
H-abstraction channels become accessible, forming benzene and
C3H3 radicals, including propargyl. Similar conclusions were
reached for the reactions of phenyl radicals with the other C3H4
isomers, as well as for the benzyl + acetylene reaction. The
strong temperature dependence emphasizes that distinct product
isomers must be included in reaction networks modeling PAH formation
in extraterrestrial environments.
), and of phenyl radicals, C6H5(X2A1),
with methylacetylene, CH3CCH(X1A1), were investigated in
crossed beam experiments, ab initio calculations, and via RRKM
theory to elucidate the underlying mechanisms of elementary reactions
relevant to the formation of polycyclic aromatic hydrocarbons
(PAHs) in extraterrestrial environments. The reactions of phenyl
radicals with allene, H2CCCH2, and with cyclopropene, cyc-C3H4,
as well as the reaction of benzyl radicals, C6H5CH2, with
acetylene, HCCH, were also investigated theoretically. The C(3Pj)
atom reacts with benzene via complex formation to a cyclic, seven
membered C7H5 doublet radical plus atomic hydrogen. Since
this pathway has neither an entrance nor an exit barrier and
is exoergic, the benzene molecule can be destroyed by carbon
atoms even in the coldest molecular clouds. On the other hand,
the reaction of phenyl radicals with methylacetylene has an entrance
barrier; at high collision energies, the dynamics are at the
boundary between an osculating complex and a direct pathway.
Statistical calculations on the phenyl plus methylacetylene reaction
demonstrate dramatic energy/temperature dependencies: at lower
temperatures, the bicyclic indene isomer is the sole reaction
product. But as the temperature increases to 2000 K, formation
of indene diminishes in favor of substituted acetylenes and allenes,
such as PhCCH, PhCCCH3, PhCHCCH2, and PhCH2CCH. Also, direct
H-abstraction channels become accessible, forming benzene and
C3H3 radicals, including propargyl. Similar conclusions were
reached for the reactions of phenyl radicals with the other C3H4
isomers, as well as for the benzyl + acetylene reaction. The
strong temperature dependence emphasizes that distinct product
isomers must be included in reaction networks modeling PAH formation
in extraterrestrial environments.
Key words: astrochemistry / ISM: atoms / ISM: molecules / ISM: jet and outflows
© ESO, 2003
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


