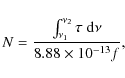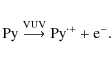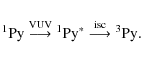| Issue |
A&A
Volume 511, February 2010
|
|
|---|---|---|
| Article Number | A33 | |
| Number of page(s) | 10 | |
| Section | Atomic, molecular, and nuclear data | |
| DOI | https://doi.org/10.1051/0004-6361/200913291 | |
| Published online | 03 March 2010 | |
Photochemistry of the PAH pyrene in water ice: the case for ion-mediated solid-state astrochemistry
J. Bouwman1 - H. M. Cuppen1 - A. Bakker1 - L. J. Allamandola2 - H. Linnartz1
1 - Raymond and Beverly Sackler Laboratory for Astrophysics, Leiden
Observatory, Leiden University, PO Box 9513, 2300 RA Leiden, The
Netherlands
2 - NASA-Ames Research Center, Space Science Division, Mail Stop 245-6,
Moffett Field, CA 94035, USA
Received 14 September 2009 / Accepted 9 November 2009
Abstract
Context. Icy dust grains play an important role in
the formation of complex molecules in the interstellar medium (ISH).
Laboratory studies have mainly focused on the physical interactions and
chemical pathways in ices containing rather simple molecules, such as H2O,
CO, CO2, CH4, and CH3OH.
Observational studies show that polycyclic aromatic hydrocarbons (PAHs)
are also abundantly present in the ISM in the gas phase. It is likely
that these non-volatile species also freeze-out onto dust grains and
participate in the astrochemical solid-state network, but additional
experimental PAH ice studies are largely lacking.
Aims. The study presented here focuses on a rather
small PAH, pyrene (C16H10),
and aims to understand and quantify photochemical reactions of PAHs in
interstellar ices upon vacuum ultraviolet (VUV) irradiation as a
function of astronomically relevant parameters.
Methods. Near UV/VIS spectroscopy is used to track
the in situ VUV driven photochemistry of pyrene containing
ices at temperatures ranging from 10 to 125 K.
Results. The main photoproducts of VUV photolyzed
pyrene ices are spectroscopically identified and their band positions
are listed for two host ices, H2Oand CO. Pyrene
ionization is found to be most efficient in H2Oices
at low temperatures. The reaction products, triplet pyrene and the
1-hydro-1-pyrenyl radical are most efficiently formed in higher
temperature water ices and in low temperature CO ice. Formation routes
and band strength information of the identified species are discussed.
Additionally, the oscillator strengths of Py, Py![]() ,
and PyH
,
and PyH![]() are derived and a quantitative kinetic analysis is performed by fitting
a chemical reaction network to the experimental data.
are derived and a quantitative kinetic analysis is performed by fitting
a chemical reaction network to the experimental data.
Conclusions. Pyrene is efficiently ionized in water
ice at temperatures below 50 K. Hydrogenation reactions
dominate the chemistry in low temperature CO ice with trace amounts of
water. The results are placed in an astrophysical context by
determining the importance of PAH ionization in a molecular cloud. We
conclude that the rate of pyrene ionization in water ice mantles is
comparable to the rate of photodesorption of H2Oice.
The photoprocessing of a sample PAH in ice described in this manuscript
indicates that PAH photoprocessing in the solid state should also be
taken into account in astrochemical models.
Key words: astrochemistry - molecular processes - methods: laboratory - techniques: spectroscopic
1 Introduction
Strong infrared emission attributed to polycyclic aromatic hydrocarbons (PAHs) is characteristic of many galactic and extragalactic objects (Tielens 2008; Smith et al. 2007; Draine et al. 2007; Draine & Li 2007). While this emission generally originates in optically thin, diffuse regions, PAHs should also be common throughout the dense interstellar medium. There, as with most other interstellar species in molecular clouds, PAHs condense out of the gas onto cold icy grain mantles, where they are expected to influence or participate in the chemistry and physics of the ice. While laboratory studies of interstellar ice analogs have shown that complex organic molecules are produced upon extended vacuum ultraviolet (VUV) photolysis (e.g., Bernstein et al. 1995; Briggs et al. 1992), the photoinduced processes occurring during the irradiation of PAH containing interstellar ice analogues have not yet been studied in detail. In optical, in situ studies of the photochemistry of naphthalene, 4-methylpyrene, and quatterylene containing water ice at 20 K, Gudipati & Allamandola (2003,2006a,b) and Gudipati (2004) showed that these PAHs are readily ionized and stabilized within the ice, suggesting that trapped ions may play important, but overlooked roles in cosmic ice processes. Beyond this, there is little information about the VUV induced, in situ photochemistry and photophysics of PAH-containing water-rich ices.
![\begin{figure}
\par\includegraphics[width=16cm,clip]{13291fg1.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg8.png)
|
Figure 1:
The spectrum of a dilute pyrene:H2O ice after
900 s of VUV irradiation at 125 K. The inset shows a
blow-up of the pyrene photoproduct bands. Band assignments are
discussed in Sect. 3.
Note the broad feature ranging from about 350 to 470 nm which
is indicated by a Gaussian fit. This is attributed to overlapping bands
from individual pyrene photoproducts. Bands with negative optical depth
indicate species destruction, those with positive optical depth show
species formation. The blue bands are Gaussian profiles which co-add to
the overall fit shown in red. Note the instrumental resolution
indicated by the profile of the H |
| Open with DEXTER | |
Here, we describe a detailed study of the VUV-induced photochemistry that takes place within pyrene (Py or C16H10) containing water ices (Py:H2O = 1:10 000-1:5000). The present study is an extension of the study of Bouwman et al. (2009) in which the focus was on the new experimental setup and where the use of PAH ice spectra was discussed to search for solid-state features of PAHs in space. In this work, the focus is on a detailed characterization of the chemical processes taking place upon VUV irradiation, particularly as a function of ice temperature ranging from 25 to 125 K. Additionally, measurements on Py:CO ices at 10 K were performed to elucidate the role of water in the reaction schemes and to clarify the formation routes of identified species. A similar study of three small PAHs is now underway to understand the general principles of PAH/ice photochemistry. This is part of an overall experimental program at the Sackler Laboratory for Astrophysics to study the fundamental processes of inter- and circumstellar ice analogues such as thermal (Acharyya et al. 2007) and photodesorption (Öberg et al. 2009c,2007b), hydogenation reactions (Ioppolo et al. 2008; Fuchs et al. 2009), photochemistry (Öberg et al. 2009b), and physical interactions in interstellar ice analogues (Öberg et al. 2009a; Bouwman et al. 2007; Öberg et al. 2007a).
The manuscript is organized as follows. The experimental technique is summarized in Sect. 2. Section 3 describes the Py:H2O and Py:CO ice photochemistry, the resulting products and their formation routes. The temperature-dependent photochemistry and derived reaction dynamics are described in Sect. 4 and astrochemical implications are discussed in Sect. 5. The main conclusions are summarized in Sect. 6.
2 Experimental technique
We use a new apparatus as described in Bouwman
et al. (2009), which follows the photochemistry in
kinetic mode during VUV irradiation by measuring the near UV-visible
absorption spectra of an ice, providing ``real-time'' tracking of the
reactants and photoproducts. Dilute Py:H2O ice
samples (![]() 1:10 000-
1:10 000-![]() 1:5000) and
a Py:CO ice sample of comparable concentration are prepared by
depositing the vapor from a pyrene sample heated to 40
1:5000) and
a Py:CO ice sample of comparable concentration are prepared by
depositing the vapor from a pyrene sample heated to 40![]() C together
with H2Ovapor or CO gas onto a cold MgF2
window. The window is cooled to 10 K in the case of CO
deposition or 25 K in the case of H2Odeposition.
The sample window is cooled by a closed cycle He refrigerator. Pyrene
(Aldrich, 99%) and CO (Praxair 99.999%) are used as
commercially available. Vapor from water, filtered through a milli-Q
purification system and purified further by three freeze-pump-thaw
cycles, is used. The sample window is mounted in a high-vacuum chamber
(
C together
with H2Ovapor or CO gas onto a cold MgF2
window. The window is cooled to 10 K in the case of CO
deposition or 25 K in the case of H2Odeposition.
The sample window is cooled by a closed cycle He refrigerator. Pyrene
(Aldrich, 99%) and CO (Praxair 99.999%) are used as
commercially available. Vapor from water, filtered through a milli-Q
purification system and purified further by three freeze-pump-thaw
cycles, is used. The sample window is mounted in a high-vacuum chamber
(
![]() mbar). The ice
growth rate and thickness are determined with a HeNe laser by
monitoring the thin-film interference fringes generated during
deposition. Simultaneously, the amount of pyrene is tracked by
measuring the integrated strength of the S
mbar). The ice
growth rate and thickness are determined with a HeNe laser by
monitoring the thin-film interference fringes generated during
deposition. Simultaneously, the amount of pyrene is tracked by
measuring the integrated strength of the S
![]() S0
neutral Py transition at 334 nm. Deposition is typically
stopped when the optical depth (OD) of Py approaches
S0
neutral Py transition at 334 nm. Deposition is typically
stopped when the optical depth (OD) of Py approaches ![]() 0.15.
0.15.
The ice samples are photolyzed with the 121.6 nm Ly![]() (10.6 eV) and the 160 nm molecular hydrogen emission
bands (centered around 7.8 eV) generated by a microwave
powered discharge in a flowing H2 gas with a
vacuum ultraviolet flux of
(10.6 eV) and the 160 nm molecular hydrogen emission
bands (centered around 7.8 eV) generated by a microwave
powered discharge in a flowing H2 gas with a
vacuum ultraviolet flux of ![]()
![]() (Muñoz Caro et al. 2002).
This results in a photon flux of
(Muñoz Caro et al. 2002).
This results in a photon flux of ![]() 1014
photons cm-2 s-1
at the sample surface (Öberg
et al. 2009c).
1014
photons cm-2 s-1
at the sample surface (Öberg
et al. 2009c).
Absorption spectra of VUV-photolyzed Py-containing ices are
measured with a Xe-arc lamp serving as a white light source. Lenses and
diaphragms direct the light through the ice sample along the optical
axis determined by the HeNe laser beam after which it is focused onto
the entrance slit of a 0.3 m spectrometer. A
150 lines mm-1 grating, blazed
at 300 nm, disperses the light onto a sensitive ![]() pixel
CCD camera with 16 bit digitization. The camera is read out in
vertical binning mode by a data acquisition computer that converts the
data to absorbance spectra (
pixel
CCD camera with 16 bit digitization. The camera is read out in
vertical binning mode by a data acquisition computer that converts the
data to absorbance spectra (
![]() .
This configuration spans the 270 to 830 nm spectral range,
which permits simultaneously monitoring of the behavior of the neutral
Py parent molecule and photoproduct bands without any adjustment of the
elements along the optical path. This is critical to obtaining reliable
and reproducible baselines in measuring the optical spectra of ices.
The spectral resolution is of the order of 0.9 nm, which is
more than sufficient to record broad solid-state absorption features.
.
This configuration spans the 270 to 830 nm spectral range,
which permits simultaneously monitoring of the behavior of the neutral
Py parent molecule and photoproduct bands without any adjustment of the
elements along the optical path. This is critical to obtaining reliable
and reproducible baselines in measuring the optical spectra of ices.
The spectral resolution is of the order of 0.9 nm, which is
more than sufficient to record broad solid-state absorption features.
The measurements described here were performed on various H2O:Py
ice samples at 25, 50, 75, 100, and 125 K. The CO ice
experiments were carried out at 10 K to avoid matrix
sublimation at higher temperatures. The sample temperature is
maintained using a resistive heater with an accuracy of ![]() .
The measured spectra are converted into units of optical depth by using
the spectrum of the freshly deposited, unphotolyzed ice at the
appropriate temperature as a reference spectrum (I0).
Recording a single spectrum typically takes about 5 ms, and
229 spectra are generally coadded to improve the S/N of a
spectrum, producing one single spectrum every 10 seconds.
.
The measured spectra are converted into units of optical depth by using
the spectrum of the freshly deposited, unphotolyzed ice at the
appropriate temperature as a reference spectrum (I0).
Recording a single spectrum typically takes about 5 ms, and
229 spectra are generally coadded to improve the S/N of a
spectrum, producing one single spectrum every 10 seconds.
The optical configuration of the apparatus is such that
spectra are recorded simultaneously with
photolysis. Thus, the short spectral recording time permits us to
monitor photoinduced changes on a roughly 10-second time scale.
Figure 1
shows the 290 to 490 nm spectrum of a Py:H2O
ice at 125 K after 900 s of in situ VUV
photolysis. Because the spectrum recorded before VUV irradiation is
taken as a reference (I0),
bands with positive OD values originate from species that are produced
by photolysis, while the bands with negative OD correspond to the
neutral pyrene that is lost upon photolysis. Comparing the Py and
photoproduct absorption bands with the narrow H![]() lamp line at 486.1 nm shows that the instrumental resolution
indeed far exceeds the ice band widths. The absolute wavelength
calibration is accurate to within
lamp line at 486.1 nm shows that the instrumental resolution
indeed far exceeds the ice band widths. The absolute wavelength
calibration is accurate to within ![]() 0.5 nm.
0.5 nm.
More than 1400 individual spectra are recorded and are reduced in a typical 4 h experiment. Spectra are individually baseline-corrected by fitting a second order polynomial through data points where no absorption occurs and subsequently subtracting the fit from the measured spectrum. Integrated absorbances of absorption features are calculated numerically for all spectra. These are corrected for the contributions of atomic hydrogen lines originating in the H2 discharge lamp. The data reduction software also allows us to plot correlation diagrams between integrated absorbances of different absorption features. All data handling and reduction is performed with LabView routines.
Integrated band areas are used, in conjunction with oscillator
strengths (f), to derive molecular abundances. The
oscillator strength is converted to integrated absorbance
(cm molecule-1) using the conversion
factor ![]() (Kjaergaard et al. 2000).
The number of molecules per cm2 (N)
is given by
(Kjaergaard et al. 2000).
The number of molecules per cm2 (N)
is given by
where
3 Band assignments and band strength analysis
The typical photolysis duration of about 4 h is the time
required for nearly complete loss of the neutral pyrene vibrational
progression at 334.0, 329.2, and 319.2 nm. Irradiating the
sample ices with VUV light produces a set of new absorption bands in
the spectra, indicating active photochemistry. The band positions, FWHM,
and assignments of the bands in the Py:H2O ice
at 25 K are listed in Table 1. The bands
appearing in the Py:CO ice at 10 K are similar to those in the
Py:H2Oice at 25 K, although, with
slightly altered band positions and FWHM and with
very different relative intensities (see also Table 1). Figure 1 presents a
spectrum from the 125 K Py:H2O series.
This figure illustrates production of the pyrene radical cation (Py
![]() ), triplet pyrene (3Py),
1-hydro-1-pyrenyl radical (PyH
), triplet pyrene (3Py),
1-hydro-1-pyrenyl radical (PyH![]() ), and a broad underlying
``residue'' feature upon VUV irradiation. Additionally, a progression
of distinct absorption features is found in the Py:CO experiment, which
indicates the formation of the (reactive intermediate) HCO
), and a broad underlying
``residue'' feature upon VUV irradiation. Additionally, a progression
of distinct absorption features is found in the Py:CO experiment, which
indicates the formation of the (reactive intermediate) HCO![]() radical. The identifications of these species and their oscillator
strengths are discussed below.
radical. The identifications of these species and their oscillator
strengths are discussed below.
3.1 Neutral pyrene bands
As in Bouwman et al. (2009),
the strong, negative bands peaking at 334.0 nm and weaker
bands at 329.2 and 319.2 nm in the H2Oice
(see Fig. 1),
and at slightly shifted positions in the CO ice, are assigned to the 1B2u
![]() 1Ag
electronic transition of neutral pyrene (S
1Ag
electronic transition of neutral pyrene (S
![]() S0)
based on previous studies of pyrene in rare gas matrices (Halasinski
et al. 2005; Vala et al. 1994). To
study the chemistry in absolute number densities, a value of f=0.33
is adopted from the literature for the oscillator strength of pyrene (Bito et al.
2000; Wang
et al. 2003). This value is used throughout this
paper both for the Py:H2Oand Py:CO experiments.
Pure pyrene ice measured at 10 K shows broader absorptions
located at 341.5, 325.3, and 312.7 nm (see Table 1). We did not
perform VUV experiments on the pure pyrene sample.
S0)
based on previous studies of pyrene in rare gas matrices (Halasinski
et al. 2005; Vala et al. 1994). To
study the chemistry in absolute number densities, a value of f=0.33
is adopted from the literature for the oscillator strength of pyrene (Bito et al.
2000; Wang
et al. 2003). This value is used throughout this
paper both for the Py:H2Oand Py:CO experiments.
Pure pyrene ice measured at 10 K shows broader absorptions
located at 341.5, 325.3, and 312.7 nm (see Table 1). We did not
perform VUV experiments on the pure pyrene sample.
3.2 Pyrene cation bands
Table 1:
Band positions (![]() )
and FWHM in nm for pure pyrene ice at
10 K, pyrene in H2Oice at
25 K, pyrene in CO ice at 10 K, and photoproduct
bands for the Py:H2O and Py:CO UV processed ices.
)
and FWHM in nm for pure pyrene ice at
10 K, pyrene in H2Oice at
25 K, pyrene in CO ice at 10 K, and photoproduct
bands for the Py:H2O and Py:CO UV processed ices.
Positive bands at 363.2, 354.0, and 344.9 nm appear
upon photolysis in the Py:H2Oexperiments. This
progression is assigned to the 2B1u
![]() 2B3g
vibronic transition of the pyrene cation (Py
2B3g
vibronic transition of the pyrene cation (Py![]() )
in accordance with the proximity to the band positions reported by Vala et al. (1994) and Halasinski et al. (2005).
This transition for Py
)
in accordance with the proximity to the band positions reported by Vala et al. (1994) and Halasinski et al. (2005).
This transition for Py![]() in H2O ice was reported in Bouwman et al. (2009).
The 2B1u
in H2O ice was reported in Bouwman et al. (2009).
The 2B1u
![]() 2B3g
transition is too weak to be detected in the Py:CO experiment. A
stronger Py
2B3g
transition is too weak to be detected in the Py:CO experiment. A
stronger Py![]() progression occurs at 445.6, 435.5, 423.0, and 413.8 nm in
water ice. Of these bands, only the strongest at 445.3 nm is
detectable in the irradiated Py:CO ice. This progression is assigned to
the 2Au
progression occurs at 445.6, 435.5, 423.0, and 413.8 nm in
water ice. Of these bands, only the strongest at 445.3 nm is
detectable in the irradiated Py:CO ice. This progression is assigned to
the 2Au
![]() 2B3g
transition of Py
2B3g
transition of Py![]() .
The much weaker absorption caused by the 2B1u
.
The much weaker absorption caused by the 2B1u
![]() 2B3g Py
2B3g Py![]() transition at 490.1 nm in H2Ois again
undetectable in CO.
transition at 490.1 nm in H2Ois again
undetectable in CO.
In these H2Oand CO ice experiments, Py![]() formation is the result of direct single photon ionization of the
neutral species, following:
formation is the result of direct single photon ionization of the
neutral species, following:
We emphasize that ionization in Py:H2Oices is far more efficient than in Py:CO ices. Additional measurements on Py:CO:H2Omixtures indicate that the presence of H2Oindeed enhances the ionization. Hence, it is possible that water contamination in the CO ice is responsible for the formation of some, if not all, of the cation species in the Py:CO experiment. The role of water contamination in CO ice will be discussed in more detail in Sect. 3.3.
![\begin{figure}
\par\includegraphics[width=8.5cm,clip]{13291fg2.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg25.png)
|
Figure 2:
Integrated absorbance of the 445.6 nm Py |
| Open with DEXTER | |
Using baseline-corrected spectra as shown in Fig. 1, the
photochemical evolution is tracked by integrating areas of bands
produced by each species in every spectrum and plotting them as a
function of photolysis time. The strongest Py![]() band at
band at ![]() 445 nm
is selected to track the number density evolution of this species. To
put the kinetic analysis (Sect. 4) on a
quantitative footing, we determine the oscillator strength of the
445 nm Py
445 nm
is selected to track the number density evolution of this species. To
put the kinetic analysis (Sect. 4) on a
quantitative footing, we determine the oscillator strength of the
445 nm Py![]() band as follows. First, the integrated absorbance of the
445 nm Py
band as follows. First, the integrated absorbance of the
445 nm Py![]() band is plotted versus that of the 334 nm Py band during the
course of VUV photolysis at different ice temperatures. These graphs
are shown in Fig. 2.
It should be noted that there is a tight, linear behavior between the
loss of neutral pyrene and growth of the pyrene cation during early
photolysis times up to 100 s (the first 10 successive
datapoints). Inspection of Fig. 2 shows that the
slope is steepest and the ratio of the integrated absorbance of the Py
band is plotted versus that of the 334 nm Py band during the
course of VUV photolysis at different ice temperatures. These graphs
are shown in Fig. 2.
It should be noted that there is a tight, linear behavior between the
loss of neutral pyrene and growth of the pyrene cation during early
photolysis times up to 100 s (the first 10 successive
datapoints). Inspection of Fig. 2 shows that the
slope is steepest and the ratio of the integrated absorbance of the Py![]() band to the Py band is optimum in the 25 K ice. Since no other
photoproduct bands are evident during the linear correlation stage, we
conclude that during this phase, neutral pyrene is converted solely
into the cation as described previously for naphthalene and
quaterrylene (Gudipati &
Allamandola 2006a). The straight-line portion, fitted through
the first 10 data points of irradiation at 25 K, is used to
determine the oscillator strength of Py
band to the Py band is optimum in the 25 K ice. Since no other
photoproduct bands are evident during the linear correlation stage, we
conclude that during this phase, neutral pyrene is converted solely
into the cation as described previously for naphthalene and
quaterrylene (Gudipati &
Allamandola 2006a). The straight-line portion, fitted through
the first 10 data points of irradiation at 25 K, is used to
determine the oscillator strength of Py![]() .
Given that the ratio of the Py
.
Given that the ratio of the Py![]() to the 334 nm Py band is 0.99 and the oscillator strength of
this Py transition is 0.33, the oscillator strength of the
443 nm Py
to the 334 nm Py band is 0.99 and the oscillator strength of
this Py transition is 0.33, the oscillator strength of the
443 nm Py![]() band in water ice is also taken to be 0.33. This conclusion is
consistent with ab initio calculations on pyrene by Weisman et al. (2005).
They calculated that the oscillator strength of the cation is only
band in water ice is also taken to be 0.33. This conclusion is
consistent with ab initio calculations on pyrene by Weisman et al. (2005).
They calculated that the oscillator strength of the cation is only ![]() 2% stronger
than that of the neutral species.
2% stronger
than that of the neutral species.
As described below, the photolysis of Py in water ices at higher temperatures produces other species in addition to the cation. This explains the different curves in Fig. 2.
3.3 HCO bands in Py:CO
VUV irradiation of a Py:CO ice also produces a vibrational progression
ranging from ![]() 500
to 650 nm. As shown in Fig. 3, these absorption
bands, located at 513.4, 535.3, 556.3, 583.0, 604.9, and
639.2 nm, are assigned to the
500
to 650 nm. As shown in Fig. 3, these absorption
bands, located at 513.4, 535.3, 556.3, 583.0, 604.9, and
639.2 nm, are assigned to the ![]() (0,0,0)
HCO
(0,0,0)
HCO![]() (
(![]() -13)
transitions based on band positions reported by van Ijzendoorn et al. (1983).
The clear HCO
-13)
transitions based on band positions reported by van Ijzendoorn et al. (1983).
The clear HCO![]() progression indicates a photolytic source of free H atoms in the ice.
In addition, it confirms the ability of this setup to record small
reactive intermediates in the ice.
progression indicates a photolytic source of free H atoms in the ice.
In addition, it confirms the ability of this setup to record small
reactive intermediates in the ice.
![\begin{figure}
\par\includegraphics[width=8.5cm]{13291fg3.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg28.png)
|
Figure 3:
Vibrational progression of HCO |
| Open with DEXTER | |
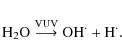
|
(3) |
An experiment on VUV irradiation of a pure CO ice indicated that HCO
| (4) |
Another possible formation route could be by means of VUV-induced hydrogen abstraction from pyrene. This pyrene photodissociation reaction, however, is unlikely to occur, since PAHs are generally highly photostable molecules.
3.4 The 400 nm band carrier
Another vibrational progression appears at 400.5, 392.5, and
378.4 nm in the CO ice experiments. As shown in Fig. 4, the
400.5 nm band dominates this progression. In contrast, a
single band appears at 399.4 nm in the Py:H2Oice
upon VUV irradiation of the samples. The relative intensity of these
bands varies with respect to the Py![]() bands. The 400 nm bands are more pronounced than the cation
bands in the H2Oice only at high
temperatures, whereas they are more pronounced in the low
temperature CO ice.
bands. The 400 nm bands are more pronounced than the cation
bands in the H2Oice only at high
temperatures, whereas they are more pronounced in the low
temperature CO ice.
![\begin{figure}
\par\includegraphics[width=9cm,clip]{13291fg4.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg31.png)
|
Figure 4:
Integrated absorbance of the 400 nm PyH |
| Open with DEXTER | |
Two additional measurements were performed to identify the carrier
responsible for these transitions. A kinetic experiment was performed
on non-VUV-irradiated Py:CO ice. This ice showed no sign of pyrene
ionization by the Xe-lamp, which is used as a spectroscopic light
source.The production of HCO![]() and the formation of the 400 nm band were not observed either.
Subsequently, the ice was irradiated by the VUV source for
10 min. The steady growth of the 400 nm band with VUV
photolysis indicates that the species responsible for the
400 nm band is a product of the VUV processing of the ice.
Moreover, when the VUV irradiation is stopped, the 400 nm band
carrier continues to grow at the expense of the
remaining neutral pyrene. This indicates that the chemical reaction
leading to the formation of this species is not directly
photon-dependent, but rather depends on the diffusion of a
photoproduct. A similar experiment on a Py:H2Oice
at 25 K indicates that the same process also takes place in H2Oice.
The detection of HCO
and the formation of the 400 nm band were not observed either.
Subsequently, the ice was irradiated by the VUV source for
10 min. The steady growth of the 400 nm band with VUV
photolysis indicates that the species responsible for the
400 nm band is a product of the VUV processing of the ice.
Moreover, when the VUV irradiation is stopped, the 400 nm band
carrier continues to grow at the expense of the
remaining neutral pyrene. This indicates that the chemical reaction
leading to the formation of this species is not directly
photon-dependent, but rather depends on the diffusion of a
photoproduct. A similar experiment on a Py:H2Oice
at 25 K indicates that the same process also takes place in H2Oice.
The detection of HCO![]() radicals in the ice and the inherent presence of free photolytic H
atoms, implies that the growth of the vibrational progression starting
at
radicals in the ice and the inherent presence of free photolytic H
atoms, implies that the growth of the vibrational progression starting
at ![]() 400 nm
could be the result of the reaction of pyrene with diffusing H atoms
400 nm
could be the result of the reaction of pyrene with diffusing H atoms
This assignment to the 1-hydro-1-pyrenyl radical (PyH
In contrast to the Py:H2Oexperiments
where pyrene is also efficiently ionized, the experiment on PyH![]() formation in CO shows no sign of other reaction products. The
integrated absorbance of the growing PyH
formation in CO shows no sign of other reaction products. The
integrated absorbance of the growing PyH![]() transition is plotted versus the integrated absorbance of the
diminishing neutral in Fig. 4. Growth is
tracked over a duration of more than 1.5 h. Since there is a
one-to-one conversion of Py to PyH
transition is plotted versus the integrated absorbance of the
diminishing neutral in Fig. 4. Growth is
tracked over a duration of more than 1.5 h. Since there is a
one-to-one conversion of Py to PyH![]() in the Py:CO ice (Eq. (5)),
as described in Sect. 3.2, we
derive an oscillator strength of 0.089 for this species by fitting a
line through the correlating absorbances in Fig. 4.
in the Py:CO ice (Eq. (5)),
as described in Sect. 3.2, we
derive an oscillator strength of 0.089 for this species by fitting a
line through the correlating absorbances in Fig. 4.
3.5 The 405 nm band carrier
Besides the Py![]() and the PyH
and the PyH![]() bands, another distinct absorption is found in the spectra of VUV
irradiated ices. This feature is located at 405.0 nm in the
Py:H2Oand at 406.2 nm in the Py:CO
experiment. In our previously published paper on low temperature Py:H2Oice,
we tentatively assigned this absorption to a negative ion, Py-
or PyO- (Bouwman
et al. 2009). The experiments on Py:CO ices
presented here enable us to exclude this assignment because of the
nearly absent Py
bands, another distinct absorption is found in the spectra of VUV
irradiated ices. This feature is located at 405.0 nm in the
Py:H2Oand at 406.2 nm in the Py:CO
experiment. In our previously published paper on low temperature Py:H2Oice,
we tentatively assigned this absorption to a negative ion, Py-
or PyO- (Bouwman
et al. 2009). The experiments on Py:CO ices
presented here enable us to exclude this assignment because of the
nearly absent Py![]() transitions. Firstly, Py- is ruled out because a
much stronger second Py- absorption band,
expected at 490 nm (Montejano
et al. 1995), is absent in our Py:CO experiment.
Secondly, PyO- is also ruled out, because it
should exhibit absorption bands down to 350 nm (Milosavljevic & Thomas 2002),
bands that are also absent in the Py:CO experiment. Additionally, in
our previous paper we assumed that PyO- was a
product of PyOH. The formation of PyO- is also
unlikely in the absence of PyOH absorption in these experiments, as
discussed below.
transitions. Firstly, Py- is ruled out because a
much stronger second Py- absorption band,
expected at 490 nm (Montejano
et al. 1995), is absent in our Py:CO experiment.
Secondly, PyO- is also ruled out, because it
should exhibit absorption bands down to 350 nm (Milosavljevic & Thomas 2002),
bands that are also absent in the Py:CO experiment. Additionally, in
our previous paper we assumed that PyO- was a
product of PyOH. The formation of PyO- is also
unlikely in the absence of PyOH absorption in these experiments, as
discussed below.
The absorption at 405 nm does not correlate with that
of the cation, nor with the PyH![]() band. The band only appears during photolysis and hence is
characterized as a VUV-photon-related product. From the literature, it
is known that a pyrene triplet-triplet (3Ag-
band. The band only appears during photolysis and hence is
characterized as a VUV-photon-related product. From the literature, it
is known that a pyrene triplet-triplet (3Ag-
![]() 3B2u+)
transition is expected at this wavelength upon laser excitation of
pyrene in solution which populates the lowest member of the triplet
manifold (e.g., Langelaar
et al. 1970; Hsiao & Webber 1992). For
the 405 nm band to originate from this triplet-triplet
transition, the lowest level must be populated and remain so with a
long enough lifetime to allow absorption to the 3Ag-
level. In the ice experiments reported here, there are a number of
possible routes for pumping the 3Py state. The
most obvious route is by means of photoexcitation followed by
intersystem crossing
3B2u+)
transition is expected at this wavelength upon laser excitation of
pyrene in solution which populates the lowest member of the triplet
manifold (e.g., Langelaar
et al. 1970; Hsiao & Webber 1992). For
the 405 nm band to originate from this triplet-triplet
transition, the lowest level must be populated and remain so with a
long enough lifetime to allow absorption to the 3Ag-
level. In the ice experiments reported here, there are a number of
possible routes for pumping the 3Py state. The
most obvious route is by means of photoexcitation followed by
intersystem crossing
Triplet formation is found to decrease with decreasing temperature in ethanol ice (Stevens et al. 1967). This translates to our experiment in a nearly absent 405 nm band in the low temperature Py:H2Oexperiment, because of the high Py
In the CO ice, on the other hand, where Py![]() production is low, formation of the 405 nm band carrier
appears to be very efficient at low temperatures. The production of the
405 nm band carrier requires VUV photons to be initiated. The
pumping of the 3Py state can again occur by
means of Eq. (6).
Moreover, CO has a dipole allowed electronic transition in the VUV.
Hence, speculating, pumping of the 3Py state by
collisional de-excitation of CO molecules exited by the VUV radiation
provides a reaction path of
production is low, formation of the 405 nm band carrier
appears to be very efficient at low temperatures. The production of the
405 nm band carrier requires VUV photons to be initiated. The
pumping of the 3Py state can again occur by
means of Eq. (6).
Moreover, CO has a dipole allowed electronic transition in the VUV.
Hence, speculating, pumping of the 3Py state by
collisional de-excitation of CO molecules exited by the VUV radiation
provides a reaction path of
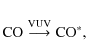
|
(7) |
followed by
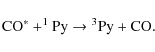
|
(8) |
In summary, while we cannot identify the carrier of the 405 nm band, the 3Ag-
3.6 Broad absorption feature
Finally, besides the narrower bands reported in the previous sections, we discuss a broad underlying feature extending from about 350 to 570 nm, which grows upon photolysis in all cases. This band probably comprises overlapping bands caused by a number of Py/H2O or Py/CO photoproducts. Part of this Py-residue feature remains even after warming up the sample window to room temperature, whereas all other features disappear at the water desorption temperature.
As discussed above, the very broad feature must be produced by a variety of similar but distinct photoproducts, all containing the pyrene chromophore. Mass spectral analysis of the species produced by the VUV photolysis of a few other PAHs in water ice show that the parent PAH is not destroyed but that OH, O, and H are added to some of the edge carbon atoms (Bernstein et al. 1999). Given the multiplicity of the side sites on pyrene that can undergo substitution, it is likely that the photoproducts produced in the experiments reported here are multiply substituted, rather than singly substituted. Thus, it is possible that a mixture of related but distinct Py-Xn species, where X may be H, OH, or O, produce the broad band.
In our previous work, we reported the production of a clear
and reproducible PyOH band at 344.9 nm in a low temperature H2Oice
(Bouwman et al. 2009).
The results presented here do not show evidence of this absorption
feature. However, in some instances the absorption was detected upon
irradiation or warm-up of the ice. The irregular appearance of the PyOH
absorption feature in these experiments indicates that the formation of
this species is highly sensitive to the sample's physical parameters,
i.e., structure of the ice, temperature, and concentration. One
possible explanation is that in the previously reported experiments,
the Py concentration was not controlled and those experiments sampled a
very different ice concentration and, by implication, physical ice
structure. While we do not have a solution for this discrepancy, we
emphasize that both measurement series have been fully reproducible
over many independent experiments for periods of months. An
experimental program to investigate the role of the PAH:H2Oconcentration
on ice photochemistry is underway.
4 Py:H O ice
photochemistry at different temperatures
O ice
photochemistry at different temperatures
![\begin{figure}
\par\includegraphics[width=9cm,clip]{13291fg5.eps}\vspace{-3mm}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg36.png)
|
Figure 5: The VUV-induced spectroscopic changes in Py:H2O ice for two different temperatures as a function of photolysis time. Comparing the spectra from the 25 K ice ( bottom) with those of the 100 K ice ( top) shows the critical role that temperature plays in determining photochemical pathways in a PAH-containing ice. In the 25 K ice, cation formation is favored over production of the pyrene residue and the 400 and 405 nm band carriers. The opposite holds for the 100 K ice. |
| Open with DEXTER | |
Figure 5 shows the spectral evolution of two different Py:H2Osamples at different temperatures. The top frame presents the 280 to 540 nm spectra of the 100 K Py:H2O ice after 0, 20, 40, 80, and 160 s of in situ photolysis and the bottom frame the corresponding spectra for the 25 K ice. These spectra are snapshots of the more than one thousand spectra collected during 4 h of photolysis. They illustrate the rapid changes that occur during the early stages in the photochemistry of these ices and the major differences in reaction products at different temperatures.
![\begin{figure}
\par\includegraphics[width=14cm,clip]{13291fg6.eps}\vspace{3mm}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg37.png)
|
Figure 6:
The integrated absorbance of the Py 334 nm, Py |
| Open with DEXTER | |
To probe the VUV-driven photophysics and reaction dynamics for a set of
selected temperatures, the production and depletion of species was
tracked as a function of irradiation time. To this end, the Py
334 nm, Py![]() 445 nm, and PyH
445 nm, and PyH![]() 400 nm bands were integrated for every spectrum. The spectra
in Figs. 1
and 5
show that it is rather straightforward to determine the boundaries
needed to integrate these bands. We estimate that the uncertainty in
most of these band areas is of the order of 10%.
400 nm bands were integrated for every spectrum. The spectra
in Figs. 1
and 5
show that it is rather straightforward to determine the boundaries
needed to integrate these bands. We estimate that the uncertainty in
most of these band areas is of the order of 10%.
The integrated absorbances of the neutral Py, strongest Py![]() ,
and PyH
,
and PyH![]() bands in H2Oice at temperatures of 25, 50, 75,
100, and 125 K are plotted versus photolysis time (VUV
fluence) in Fig. 6.
The spectra in Fig. 5
and photochemical behavior in Fig. 6 show that, upon
photolysis, neutral pyrene loss is immediate and rapid. The initial
growth of Py
bands in H2Oice at temperatures of 25, 50, 75,
100, and 125 K are plotted versus photolysis time (VUV
fluence) in Fig. 6.
The spectra in Fig. 5
and photochemical behavior in Fig. 6 show that, upon
photolysis, neutral pyrene loss is immediate and rapid. The initial
growth of Py![]() mirrors the rapid, initial loss of Py. However, while Py steadily
decreases, and several other Py photoproduct bands increase during some
4 h of photolysis, the production of Py
mirrors the rapid, initial loss of Py. However, while Py steadily
decreases, and several other Py photoproduct bands increase during some
4 h of photolysis, the production of Py![]() reaches a maximum and then slowly diminishes. From Fig. 6, one can clearly
see that ionization of pyrene is most efficient in the low temperature
ice. Formation of PyH
reaches a maximum and then slowly diminishes. From Fig. 6, one can clearly
see that ionization of pyrene is most efficient in the low temperature
ice. Formation of PyH![]() ,
on the other hand, is far more efficient at higher temperatures.
,
on the other hand, is far more efficient at higher temperatures.
For comparison, the integrated absorbances for the irradiated
Py:CO ice are plotted as a function of time in the right bottom frame
of Fig. 6.
It should be noted that the PyH![]() band is multiplied by a factor of 10 in the Py:CO experiment, compared
to a factor of 20 in the Py:H2Oexperiment. The
PyH
band is multiplied by a factor of 10 in the Py:CO experiment, compared
to a factor of 20 in the Py:H2Oexperiment. The
PyH![]() band is clearly more prominent in the CO ice experiment than in the H2Oice
experiments. The Py+ signal on the other hand is
negligible. This indicates that the H2Oice plays
a role in ion formation and stabilization.
band is clearly more prominent in the CO ice experiment than in the H2Oice
experiments. The Py+ signal on the other hand is
negligible. This indicates that the H2Oice plays
a role in ion formation and stabilization.
To place this behavior on a quantitative footing, the
integrated areas for the Py and Py![]() bands are converted to number densities using Eq. (1). Here, an
oscillator strength of 0.33 is used for the 334 nm Py bands.
The values used for the oscillator strengths of the Py
bands are converted to number densities using Eq. (1). Here, an
oscillator strength of 0.33 is used for the 334 nm Py bands.
The values used for the oscillator strengths of the Py![]() and PyH
and PyH![]() bands are 0.33 and 0.089, respectively, as determined in
Sects. 3.2
and 3.4.
Perusal of Fig. 6
shows that Py behaves similarly in all of the ices considered here.
Regardless of temperature, its signal drops quickly with the onset of
irradiation and continues to diminish with ongoing photolysis.
Likewise, Py
bands are 0.33 and 0.089, respectively, as determined in
Sects. 3.2
and 3.4.
Perusal of Fig. 6
shows that Py behaves similarly in all of the ices considered here.
Regardless of temperature, its signal drops quickly with the onset of
irradiation and continues to diminish with ongoing photolysis.
Likewise, Py![]() grows rapidly with initial photolysis but peaks after a relatively
short time interval corresponding to a fluence of roughly
grows rapidly with initial photolysis but peaks after a relatively
short time interval corresponding to a fluence of roughly ![]() photons
and then drops continuously. While the Py
photons
and then drops continuously. While the Py![]() growth and loss curves resemble each other, cation production
efficiency is strongest in the 25 K ice. This efficiency
remains of the same order at even lower temperatures (not shown here).
The photolysis time required for the cation to reach a maximum shortens
with increasing temperature. The PyH
growth and loss curves resemble each other, cation production
efficiency is strongest in the 25 K ice. This efficiency
remains of the same order at even lower temperatures (not shown here).
The photolysis time required for the cation to reach a maximum shortens
with increasing temperature. The PyH![]() band contribution is minor with respect to the Py
band contribution is minor with respect to the Py![]() band for ices below 50 K. This reverses between 50 and
75 K, suggesting that there is a change in the dominant Py:H2O
ice photochemical channel in this temperature range.
band for ices below 50 K. This reverses between 50 and
75 K, suggesting that there is a change in the dominant Py:H2O
ice photochemical channel in this temperature range.
A kinetic analysis of the plots in Fig. 6 is carried out
using the reaction scheme indicated in Fig. 7. Here, k11
is the photoionization rate of Py to Py![]() ,
k12 the electron-ion
recombination rate of Py
,
k12 the electron-ion
recombination rate of Py![]() ,
k21 the production rate of
the PyH
,
k21 the production rate of
the PyH![]() feature, and k22 the rate of
the reverse reaction of PyH
feature, and k22 the rate of
the reverse reaction of PyH![]() to Py. The rates designated k1,
k2, and k3
are the production rates for the different products that comprise the
Py-residue band. The oscillator strengths for the Py
to Py. The rates designated k1,
k2, and k3
are the production rates for the different products that comprise the
Py-residue band. The oscillator strengths for the Py![]() and PyH
and PyH![]() bands are also fitted, but are restricted to remain within
bands are also fitted, but are restricted to remain within ![]() 10% of the
experimentally determined values of 0.33 and 0.089. All reactions are
assumed to be first order in the reactant. The relative abundances of
free or solvated electrons and O, H, and OH radicals in the
ice are not considered.
10% of the
experimentally determined values of 0.33 and 0.089. All reactions are
assumed to be first order in the reactant. The relative abundances of
free or solvated electrons and O, H, and OH radicals in the
ice are not considered.
The fits to the growth and decay curves are included in
Fig. 6
and the temperature dependence of the derived rate constants is
presented in Fig. 8.
The agreement between the fit and the experimental data in terms of
curve shape and absolute intensity is good. The fitted oscillator
strengths of the Py![]() and PyH
and PyH![]() bands amount to 0.31 and 0.082, respectively, and hence do not deviate
much from the experimentally determined values.
bands amount to 0.31 and 0.082, respectively, and hence do not deviate
much from the experimentally determined values.
The graph in Fig. 8
indicates that the Py photoionization rate (k11)
drops rapidly between 25 and 50 K. The electron recombination
rate (k12) decreases only
slightly, if at all, within the errors over the entire temperature
range. As mentioned above, the production of the PyH![]() becomes more important at higher temperatures. Its formation rate (k21)
is low in all ices up to 50 K (<
becomes more important at higher temperatures. Its formation rate (k21)
is low in all ices up to 50 K (<
![]() ), but jumps to >
), but jumps to >
![]() in the ices with temperatures of 75 K and higher. The back
channel from PyH
in the ices with temperatures of 75 K and higher. The back
channel from PyH![]() to Py, k22, also shows a
temperature dependence. It increases almost linearly in going from cold
to warm ices. The formation rate of a photoproduct produced directly
from Py (k1) also seems to
jump at 50 K. The formation rate of products originating in
the Py
to Py, k22, also shows a
temperature dependence. It increases almost linearly in going from cold
to warm ices. The formation rate of a photoproduct produced directly
from Py (k1) also seems to
jump at 50 K. The formation rate of products originating in
the Py![]() species, on the other hand, seems to lower with increasing temperature.
Finally, the rate of product formation from the PyH
species, on the other hand, seems to lower with increasing temperature.
Finally, the rate of product formation from the PyH![]() channel is low throughout the entire temperature range. The jump in
rate of the formation of P1 and PyH
channel is low throughout the entire temperature range. The jump in
rate of the formation of P1 and PyH![]() with temperature probably reflects the diffusion barrier of radical
species (H
with temperature probably reflects the diffusion barrier of radical
species (H![]() and OH
and OH![]() )
in the ice.
)
in the ice.
Since published studies of the processes induced by the photolysis of other PAH:H2O ices are limited, not much information is available with which to compare these results. While, to the best of our knowledge, there are no reports of the photochemistry that takes place as a function of ice temperature or of long-term fluence, the VUV photochemistry of the PAHs naphthalene, 4-methylpyrene (4MP), and quatterrylene in water ice at 10 K has been studied (Gudipati & Allamandola 2003,2006a; Gudipati 2004; Gudipati & Allamandola 2006b). The results obtained are in good agreement with the low temperature (25 K) case reported here. Namely, the parent PAH is easily and efficiently ionized, by quantitative conversion of the neutral species to the cation form. The focus of the earlier studies was on cation production and stabilization and not on long-duration photolysis experiments. In their study of 4MP:H2O (1:>500) ice at 15 K, Gudipati & Allamandola (2003) utilized a reaction scheme similar to that on the right half of that presented in Fig. 7. Table 2 compares the reaction rates that they determined with those of the 25 K ice reported here. Except for the production of P2, which differs by one order of magnitude, there is very good agreement between the rate constants for each step in the two experiments.
| Figure 7: Reaction scheme used to fit the experimental data. |
|
| Open with DEXTER | |
The growth and decay curves in Fig. 6, taken together with the temperature dependence of the reaction rates in Fig. 8, show that the VUV-driven PAH photochemistry depends strongly on ice temperature. The influence of the ice morphology on this chemistry was also investigated, to understand the origin of the temperature dependence. An experiment on an ice deposited at 25 K, annealed to 125 K, and subsequently cooled to 25 K before photolysis, showed that the ionization rate and efficiency are similar to that of an unannealed ice. Apparently, it is not the morphology but the temperature of the ice that primarily determines which process dominates. We discriminate between two temperature regimes. One governed by ion-mediated processes that dominate at 25 K and slightly higher temperatures, and a second, presumably radical-driven regime, that becomes increasingly more important at higher temperatures.
![\begin{figure}
\par\includegraphics[width=8.5cm,clip]{13291fg8.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg42.png)
|
Figure 8:
Parameters (
|
| Open with DEXTER | |
Table 2:
The reaction rates for the VUV photolysis of Py:H2O
(![]() 1:5000) ice
at 25 K compared to those for 4-methylypyrene:H2O
(1:>500) ice at 15 K (Gudipati
& Allamandola 2003).
1:5000) ice
at 25 K compared to those for 4-methylypyrene:H2O
(1:>500) ice at 15 K (Gudipati
& Allamandola 2003).
5 Astrochemical implications
As shown in the previous sections, ionization and chemistry of a rather
small PAH species, pyrene, trapped in H2Oice
turns out to be very efficient in a laboratory setting. Here, we extend
these findings to interstellar conditions, with the aim of including
the calculated rates in astrochemical models. For this, it is crucial
to distinguish pure photochemical processes from diffusion, since the
latter will be highly dependent on the number density of radicals and
electrons in the ice. As mentioned in the previous section, the
photoionization of Py is probably a single-photon process, whereas
protonation of Py and the electron recombination of Py![]() are the results of both VUV photolysis and diffusion. The mechanism for
PyH
are the results of both VUV photolysis and diffusion. The mechanism for
PyH![]() deprotonation is unclear, since it can proceed by means of either VUV
processing or through hydrogen abstraction by diffusing species.
Diffusion of radicals through the ice is a thermally activated process
and will therefore increase with temperature. Recombination, however,
is largely temperature-independent in our experiments, indicating that
the rate of Py
deprotonation is unclear, since it can proceed by means of either VUV
processing or through hydrogen abstraction by diffusing species.
Diffusion of radicals through the ice is a thermally activated process
and will therefore increase with temperature. Recombination, however,
is largely temperature-independent in our experiments, indicating that
the rate of Py![]() recombination is not dominated by the diffusion of electrons in the
ice. If Py
recombination is not dominated by the diffusion of electrons in the
ice. If Py![]() loss occurs by means of electron recombination and not Py
loss occurs by means of electron recombination and not Py![]() reaction with H2O or one of its photoproducts,
the electron most likely originates from the initial photoionization
event after which electrons remain in the vicinity of the recombining Py
reaction with H2O or one of its photoproducts,
the electron most likely originates from the initial photoionization
event after which electrons remain in the vicinity of the recombining Py![]() species. Hence, this local process can be, although indirectly,
regarded as a single-photon process.
species. Hence, this local process can be, although indirectly,
regarded as a single-photon process.
The rates of protonation of Py and deprotonation of PyH![]() show a temperature dependence and the importance of diffusion can
therefore not be excluded. This makes it harder to directly translate
the rates (s-1) into photon rates (cm2photon-1).
However, we can determine astrochemical photon rates for both
ionization and recombination of pyrene in interstellar H2Oice
(see Table 2).
show a temperature dependence and the importance of diffusion can
therefore not be excluded. This makes it harder to directly translate
the rates (s-1) into photon rates (cm2photon-1).
However, we can determine astrochemical photon rates for both
ionization and recombination of pyrene in interstellar H2Oice
(see Table 2).
Now, to translate this to the astrochemical situation and with other processes, we assume that PAHs generally have an ionization rate similar to that of pyrene. How do ionization and chemistry compare with other processes such as the photodesorption of the icy grain mantle, in which the PAHs are embedded? To exemplify this, the rate of ionization of a PAH in water ice at 25 K (in photon-1) is calculated anywhere in a dense cloud where AV=3 and compared with the VUV photodesorption rate of H2Oderived by Öberg et al. (2009c). It is well established that the onset of ice formation occurs in clouds with an edge-to-edge (through the cloud) magnitude of AV=3 (e.g. Whittet et al. 2001). Thus, inside our hypothetical dense cloud at AV=3 (from cloud edge to within the cloud), ices are present.
The experimentally determined PAH ionization rate in H2Oat
25 K, normalized to the total amount of deposited PAH is given
by
![\begin{displaymath}{k}_{11}=\frac{{\rm d}\frac{\displaystyle[{\rm PAH}^+]}{\displaystyle[{\rm PAH}]_0}}{{\rm d}t}= 10^{-3}~{\rm s}^{-1}.
\end{displaymath}](/articles/aa/full_html/2010/03/aa13291-09/img44.png)
|
(9) |
Consider a typical interstellar grain, covered by a 100 monolayer (ML) thick ice. The number of sites on a grain is 1015 cm-2. If we assume that one in every 104 particles on the grain is a PAH, the total number of PAH molecules on the grain is
However, in our dense cloud the number of photons available for PAH
photoionization is larger than the number of photons available for
photodesorption of H2Oice. This is because H2Ophotodesorption
is primarily caused by VUV photons, whereas PAH ionization can occur
for much lower energy photons. To quantify the radiation field in a
dense cloud at AV=3
as a function of wavelength (![]() ), we take the average UV
interstellar radiation field (
), we take the average UV
interstellar radiation field (![]() )
from Sternberg (1988)
and rewrite the expression to
)
from Sternberg (1988)
and rewrite the expression to ![]() with units photons cm-2 s-1 nm-1
with units photons cm-2 s-1 nm-1
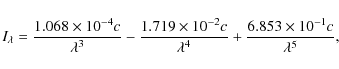
|
(10) |
where c is the speed of light in nm s-1. The attenuation of the radiation field by dust as a function of wavelength is given by
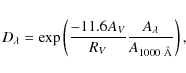
|
(11) |
from Draine & Bertoldi (1996), where we assume that RV=3.1 and
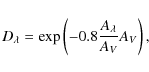
|
(12) |
where the table of
| (13) |
Water ice absorbs photons with wavelengths ranging from 130 to 150 nm (Andersson & van Dishoeck 2008; Kobayashi 1983). The ionization energy of PAHs on the other hand, is lowered by about 2 eV when in H2Oice (Gudipati & Allamandola 2004; Woon & Park 2004). For the wavelength range available for ionization of PAHs, assuming that H2Oblocks all photons below 150 nm, we take 150 to 250 nm (Li & Draine 2001). By integrating the photon flux in a cloud of AV=3 over both wavelength intervals a number of photons available for PAH ionization is found that is 6 times larger than the number of photons available for photodesorption of H2O. Additionally, at AV=3, the cosmic-ray-induced UV field is negligible compared to the interstellar UV field (Shen et al. 2004). Therefore, the occurrence of photoionization is of similar order as photodesorption of the main component in the grain mantle in a dense cloud. The ionization rates from Table 2 can be directly included in astrochemical models in the form
![\begin{displaymath}\frac{{\rm d[PAH^+]}}{{\rm d}t}=k_{11}\Psi{\rm [PAH]},
\end{displaymath}](/articles/aa/full_html/2010/03/aa13291-09/img59.png)
|
(14) |
where [PAH+] is the concentration of the PAH (pyrene) cation in the ice, k11 is the photon rate in cm2 photon-1,
In the above calculation, we assume that all PAHs exhibit the ionization behavior of the pyrene chromophore. Of course, more PAHs need to be investigated experimentally before drawing conclusions on their general photochemical behavior in interstellar ices. However, if all PAHs have an ionization rate similar to that of pyrene, photoionization and subsequent chemical reactions of PAHs trapped in ices are important processes in dense clouds. When frozen-out in ices, PAHs have an important impact on the radical and electron budget in solid state chemistry. Hence, the processes described here may be more important than previously assumed in modeling complex interstellar grain chemistry.
6 Conclusions
A recently constructed setup has been used to track, on a sub-second timescale, the photochemistry of a PAH in H2Oand CO ices as a function of temperature. The setup used here clearly has advantages compared to relatively slow infrared photochemical ice studies. The conclusions from this work on a PAH, pyrene, trapped in an interstellar ice analogue are summarized below:
- 1.
- A set of photochemical reaction products has been
identified in both irradiated Py:H2Oand Py:CO
ice experiments. The reaction products result from direct
photoionization of pyrene, or from a reaction of the parent, pyrene,
with free H atoms produced in the matrix. Additionally, an absorption
band is tentatively assigned to a triplet-triplet transition of pyrene.
A vibrational progression assigned to HCO
 is found in spectra of the VUV-irradiated Py:CO ice.
is found in spectra of the VUV-irradiated Py:CO ice. - 2.
- Pyrene is easily and efficiently ionized when trapped in H2Oice.
Photoionization is a non-diffusion-related reaction and hence a
photonrate of
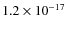 cm2 photon-1,
which can serve as input for astrochemical models, is derived.
cm2 photon-1,
which can serve as input for astrochemical models, is derived.
- 3.
- When trapped in CO ice, pyrene ionization is inefficient compared to that in water ice.
- 4.
- Electron-ion recombination is independent of ice
temperature and is characterized as a non-diffusion-dominated reaction.
For this process, a photon rate of
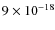 cm2 photon-1
is derived, which can be directly used in astrochemical models.
cm2 photon-1
is derived, which can be directly used in astrochemical models. - 5.
- There are two distinct reaction paths in the photochemistry of pyrene trapped in H2Oice. At low temperatures (<50 K), the chemistry is dominated by ion-molecule interactions and processes. At temperatures above 50 K, reactions are dominated by diffusing radical species.
- 6.
- A simple model indicates that, in dense clouds where AV=3, the rate of pyrene ionization is comparable to the rate of photodesorption in water-rich ices. Hence, chemical reactions involving pyrene and its cation, and other PAHs in general, may be important and should be taken into account in modeling grain chemistry in these environments.
This work is financially supported by ``Stichting voor Fundamenteel Onderzoek der Materie'' (FOM), ``the Netherlands Research School for Astronomy'' (NOVA) and NASA's Laboratory Astrophysics and Astrobiology Programs. L. J. Allamandola is especially grateful to the ``Nederlandse Organisatie voor Wetenschappelijk Onderzoek'' (NWO) for a visitors grant.
References
- Acharyya, K., Fuchs, G. W., Fraser, H. J., van Dishoeck, E. F., & Linnartz, H. 2007, A&A, 466, 1005 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Andersson, S., & van Dishoeck, E. F. 2008, A&A, 491, 907 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bernstein, M. P., Sandford, S. A., Allamandola, L. J., Chang, S., & Scharberg, M. A. 1995, ApJ, 454, 327 [NASA ADS] [CrossRef] [Google Scholar]
- Bernstein, M. P., Sandford, S. A., Allamandola, L. J., et al. 1999, Science, 283, 1135 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Bito, Y., Shida, N., & Toru, T. 2000, Chem. Phys. Lett., 328, 310 [NASA ADS] [CrossRef] [Google Scholar]
- Bouwman, J., Ludwig, W., Awad, Z., et al. 2007, A&A, 476, 995 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Bouwman, J., Paardekooper, D. M., Cuppen, H. M., et al. 2009, ApJ, 700, 56 [NASA ADS] [CrossRef] [Google Scholar]
- Briggs, R., Ertem, G., Ferris, J. P., et al. 1992, Origins of Life and Evolution of the Biosphere, 22, 287 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Draine, B. T., & Bertoldi, F. 1996, ApJ, 468, 269 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Draine, B. T., & Li, A. 2007, ApJ, 657, 810 [NASA ADS] [CrossRef] [Google Scholar]
- Draine, B. T., Dale, D. A., Bendo, G., et al. 2007, ApJ, 663, 866 [NASA ADS] [CrossRef] [Google Scholar]
- Fuchs, G. W., Cuppen, H. M., Ioppolo, S., et al. 2009, A&A, 505, 629 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Gudipati, M. 2004, J. Phys. Chem. A., 108, 4412 [CrossRef] [Google Scholar]
- Gudipati, M. S., & Allamandola, L. J. 2003, ApJ, 596, L195 [NASA ADS] [CrossRef] [Google Scholar]
- Gudipati, M. S., & Allamandola, L. J. 2004, ApJ, 615, L177 [NASA ADS] [CrossRef] [Google Scholar]
- Gudipati, M. S., & Allamandola, L. J. 2006a, J. Phys. Chem. A., 110, 9020 [CrossRef] [PubMed] [Google Scholar]
- Gudipati, M. S., & Allamandola, L. J. 2006b, ApJ, 638, 286 [NASA ADS] [CrossRef] [Google Scholar]
- Halasinski, T. M., Salama, F., & Allamandola, L. J. 2005, ApJ, 628, 555 [NASA ADS] [CrossRef] [Google Scholar]
- Hsiao, J. S., & Webber, S. E. 1992, J. Phys. Chem., 96, 2892 [CrossRef] [Google Scholar]
- Ioppolo, S., Cuppen, H. M., Romanzin, C., van Dishoeck, E. F., & Linnartz, H. 2008, ApJ, 686, 1474 [NASA ADS] [CrossRef] [Google Scholar]
- Kjaergaard, H. G., Robinson, T. W., & Brooking, K. A. 2000, J. Phys. Chem. A, 104, 11297 [CrossRef] [Google Scholar]
- Kobayashi, K. 1983, J. Phys. Chem., 87, 4317 [CrossRef] [Google Scholar]
- Langelaar, J., Wegdam-van Beek, J., Ten Brink, H., et al. 1970, Chem. Phys. Lett., 7, 368 [NASA ADS] [CrossRef] [Google Scholar]
- Li, A., & Draine, B. T. 2001, ApJ, 554, 778 [Google Scholar]
- Mathis, J. S. 1990, ARA&A, 28, 37 [NASA ADS] [CrossRef] [Google Scholar]
- Milosavljevic, B., & Thomas, J. 2002, Photochem. Photobiol. Sci., 1, 100 [CrossRef] [Google Scholar]
- Montejano, H. A., Cosa, J. J., Garrera, H. A., et al. 1995, J. Photochem. Photobiol. A: Chem., 86, 115 [CrossRef] [Google Scholar]
- Muñoz Caro, G. M., Meierhenrich, U. J., Schutte, W. A., et al. 2002, Nature, 416, 403 [NASA ADS] [CrossRef] [PubMed] [Google Scholar]
- Öberg, K. I., Fraser, H. J., Boogert, A. C. A., et al. 2007a, A&A, 462, 1187 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Öberg, K. I., Fuchs, G. W., Awad, Z., et al. 2007b, ApJ, 662, L23 [NASA ADS] [CrossRef] [Google Scholar]
- Öberg, K. I., Fayolle, E. C., Cuppen, H. M., van Dishoeck, E. F., & Linnartz, H. 2009a, A&A, 505, 183 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Öberg, K. I., Garrod, R. T., van Dishoeck, E. F., et al. 2009b, A&A, 504, 891 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Öberg, K. I., Linnartz, H., Visser, R., et al. 2009c, ApJ, 693, 1209 [NASA ADS] [CrossRef] [Google Scholar]
- Okada, T., Mori, T., & Mataga, N. 1976, Bull. Chem. Soc. Japan, 49, 3398 [CrossRef] [Google Scholar]
- Okada, T., Tashita, N., & Mataga, N. 1980, Chem. Phys. Lett., 75, 220 [NASA ADS] [CrossRef] [Google Scholar]
- Shen, C. J., Greenberg, J. M., Schutte, W. A., et al. 2004, A&A, 415, 203 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Smith, J. D. T., Draine, B. T., Dale, D. A., et al. 2007, ApJ, 656, 770 [NASA ADS] [CrossRef] [Google Scholar]
- Sternberg, A. 1988, ApJ, 332, 400 [NASA ADS] [CrossRef] [Google Scholar]
- Stevens, B., Thomaz, M. F., & Jones, J. 1967, J. Chem. Phys., 46, 405 [NASA ADS] [CrossRef] [Google Scholar]
- Tielens, A. G. G. M. 2008, ARA&A, 46, 289 [NASA ADS] [CrossRef] [EDP Sciences] [Google Scholar]
- Vala, M., Szczepanski, J., Pauzat, F., et al. 1994, J. Phys. Chem., 98, 9187 [Google Scholar]
- van Ijzendoorn, L. J., Allamandola, L. J., Baas, F., et al. 1983, J. Chem. Phys., 78, 7019 [NASA ADS] [CrossRef] [Google Scholar]
- Wang, B. C., Chang, J. C., Tso, H. C., Hsu, H. F., & Cheng, C. Y. 2003, J. Mol. Struct. (Theochem), 629, 11 [CrossRef] [Google Scholar]
- Weisman, J. L., Mattioda, A., Lee, T. J., et al. 2005, Phys. Chem. Chem. Phys. (Incorporating Faraday Transactions), 7, 109 [NASA ADS] [Google Scholar]
- Whittet, D. C. B. 2003, Dust in the galactic environment (Bristol: IOP Publ.) [Google Scholar]
- Whittet, D. C. B., Gerakines, P. A., Hough, J. H., et al. 2001, ApJ, 547, 872 [NASA ADS] [CrossRef] [Google Scholar]
- Woon, D. E., & Park, J.-Y. 2004, ApJ, 607, 342 [NASA ADS] [CrossRef] [Google Scholar]
All Tables
Table 1:
Band positions (![]() )
and FWHM in nm for pure pyrene ice at
10 K, pyrene in H2Oice at
25 K, pyrene in CO ice at 10 K, and photoproduct
bands for the Py:H2O and Py:CO UV processed ices.
)
and FWHM in nm for pure pyrene ice at
10 K, pyrene in H2Oice at
25 K, pyrene in CO ice at 10 K, and photoproduct
bands for the Py:H2O and Py:CO UV processed ices.
Table 2:
The reaction rates for the VUV photolysis of Py:H2O
(![]() 1:5000) ice
at 25 K compared to those for 4-methylypyrene:H2O
(1:>500) ice at 15 K (Gudipati
& Allamandola 2003).
1:5000) ice
at 25 K compared to those for 4-methylypyrene:H2O
(1:>500) ice at 15 K (Gudipati
& Allamandola 2003).
All Figures
![\begin{figure}
\par\includegraphics[width=16cm,clip]{13291fg1.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg8.png)
|
Figure 1:
The spectrum of a dilute pyrene:H2O ice after
900 s of VUV irradiation at 125 K. The inset shows a
blow-up of the pyrene photoproduct bands. Band assignments are
discussed in Sect. 3.
Note the broad feature ranging from about 350 to 470 nm which
is indicated by a Gaussian fit. This is attributed to overlapping bands
from individual pyrene photoproducts. Bands with negative optical depth
indicate species destruction, those with positive optical depth show
species formation. The blue bands are Gaussian profiles which co-add to
the overall fit shown in red. Note the instrumental resolution
indicated by the profile of the H |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=8.5cm,clip]{13291fg2.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg25.png)
|
Figure 2:
Integrated absorbance of the 445.6 nm Py |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=8.5cm]{13291fg3.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg28.png)
|
Figure 3:
Vibrational progression of HCO |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{13291fg4.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg31.png)
|
Figure 4:
Integrated absorbance of the 400 nm PyH |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=9cm,clip]{13291fg5.eps}\vspace{-3mm}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg36.png)
|
Figure 5: The VUV-induced spectroscopic changes in Py:H2O ice for two different temperatures as a function of photolysis time. Comparing the spectra from the 25 K ice ( bottom) with those of the 100 K ice ( top) shows the critical role that temperature plays in determining photochemical pathways in a PAH-containing ice. In the 25 K ice, cation formation is favored over production of the pyrene residue and the 400 and 405 nm band carriers. The opposite holds for the 100 K ice. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=14cm,clip]{13291fg6.eps}\vspace{3mm}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg37.png)
|
Figure 6:
The integrated absorbance of the Py 334 nm, Py |
| Open with DEXTER | |
| In the text | |
| |
Figure 7: Reaction scheme used to fit the experimental data. |
| Open with DEXTER | |
| In the text | |
![\begin{figure}
\par\includegraphics[width=8.5cm,clip]{13291fg8.eps}
\end{figure}](/articles/aa/full_html/2010/03/aa13291-09/Timg42.png)
|
Figure 8:
Parameters (
|
| Open with DEXTER | |
| In the text | |
Copyright ESO 2010
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.