| Issue |
A&A
Volume 526, February 2011
|
|
|---|---|---|
| Article Number | L11 | |
| Number of page(s) | 5 | |
| Section | Letters | |
| DOI | https://doi.org/10.1051/0004-6361/201016035 | |
| Published online | 14 January 2011 | |
Online material
Appendix A: Chemical model and new key processes
The present chemical model is based on that of Willacy & Cherchneff (1998, hereafter WC98) and includes all processes relevant to the hot and dense gas pertaining to the inner wind between 1 and 5 R ⋆ . However, many processes and their rates have been updated and/or added in view of the progresses made in chemical kinetics, and combustion and aerosol chemistry since 1998. The various types of chemical pathways considered in the model are summarised in Table A.1. Thermal fragmentation, i.e., destruction of molecules by collision with the ambient gas, unimolecular decomposition of aromatics and hydrocarbons, and radiative association reactions were added to the chemistry. The chemical network includes 59 species (some of which are listed in Table 2) and 370 chemical reactions. The rates for these chemical processes are taken from the National Institute of Standards and Technology database (NIST chemical kinetics database), and from the literature of combustion, atmopsheric, and material sciences.
In terms of formalism, three major changes are implemented with respect to WC98 and Cherchneff (2006). Firstly, the treatment of the reverse reaction of a specific process is changed. Several new rates were measured in combustion and aerosol chemistry and are now available. Therefore, instead of calculating the rate of the reverse process from detailed balance and the equilibrium constant (see Eq. (4) in WC98), we directly enter the available measured or calculated rate values in the chemical network. When the information is not available, we make “educated” guesses depending on the type of the reaction.
Secondly, the chemistry involving atomic silicon, Si, and Si-bearing species is restricted to reactions for which rates are measured or calculated. The reactions and rates derived from the isovalence of Si with carbon as stated in WC98 are abandoned. The prevalent formation reactions for SiO are 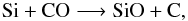 (A.1)and
(A.1)and 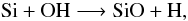 (A.2)\newpageReaction (A.1) is effective at forming SiO at temperatures ≥ 3000 K, whereas Reaction (A.2) contributes to most of the SiO synthesis at temperatures <3000 K. Destruction of SiO is mainly triggered by the opposite process of Reaction (A.2) at all temperatures. Reaction (A.2) is a competitive channel to the formation of H2O via Reaction (2), because it depletes OH radicals. But because of the high H2 content of the wind, the net formation rate of H2O according to Reaction (2) is much higher than that for SiO. Furthermore, a large quantity of atomic Si is tied up in silicon sulphide, SiS, leaving a reduced pool of available Si to form SiO. These combined effects result in water abundances higher than those of SiO, as seen in Fig. 2.
(A.2)\newpageReaction (A.1) is effective at forming SiO at temperatures ≥ 3000 K, whereas Reaction (A.2) contributes to most of the SiO synthesis at temperatures <3000 K. Destruction of SiO is mainly triggered by the opposite process of Reaction (A.2) at all temperatures. Reaction (A.2) is a competitive channel to the formation of H2O via Reaction (2), because it depletes OH radicals. But because of the high H2 content of the wind, the net formation rate of H2O according to Reaction (2) is much higher than that for SiO. Furthermore, a large quantity of atomic Si is tied up in silicon sulphide, SiS, leaving a reduced pool of available Si to form SiO. These combined effects result in water abundances higher than those of SiO, as seen in Fig. 2.
Thirdly, the chemistry now includes the formation of a larger set of molecules, some of them listed in Table 2. As explained in Sect. 3, the formation of single aromatic ring compounds (benzene and phenyl) is considered. Previous studies of the formation of polycyclic aromatic hydrocarbons in AGB winds never took into account the presence of O-bearing species in the shocked regions (Cherchneff 2010). The coupling of these C- and O-rich chemistries is important, because the unimolecular decomposition of hydrocarbons and aromatics replenish the gas in acetylene. Since C2H2 has large abundances in the inner wind and its formation and destruction processes are coupled to the H2 chemistry, it indirectly impacts on the formation of OH, H2O, and SiO through Reactions (1), (2), and (A.2). Furthermore, the potential oxidation of hydrocarbon precursors by O-bearing species, namely OH, is included and gives back H2O to the gas phase, albeit its impact is minor in replenishing water.
Chemical reaction types included in the chemical model of IRC+10216 inner wind.
© ESO, 2011
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


